The antibacterial properties of oral mucosal lamina propria-progenitor cells (OMLP-PCs) was investigated. Analysis of OMLP-PC conditioned media indicated constitutive secretion of osteoprotegerin and haptoglobin, and exposure of bacteria to these factors demonstrated their differential antibacterial properties. OMLP-PCs offer great potential in the development of novel cell- or soluble factor-based therapies for the treatment of infectious illnesses and chronic wounds.
Keywords: Oral mucosa, Progenitor cell, Antibacterial agents, Osteoprotegerin, Haptoglobin
Abstract
The oral cavity possesses a diverse microflora, yet recurrent infections within healthy individuals are rare. Wound healing within the buccal mucosa is preferential, potentially because of the presence of oral mucosal lamina propria-progenitor cells (OMLP-PCs). In addition to their multipotency, OMLP-PCs demonstrate potent immunosuppressive properties. The present study investigated whether OMLP-PCs possess antibacterial properties, directly interacting with microorganisms and contributing to the maintenance of a balanced oral microflora. Gram-positive and -negative bacteria were cocultured with OMLP-PCs, buccal mucosal fibroblasts, or their respective conditioned media (CM). Bacterial growth was significantly inhibited when cocultured with OMLP-PCs or their CM. No antibacterial activity was apparent within the fibroblasts. Analysis of the OMLP-PC CM indicated constitutive secretion of osteoprotegerin (OPG) and haptoglobin (Hp). Exposure of the bacteria to OPG or Hp demonstrated their differential antibacterial properties, with neutralization/blocking studies confirming that the growth of Gram-positive bacteria was partially restored by neutralizing OPG within OMLP-PC CM; blocking Hp restored the growth of Gram-negative bacteria. The present study demonstrates, for the first time, the broad-spectrum antibacterial properties of OMLP-PCs. We report the direct and constitutive antibacterial nature of OMLP-PCs, with retention of this effect within the CM suggesting a role for soluble factors such as OPG and Hp. Knowledge of the immunomodulatory and antibacterial properties of these cells could potentially be exploited in the development of novel cell- or soluble factor-based therapeutics for the treatment of infectious diseases such as pneumonia or ailments such as chronic nonhealing wounds.
Significance
Oral mucosal lamina propria-progenitor cells (OMLP-PCs) are a cell source with known immunomodulatory properties. The present report demonstrates the novel finding that OMLP-PCs possess potent antibacterial properties, halting the growth of Gram-positive and -negative bacteria through the secretion of soluble factors. OMLP-PCs constitutively secrete osteoprotegerin (OPG) and haptoglobin (Hp) at levels high enough to exert antibacterial action. OPG, a glycoprotein not previously known to be antibacterial, can suppress Gram-positive bacterial growth. Hp is only active against Gram-negative microorganisms. These findings indicate that OMLP-PCs could offer great potential in the development of novel cell- or soluble factor-based therapies for the treatment of infectious illness, such as bacterial pneumonia, through systemic infusion and of chronic wounds through local administration.
Introduction
Oral soft tissues are continually exposed to the external environment and are thus a critical entry point for potentially harmful bacteria [1]. However, chronic infections are rare, potentially because of antimicrobial factors secreted by resident cells within tissues such as the salivary glands [2]. A decrease in specific antimicrobial factors has been linked to the onset of oral diseases such as periodontitis and deficiencies in the human cathelicidin protein LL-37 [3].
Oral mucosal lamina propria-progenitor cells (OMLP-PCs) are isolated from buccal mucosal biopsies by differential adhesion to fibronectin [4]. These cells are clonally expanded and are CD44+CD90+CD105+CD166+ and CD34−CD45−. They are of neural crest origin (Slug+, Snail+, Sox10+, and Twist+) and are multipotent (mesenchymal and neural lineages) [4]. In addition to their plasticity, OMLP-PCs demonstrate potent immunosuppressive properties, suppressing lymphocyte proliferation in a contact- and dose-independent manner, indicating the importance of secreted factors in mediating their mechanism of action [5].
The immunosuppressive properties of bone marrow-derived mesenchymal stem cells (BMMSCs) are well documented [6]. BMMSCs require licensing to an anti-inflammatory phenotype, termed “MSC2,” by exposure to proinflammatory cytokines, such as interferon-γ (IFN-γ), to display these properties [7]. IFN-γ induces the expression of indoleamine 2,3-dioxygenase (IDO), the enzyme responsible for depleting tryptophan, an essential factor in suppressing lymphocyte proliferation [8].
Immunosuppressive factors, such as IDO, are also known to have prominent antibacterial actions. This enzyme also inhibits tryptophan-dependent bacteria such as group B streptococci [9] and Escherichia coli [10, 11] by the depletion of tryptophan. This knowledge has led to the hypothesis that stem cells can act with dual properties, displaying both immunomodulatory and antibacterial characteristics. However, limited studies have been reported with respect to the potential antibacterial properties of stem/progenitor cells. BMMSCs have been demonstrated to decrease the growth of a restricted number of bacteria in vitro by the secretion of antibacterial factors such as LL-37 [12]. In vivo studies have also documented the antibacterial nature of BMMSCs for the treatment of conditions such as sepsis. For example, BMMSCs stimulate bacterial clearance within the blood in both polymicrobial [13] and Gram-negative sepsis mouse models [14], potentially owing to an increase in the phagocytosis of bacteria.
Haptoglobin (Hp) is a multifunctional glycoprotein with known antibacterial and immunomodulatory properties affecting both the innate and the adaptive immune systems [15]. It is composed of two α and two β chains, with the type of α chain (α1 or α2) conveying the level of Hp activity. Three human forms are known: Hp1-1, Hp2-1, and Hp2-2. Hp1-1 with two α1 chains demonstrating the highest level of activity. Its ability to effectively bind hemoglobin and thereby deplete iron from the environment provides Hp with excellent antibacterial effects against multiple bacterial and fungal pathogens [10]. Normally associated as a positive acute phase protein, produced by the liver and found in increasing concentrations within the plasma as inflammation occurs, no association with stem/progenitor cells has yet been reported [16].
Osteoprotegerin (OPG) is a glycoprotein predominantly described as a decoy receptor for the receptor activator of nuclear factor κB ligand (RANKL), preventing nuclear κB activation. Activation of this pathway is central to the regulation of a number of key immune pathways and the process of osteoclastogenesis [17]. OPG is known to be a component of the BMMSC secretome, although it has been reported to not play a significant role in MSC-mediated immunosuppression [18], but rather in regulating bone resorption [19]. Numerous reports have highlighted a correlation among bone resorption, OPG, and periodontitis; a condition associated with the bacteria Porphyromonas gingivalis [20–22].
In the present report, we demonstrate, for the first time, the broad-spectrum antibacterial properties of OMLP-PCs. These findings suggest that this is an inherent and distinct property of this PC population that does not extend to stromal fibroblasts. We demonstrate that OMLP-PC-mediated antibacterial activity is through the constitutive secretion of soluble factors, including Hp and OPG, confirming that the OMLP-PC secretome is able to directly modulate bacterial growth in the absence of immune cell involvement. To our knowledge, this is the first report documenting the antibacterial properties of OPG and its ability to inhibit Gram-positive bacterial growth through a bacteriostatic mechanism. Future investigations will focus on identifying the potential for OMLP-PC usage as a cell- or soluble factor-based therapeutic in the treatment of infectious diseases.
Materials and Methods
Isolation and Maintenance of OMLP-PCs and Enriched Fibroblasts in Culture
Disease-free buccal mucosa biopsies (n = 4) were obtained from healthy donors undergoing orthognathic surgery at Cardiff and Vale University Health Board (Cardiff, U.K.). The local research ethical committee had previously approved the present study, and the donors were informed in accordance with the Declaration of Helsinki.
Single cell suspensions of the lamina propria were obtained by enzymatic digestion of the biopsy specimens, and OMLP-PCs were isolated and characterized as previously described [4, 23]. In brief, OMLP-PCs were separated from the lamina propria cell suspension by differential adhesion to fibronectin and expanded in monolayer culture. Cells remaining in suspension after multiple adhesion steps on fibronectin (i.e., the OMLP-PC-depleted stromal fraction) were termed “enriched fibroblasts” (EFs) and used as a control oral fibroblast cell population throughout the present study.
OMLP-PCs and EFs were maintained at 37°C in a 5% CO2 humidified atmosphere in Dulbecco’s modified Eagle’s medium supplemented with 10% (vol/vol) fetal calf serum (FCS), 2 mM l-glutamine, and antibiotics/antimycotics (100 U/ml penicillin G, 100 µg/ml streptomycin sulfate, and 0.25 µg/ml amphotericin B; Life Technologies, Paisley, U.K., http://www.thermofisher.com). To remove cells from plastic for subsequent experimental procedures, 0.05% (wt/vol) trypsin/0.53 mM EDTA (Life Technologies) was used, unless otherwise stated.
Maintenance of Microbiology Stocks
Enterococcus faecalis (NCTC 775), Pseudomonas aeruginosa (ATCC 15692), and Staphylococcus pyogenes (NCTC 8198) were maintained on tryptone soya agar (TSA; Oxoid Ltd., Basingstoke, U.K., http://www.oxoid.com). Proteus mirabilis (NCTC 11938) was maintained on cysteine lactose electrolyte deficient (CLED; Oxoid Ltd.) agar to prevent swarming of the bacteria. Bacteria were subcultured onto fresh agar plates weekly and grown overnight at 37°C. Agar plates were then stored at 4°C.
Coculture of OMLP-PCs, EFs, OPG, and Human Hp With Live Bacteria
OMLP-PCs and EFs (n = 4 donors) were used at 24–30 population doublings for the following experiments. OMLP-PCs were treated ±100 U/ml recombinant human IFN-γ (Sigma-Aldrich, Gillingham, U.K., http://www.sigmaaldrich.com) for 7 days before coculture with bacteria to induce an anti-inflammatory phenotype as confirmed by quantification of the IDO-inducing metabolite l-kynurenine [5].
OMLP-PCs with or without IFN-γ and EFs were seeded into 24-well plates at a density of 1 × 105 cells per well in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% (vol/vol) FCS and 2 mM l-glutamine. The cells were allowed to adhere overnight at 37°C in 5% CO2. Subsequent to adhesion, the culture medium was removed and exchanged for RPMI supplemented with 10% (vol/vol) FCS, 20% (vol/vol) brain heart infusion broth, and 2 mM l-glutamine with or without inoculation with 150 colony-forming units (CFUs) of bacteria. For the OPG and human Hp coculture experiments, recombinant human OPG (rhOPG; R&D Systems, Abingdon, U.K., http://www.rndsystems.com) or Hp (Sigma-Aldrich) was added to 150 CFU bacteria in suspension at 1–100 ng/ml or 10 pg/ml to 1 µg/ml, respectively.
The CFUs of each bacterium were previously calculated from 10- or 20-ml (S. pyogenes only) overnight cultures derived from single colonies. Cultures were spiral plated using a Whitley automatic spiral plater (Don Whitley Scientific, Shipley, U.K., http://www.dwscientific.co.uk) onto appropriate agar after 16 hours (for cocultures) and 23 hours (for susceptibility testing) at 37°C (and 5% CO2 for S. pyogenes). The bacterial colonies were subsequently counted in accordance with the manufacturer’s instructions, and the dilution for 150 CFUs was determined for each culture period.
The cocultures were incubated at 37°C and 5% CO2 for the calculated time to reach the mid-log phase for the control cultures (individual mid-log time points were previously determined for each bacterium using growth curves starting from an inoculation of 150 CFUs). Bacteria and OMLP-PC/EF-only cultures served as controls. The conditioned media (CM) was removed, serially diluted, and spiral plated onto TSA or CLED agar (P. mirabilis) and incubated overnight at 37°C before counting the bacterial colonies. The remaining CM was centrifuged at 500g for 5 minutes to remove any cell debris. For samples containing bacteria, the CM was further centrifuged at 14,000g for 2 minutes through a Pall Nanosep MF centrifugal device, Bio-Inert pore size of 0.2 µm (Sigma-Aldrich) to remove bacteria, and stored at −80°C.
Susceptibility Testing of Live Bacteria With OMLP-PC Secretome
A total of 100 CFUs of bacteria in phosphate-buffered saline was added to 90 µl of CM from the above coculture experiments. The cocultures were then incubated at 37°C and 5% CO2 for 16 hours. The bacterial cultures were serially diluted, spiral plated onto TSA or CLED (P. mirabilis) agar, and incubated overnight at 37°C before the bacterial colonies were counted as described above. The experiments were repeated with the addition of 0.6 µg/ml of human osteoprotegerin/TNFRSF11B neutralizing antibody (R&D Systems) or 24 µg/ml of the polyclonal rabbit anti-human Hp antibody (Dako, Ely, U.K., http://www.dako.com).
Quantification of OPG Gene Expression by OMLP-PCs
Total RNA was extracted from the OMLP-PCs after coculture with bacteria using the Illustra RNAspin Mini RNA isolation kit (GE Healthcare Life Sciences, Little Chalfont, U.K., http://www.gelifesciences.com) and stored at −80°C before use. cDNA was synthesized from 0.5 µg of total RNA using random hexamer primers and Moloney murine leukemia virus reverse transcriptase in accordance with the manufacturer’s instructions (Promega, Southampton, U.K., http://www.promega.com). The genomic level of OPG (forward: 5′-GAAGGGCGCTACCTTGAGAT-3′ and reverse: 5′-GCAAATGTATTTCGCTCTGG-3′) and β-actin (forward: 5′-AGCTACGAGCTGCCTGAC-3′ and reverse: 5′-AAGGTAGTTTCGTGGATGC-3′) within OMLP-PCs was determined by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) using Fast SYBR Green Master Mix (Life Technologies) according to the manufacturer’s instructions.
Quantification of OPG Level in OMLP-PC Secretome
The protein levels of secreted OPG were determined in CM samples from the coculture experiments using the human osteoprotegerin/TNFRSF11B DuoSet enzyme-linked immunosorbent assay (ELISA) (R&D Systems). The concentration of OPG in the samples was assessed using a standard curve of rhOPG from 0 to 4000 pg/ml.
Detection of Hp in OMLP-PC Secretome
CM derived from OMLP-PCs cultured with and without 100 U/ml IFN-γ for 7 days was combined with Laemmli buffer (62 mM Tris-HCL [pH 6.8], 10% [vol/vol] glycerol, 5% sodium dodecyl sulfate, 0.005% bromophenol blue, and 5% β-mercaptoethanol) and reduced by heating to 100°C for 2 minutes. The total protein concentration was determined using the bicinchoninic acid protein assay (ThermoFisher) to ensure equal loading.
Samples (35 µg) of total protein were separated on NuPAGE Novex 4%–12% Bis-Tris gels (Life Technologies). A positive control of 1 µg human Hp was added to one well (Sigma-Aldrich). The proteins were transferred to nitrocellulose membrane (HybondECL; GE Healthcare Life Sciences) overnight at 4°C, before rinsing the membrane in deionized water. The membrane was placed in 5% (wt/vol) nonfat milk for 1 hour at room temperature with agitation before probing with polyclonal rabbit anti-human Hp (1.2 µg/ml; Dako) diluted in 2% (wt/vol) nonfat milk in phosphate-buffered saline (PBS) with 0.1% Tween20 (PBS-T) for 1 hour at room temperature with agitation. The membrane was washed in PBS-T before being incubated with horseradish peroxidase-conjugated polyclonal swine anti-rabbit immunoglobulins (0.1 µg/ml; Dako) in 2% (wt/vol) nonfat milk diluted in PBS-T for 1 hour at room temperature. The membrane was washed in PBS-T before development using ECL Prime and exposure to Hyperfilm (GE Healthcare Life Sciences).
Assessing Effect of OMLP-PC Secretome, OPG, and Hp on Bacterial Viability
Bacteria were assessed for viability after exposure to OMLP-PC CM, rhOPG, and Hp, as detailed above. Bacteria were stained using the LIVE/DEAD BacLight Bacterial Viability Kit (Life Technologies) as per the manufacturer’s instructions. Bacteria were stained for 15 minutes before mounting onto poly-l-lysine microscope slides under a coverslip, using the BacLight mounting oil provided. The bacteria were visualized using a fluorescent microscope at ×100 objective under oil immersion (Olympus, Southend-on-Sea, U.K., http://www.olympus-lifescience.com). Live (green) and dead (red) bacterial cells were subsequently counted using Image Pro-Plus software, version 6.0 (Media Cybernectics, Bethesda, MD, http://www.mediacy.com). Individual bacteria in a minimum of four fields of view were counted per condition.
Statistical Analysis
All statistical analyses were performed using SPSS statistics, version 20 (IBM Corp., Armonk, NY, http://www-01.ibm.com/software/analytics/spss/). Statistical analysis to compare mean values was performed using one-way analysis of variance with post hoc Tukey test (equal variances assumed) or a Games-Howell test (unequal variances determined). Variance analysis was performed using Levene’s test. Significance was assumed at p ≤ .05.
Results
OMLP-PCs Significantly Reduce Growth of Both Gram-Positive and Gram-Negative Bacteria
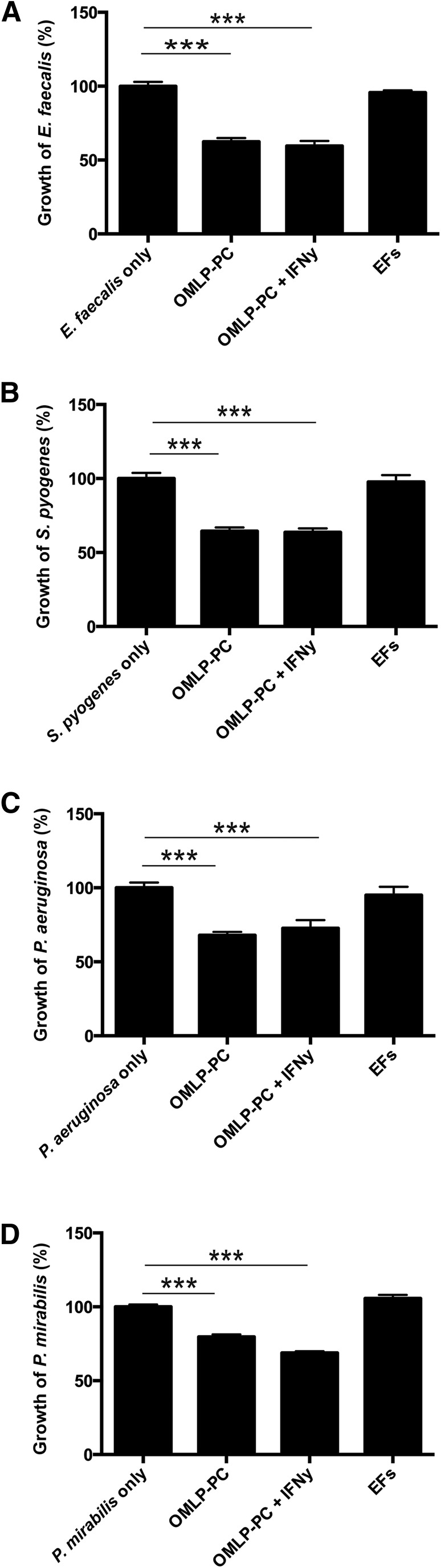
OMLP-PCs were cultured with Gram-positive (E. faecalis and S. pyogenes) and Gram-negative (P. aeruginosa and P. mirabilis) bacteria to the calculated mid-log time point. The growth of each bacterium, both Gram-positive (p < .001; Fig. 1A, 1B) and Gram-negative (p < .001; Fig. 1C, 1D), was significantly reduced when cocultured with OMLP-PCs. No further inhibitory effect was evident with previous IFN-γ licensing of the PCs (confirmation of IDO activity on IFN-γ licensing shown in supplemental online Fig. 1). Coculturing of the PC-depleted EFs with bacteria had no significant effect on the growth of any bacterium (Fig. 1A–1D).
Figure 1.
OMLP-PCs demonstrate broad-spectrum antibacterial effects in coculture with bacteria. OMLP-PCs significantly reduced the growth of Gram-positive E. faecalis (A) and S. pyogenes (B) and Gram-negative P. aeruginosa (C) and P. mirabilis (D) bacteria. This effect was observed irrespective of OMLP-PCs prestimulation with IFN-γ or not. EFs had no effect on bacterial growth. Data are expressed as the percentage of growth ± SEM, with bacteria-only cultures set to 100%. ∗∗∗, p ≤ .001. Abbreviations: EFs, enriched fibroblasts; IFN-γ, interferon-γ; OMLP-PCs, oral mucosal lamina propria-progenitor cells.
OMLP-PC Secretome Constitutively Reduces Growth of Gram-Positive and Gram-Negative Bacteria
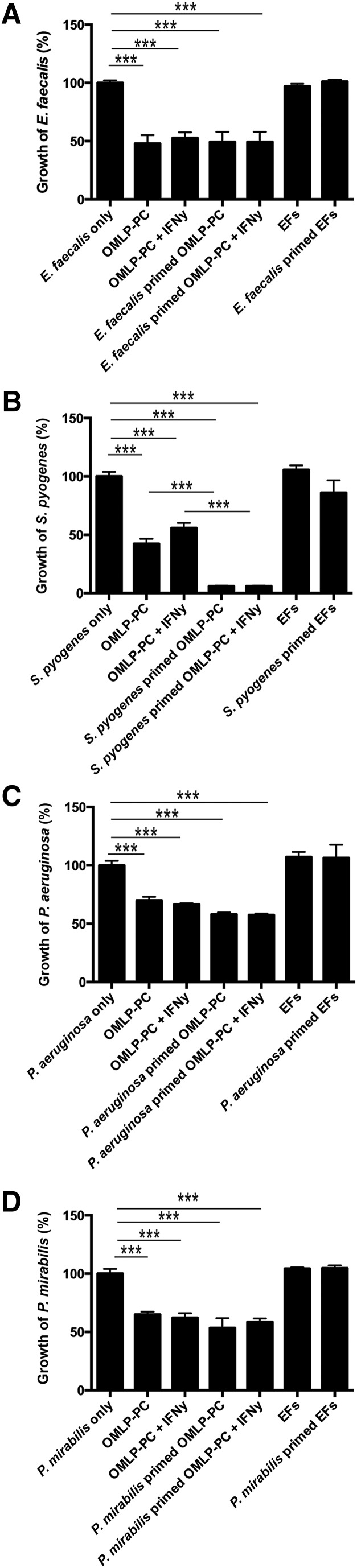
CM from OMLP-PCs significantly reduced the growth of each bacterium (p < .001; Fig. 2A–2D). No further inhibitory effect was demonstrated with CM derived from OMLP-PCs previously licensed with IFN-γ or primed by pre-exposure to the bacterium, with the exception of S. pyogenes. CM derived from OMLP-PCs previously exposed to S. pyogenes further suppressed the growth of this bacterium compared with CM derived from unprimed OMLP-PCs (p < .001; Fig. 2B). CM from EFs with or without previous exposure to each bacterium had no significant effect on the growth of any of the bacteria (Fig. 2A–2D).
Figure 2.
OMLP-PCs exert antibacterial effects through the secretion of soluble factors. Conditioned media (CM) derived from OMLP-PCs significantly reduced the growth of Gram-positive E. faecalis(A) and S. pyogenes (B) and Gram-negative P. aeruginosa (C) and P. mirabilis (D) bacteria. CM from EFs had no significant effect on bacterial growth. Data are expressed as the percentage of growth ± SEM, with bacteria-only cultures set to 100%. ∗∗∗, p ≤ .001. Abbreviations: EFs, enriched fibroblasts; IFN-γ, interferon-γ; OMLP-PCs, oral mucosal lamina propria-progenitor cells.
OPG Is Constitutively Expressed and Secreted by OMLP-PCs
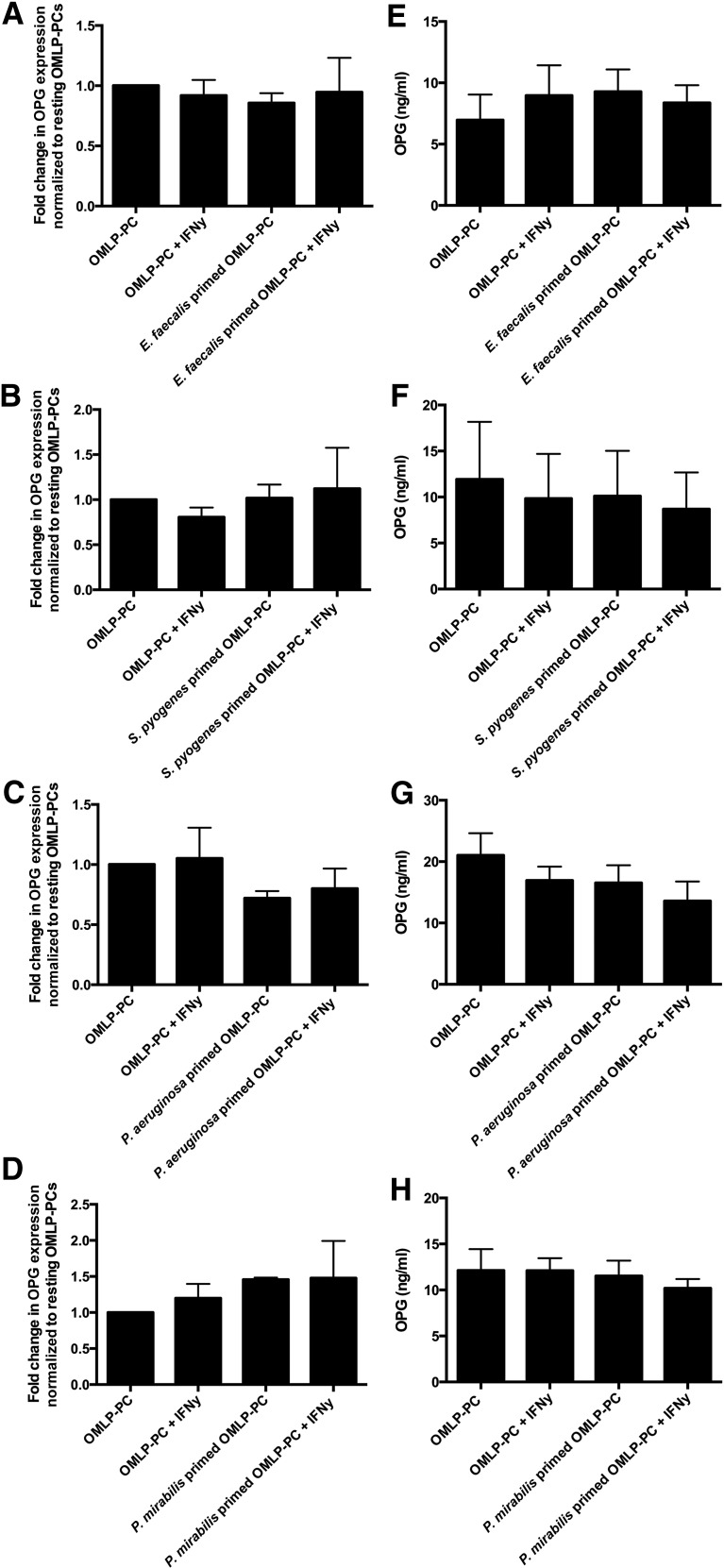
mRNA expression levels of OPG from OMLP-PCs were examined using qRT-PCR, and the secreted levels within CM were measured using ELISA. OPG was constitutively expressed (Fig. 3A–3D) and secreted (average secretion of 13 ng/ml in resting OMLP-PCs; Fig. 3E–3H) by OMLP-PCs, with IFN-γ and/or bacterial exposure having no further effect on these levels.
Figure 3.
OMLP-PCs constitutively express and secrete OPG. OPG expression and secretion was unchanged by IFN-γ priming of OMLP-PCs and/or exposure to Gram-positive E. faecalis (A, E) and S. pyogenes (B, F) or Gram-negative P. aeruginosa (C, G) and P. mirabilis (D, H) bacteria. Genomic expression data are presented as the fold change from resting OMLP-PCs ± SEM and OPG secretion data as ng/ml ± SEM. Abbreviations: EFs, enriched fibroblasts; IFN-γ, interferon-γ; OMLP-PCs, oral mucosal lamina propria-progenitor cells; OPG, osteoprotegerin.
OPG Exerts Antibacterial Activity Against Gram-Positive Bacteria
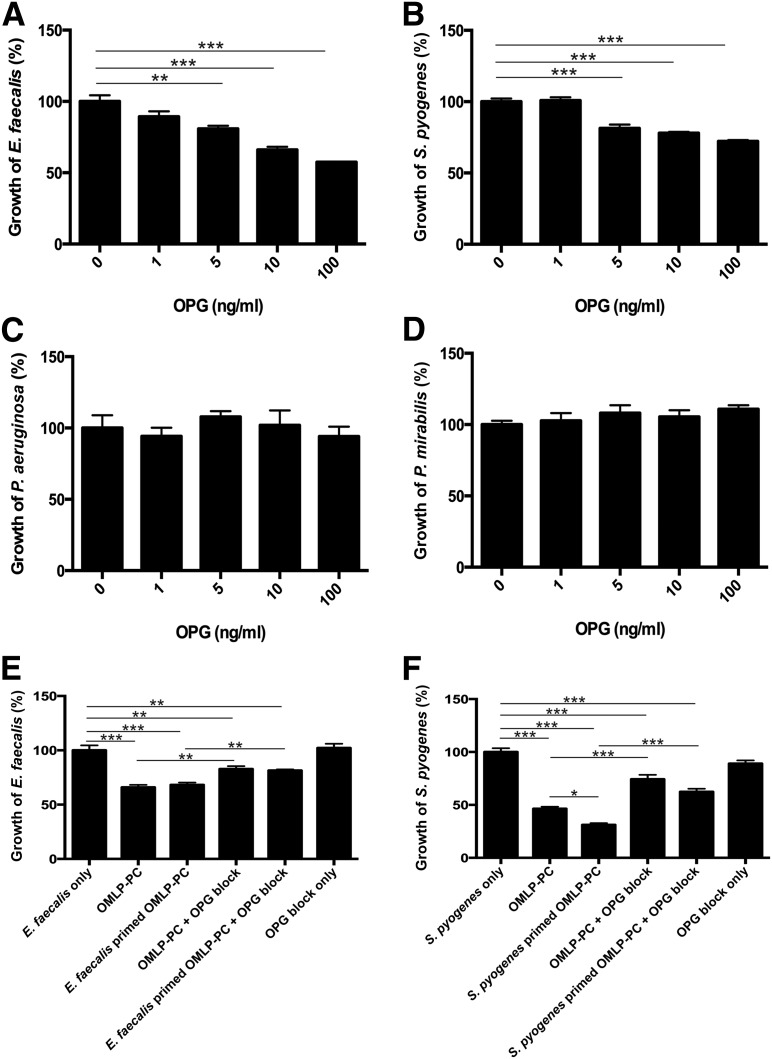
Bacteria were incubated with rhOPG at concentrations relevant to those secreted by OMLP-PCs to examine whether OPG possesses antibacterial properties. OPG significantly decreased the growth of Gram-positive bacteria (E. faecalis significant at 5 ng/ml, p ≤ .01; Fig. 4A; and S. pyogenes significant at 5 ng/ml, p ≤ .001; Fig. 4B). However, OPG demonstrated no significant effect on the growth of either Gram-negative bacteria (Fig. 4C, 4D).
Figure 4.
OPG demonstrated antibacterial properties against Gram-positive bacteria. OPG reduced the growth of Gram-positive bacteria E. faecalis (A) and S. pyogenes (B) only (no effect on Gram-negative bacteria [C, D]). Blocking OPG within the conditioned media of OMLP-PCs significantly restored the growth of Gram-positive E. faecalis (E) and S. pyogenes (F) bacteria. Data are expressed as the percentage of growth ± SEM, with bacteria-only cultures set to 100%. ∗, p ≤ .05; ∗∗, p ≤ .01; ∗∗∗, p ≤ .001. Abbreviations: EFs, enriched fibroblasts; IFN-γ, interferon-γ; OMLP-PCs, oral mucosal lamina propria-progenitor cells; OPG, osteoprotegerin.
Neutralizing OPG Partially Inhibits Antibacterial Properties of OMLP-PCs Against Gram-Positive Bacteria
CM derived from OMLP-PCs (with or without previous bacterial exposure) was incubated with live, Gram-positive bacteria in the presence or absence of an OPG-neutralizing antibody. Neutralization of the OMLP-PC (with or without bacterial exposure) secreted OPG significantly increased the growth of E. faecalis (OMLP-PC CM, p ≤ .01, and bacterially exposed OMLP-PC CM, p ≤ .05; Fig. 4E) and S. pyogenes (OMLP-PC CM, p ≤ .001, and bacterially exposed OMLP-PC CM, p ≤ .001; Fig. 4F) compared with CM that had not been neutralized. However, neither the growth of E. faecalis nor the growth of S. pyogenes was fully restored to control levels by neutralization of OPG (p ≤ .01, Fig. 4E; and p ≤ .001, Fig. 4F, respectively).
Hp Is Constitutively Secreted by OMLP-PCs
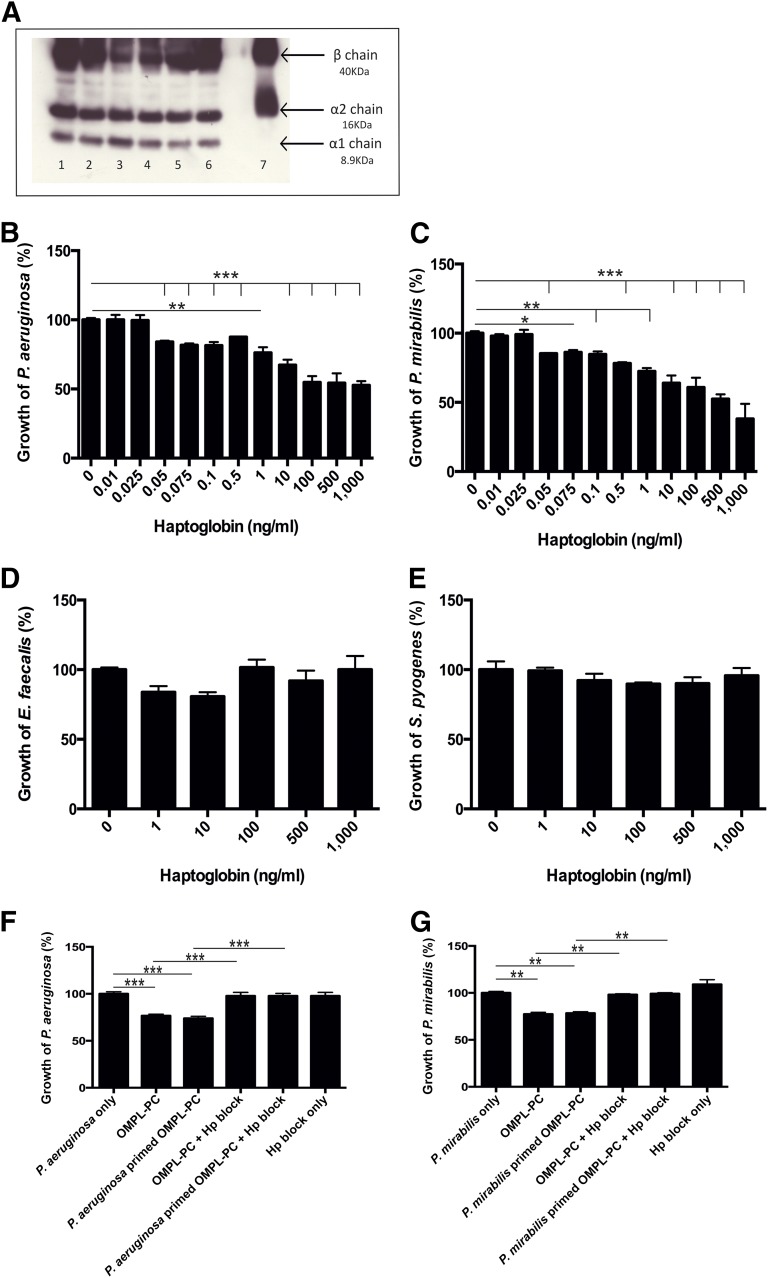
Hp secretion from OMLP-PCs was detected in the CM by Western blot, with all three chain variants detected, suggesting the constitutive secretion of the higher activity forms, Hp1-1 and Hp2-1 (Fig. 5A). Hp was secreted from OMLP-PCs, irrespective of IFN-γ stimulation of the cells.
Figure 5.
Haptoglobin is constitutively secreted by OMLP-PCs and demonstrates antibacterial properties against Gram-negative bacteria. (A): Hp was constitutively detected in the conditioned media (CM) of OMLP-PCs (n = 3 donors; lanes 2, 4, and 6, irrespective of IFN-γ stimulation of the cells [lanes 1, 3, 5]). Lane 7 indicates the Hp-positive control. Hp reduced the growth of P. aeruginosa (B) and P. mirabilis (C) bacteria only (no effect was seen on Gram-positive bacteria [D, E]). Blocking Hp in the CM restored the growth of the Gram-negative P. aeruginosa (F) and P. mirabilis (G) bacteria. Data are expressed as the percentage of growth ± SEM, with bacteria-only cultures set to 100%. ∗, p ≤ .05; ∗∗, p ≤ .01; ∗∗∗, p ≤ .001. Abbreviations: EFs, enriched fibroblasts; Hp, haptoglobin; IFN-γ, interferon-γ; OMLP-PCs, oral mucosal lamina propria-progenitor cells.
Hp Exerts Antibacterial Activity Against Gram-Negative Bacteria
Bacteria were incubated with Hp to examine its antibacterial properties. Hp significantly decreased the growth of both Gram-negative bacteria (P. aeruginosa was significant at 50 pg/ml, p ≤ .001 [Fig. 5B] and P. mirabilis was significant at 50 pg/ml, p ≤ .001 [Fig. 5C]). However, Hp demonstrated no significant effect on the growth of either Gram-positive bacterium (Fig. 5D, 5E).
Blocking Hp Inhibits Antibacterial Properties of OMLP-PCs Against Gram-Negative Bacteria
CM derived from OMLP-PCs (with or without previous bacterial exposure) was incubated with live, Gram-negative bacteria in the presence or absence of an anti-human Hp antibody. By blocking the secreted Hp in the CM samples of OMLP-PCs (with or without bacterial exposure), the growth of P. aeruginosa (p ≤ .001 for both, with or without bacterial exposure; Fig. 5F) and P. mirabilis (p ≤ .01 for both, with or without bacterial exposure; Fig. 5G) was significantly increased compared with samples in which Hp had not been blocked. The growth of P. aeruginosa and P. mirabilis was fully restored to control levels by blocking Hp.
OMLP-PC-Mediated Antibacterial Action Is Bacteriostatic
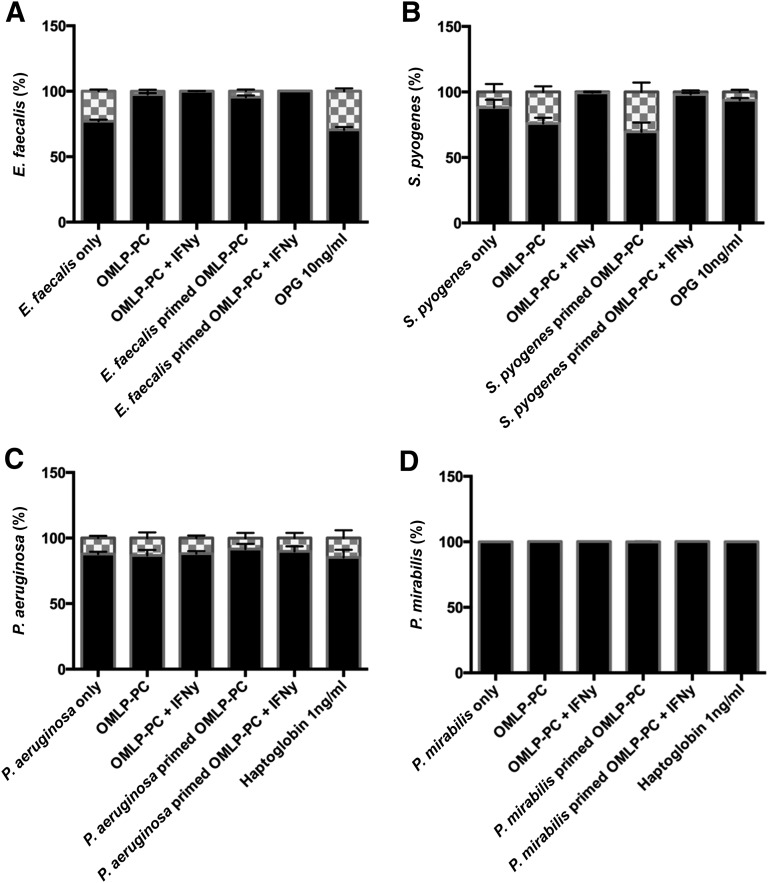
Bacterial cultures were incubated with CM derived from OMLP-PCs, rhOPG (Gram-positive bacteria), or Hp (Gram-negative bacteria), as previously stated. Subsequent to incubation, the bacterial cultures were stained using the LIVE/DEAD BacLight staining kit. Bacterial viability was not compromised when bacteria were incubated with CM with or without bacterial exposure, rhOPG, or Hp (Fig. 6A–6D).
Figure 6.
OMLP-PCs exert their antimicrobial actions via bacteriostatic mechanisms. LIVE/DEAD staining of E. faecalis (A), S. pyogenes (B), P. aeruginosa (C), and P. mirabilis (D) cultures after incubation with OMLP-PC conditioned media with or without bacterial exposure, recombinant human OPG and haptoglobin. No bactericidal effect was seen with any of the test conditions. Data are expressed as the percentage of live/dead cells ± SEM. Black bars indicate the percentage of live cells; and checkered bars, the percentage of dead cells. Abbreviations: EFs, enriched fibroblasts; IFN-γ, interferon-γ; OMLP-PCs, oral mucosal lamina propria-progenitor cells; OPG, osteoprotegerin.
Discussion
We have previously reported the potent immunomodulatory potential of OMLP-PCs and their mode of action through the release of soluble factors [5]. In addition to their immunosuppressive properties, BMMSCs have recently been reported to display antibacterial properties [12, 24], leading to the hypothesis that immunomodulatory OMLP-PCs might also exhibit equivalent or potentially enhanced antibacterial properties because of their anatomical site of origin. The present study has demonstrated a reproducible direct antibacterial action by OMLP-PCs against both Gram-positive and Gram-negative bacteria in a bacteriostatic manner through the constitutive release of the differentially acting soluble factors, OPG and Hp.
To date, limited evidence of stem cell-mediated antibacterial action has been documented. Both IDO and LL-37 have been implicated in the BMMSC mechanism of action [12, 24]. Within the present study, we found evidence for the importance of soluble factors in OMLP-PC-mediated antibacterial activity. However, in contrast to the BMMSC reports, no expression of LL-37 was seen within the OMLP-PCs (data not shown), and IDO, as we have previously reported, is only inducible by inflammatory stimuli and not constitutively expressed, as per the antibacterial effect shown [5].
Similar antibacterial effects of MSCs have been noted in murine models, with an increase in bacterial clearance on MSC treatment of sepsis [25] and E. coli-induced pneumonia [26], with lipocalin 2 thought to play a role in the latter. Our data have demonstrated that OMLP-PCs are antibacterial via the release of soluble factors. Additionally, we have shown that CM from OMLP-PCs is antibacterial through a bacteriostatic mechanism, ruling out soluble factors, which act in a bactericidal manner, such as the family of defensins and LL-37 [27]. The constitutive antibacterial nature of OMLP-PCs also differs from the reported mechanism seen in BMMSCs, for which exposure to bacteria is necessary for the cells to exert their effect by secreting soluble factors [12]. These differential modes of action among sources of stem cells is to be expected, and we hypothesize that in vivo priming and the niche environment from which the cells are derived will have a significant effect on their antibacterial properties.
OPG is vital in bone remodeling, regulating the process by acting as a decoy receptor for RANKL and influencing osteoclastogenesis [28]. The major bacterium involved in periodontal disease, P. gingivalis [29], is known to be capable of inducing OPG in human cells. The induction of OPG by P. gingivalis has been demonstrated in both endothelial cells [30] and gingival fibroblasts from both healthy and periodontitis patients, with the varying levels potentially correlating with disease status [31]. Patients with periodontitis display lower levels of OPG within the gingival crevicular fluid [20, 21] and gingival tissue [22] compared with healthy controls. However, each published study to date has compared OPG to RANKL within the context of bone remodeling and osteoclastogenesis. The clinical importance of OPG alone regarding bacterial infection during periodontal disease has not yet been explored.
We have demonstrated, for the first time, the novel antibacterial properties of OPG, which exerts a direct antibacterial action specifically against Gram-positive bacteria, through a bacteriostatic mechanism. OPG is constitutively secreted by OMLP-PCs at concentrations high enough to exert antibacterial effects. Furthermore, neutralization of OPG within the CM significantly restored the growth of the Gram-positive bacteria tested. However, because complete restoration in the growth of either bacterium to control levels was not observed, we hypothesize that OPG is not the only factor involved in this antibacterial mechanism against S. pyogenes and E. faecalis.
In contrast to the other Gram-positive bacteria used in the present study, the antibacterial effect of the CM was greater against S. pyogenes when previous exposure of the OMLP-PCs to the bacteria had occurred. We hypothesize that this might be a consequence of in vivo priming. OMLP-PCs will have been exposed to streptococcal species in vivo, because these are one of the predominate bacterial genera within the oral cavity [32]. This could explain the heightened response of the primed CM to S. pyogenes, because the cells might have been sensitized to recognize this genus. Further investigations will determine whether the CM acts in a similar manner to other bacteria commonly found within the oral cavity.
Hp has long been known to display antibacterial properties against the Gram-negative bacterium E. coli [11]. Hp’s mechanism of action is simple, sequestering iron and, therefore, reducing the iron available for bacteria [10]. Importantly, iron is crucial for bacterial growth, with a deficiency causing bacterial growth inhibition [33]. Although iron is essential for most bacteria, the levels required and uptake mechanisms vary considerably between microorganisms. Generally, Gram-negative bacteria recognize iron sources via an outer membrane receptor. The iron is then transported into the cell by an ATP-binding cassette transporter within the inner membrane [34]. Because Gram-positive bacteria lack an outer membrane, iron uptake mechanisms differ from that of the Gram-negative bacteria. The differences in iron uptake mechanisms are the likely reason Hp only demonstrated antibacterial properties against the Gram-negative bacteria examined. The studies confirmed the efficacy of Hp’s antibacterial properties, with bacteriostatic effects evident down to levels as low as 50 pg/ml. Western blot analysis identified multiple isoforms of Hp constitutively expressed within the secretome of the OMLP-PCs, with the presence of the α1 chain suggesting the production of the higher affinity Hp1-1 and/or Hp2-1 isoforms. Blocking of this protein was sufficient to completely restore bacterial growth, suggesting this is the major mechanism of OMLP-PC antibacterial action against Gram-negative bacteria.
Wound healing within the oral mucosa is well documented to be preferential, characterized by rapid re-epithelialization, remodeling of the extracellular matrix, and rapid transition through the inflammatory phase [23, 35–38]. Our previous work suggested a potential role for immunomodulatory OMLP-PCs resident within the lamina propria in orchestrating this healing response [4, 5]. The finding that this cell source possesses additional innate antibacterial properties supports the hypothesis that OMLP-PCs offer a novel cell source for the development of cell- or soluble factor-based local treatment of chronic nonhealing wounds or systemic infusions for infectious disorders such as bacterial pneumonia.
Additional studies are required to examine further the antibacterial factors secreted by the OMLP-PCs, particularly against Gram-positive bacteria as the presence of OPG did not fully explain the antibacterial properties of the cells. Future work will concentrate on investigating the potential factors involved in these antibacterial mechanisms and how these findings can be exploited for clinical translation.
Conclusion
Within the present study, we have demonstrated, for the first time, the broad spectrum antibacterial properties of OMLP-PCs. These PCs constitutively secrete antimicrobial soluble factors with bacteriostatic activity against both Gram-positive and Gram-negative bacteria. Analysis of the OMLP-PC secretome confirmed a role for OPG in the suppression of Gram-positive bacterial growth and for Hp in inhibiting Gram-negative bacteria. The findings we have reported illustrate the potential for OMLP-PCs in the development of novel antibacterial cell- or soluble factor-based therapies for conditions such as pneumonia or chronic nonhealing wounds.
Supplementary Material
Acknowledgments
This work was funded by Cardiff University, the Medical Research Council (Grant G0901562), Cardiff Institute of Tissue Engineering and Repair, and the Welsh Livery Guild. Buccal mucosal biopsy samples were kindly obtained from patients undergoing routine oral surgery by Andrew Cronin, Consultant Oral and Maxillofacial Surgeon (Cardiff and Vale University Health Board, Cardiff, U.K.). The present work is subjected to patent filing (patent application nos. PCT/GB2009/001443, AU2009259053, EP09761964.7, CN200980122121.0, JP501125275, HK1155775, and US12/997,363).
Author Contributions
E.B.-D.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; R.M.: collection and assembly of data; A.S.: conception and design, financial support, final approval of manuscript; P.S.: conception and design, financial support, provision of study material or patients, final approval of manuscript; L.C.D.: conception and design, financial support, provision of study material or patients, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
L.C.D. and P.S. have filed for patents. The other authors indicated no potential conflicts of interest.
References
- 1.Parahitiyawa NB, Scully C, Leung WK, et al. Exploring the oral bacterial flora: Current status and future directions. Oral Dis. 2010;16:136–145. doi: 10.1111/j.1601-0825.2009.01607.x. [DOI] [PubMed] [Google Scholar]
- 2.Gorr SU. Antimicrobial peptides of the oral cavity. Periodontol 2000. 2009;51:152–180. doi: 10.1111/j.1600-0757.2009.00310.x. [DOI] [PubMed] [Google Scholar]
- 3.Pütsep K, Carlsson G, Boman HG, et al. Deficiency of antibacterial peptides in patients with morbus Kostmann: An observation study. Lancet. 2002;360:1144–1149. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- 4.Davies LC, Locke M, Webb RD, et al. A multipotent neural crest-derived progenitor cell population is resident within the oral mucosa lamina propria. Stem Cells Dev. 2010;19:819–830. doi: 10.1089/scd.2009.0089. [DOI] [PubMed] [Google Scholar]
- 5.Davies LC, Lönnies H, Locke M, et al. Oral mucosal progenitor cells are potently immunosuppressive in a dose-independent manner. Stem Cells Dev. 2012;21:1478–1487. doi: 10.1089/scd.2011.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abumaree M, Al Jumah M, Pace RA, et al. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. 2012;8:375–392. doi: 10.1007/s12015-011-9312-0. [DOI] [PubMed] [Google Scholar]
- 7.Waterman RS, Tomchuck SL, Henkle SL, et al. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krampera M, Pasini A, Pizzolo G, et al. Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr Opin Pharmacol. 2006;6:435–441. doi: 10.1016/j.coph.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Hucke C, MacKenzie CR, Adjogble KD, et al. Nitric oxide-mediated regulation of gamma interferon-induced bacteriostasis: Inhibition and degradation of human indoleamine 2,3-dioxygenase. Infect Immun. 2004;72:2723–2730. doi: 10.1128/IAI.72.5.2723-2730.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barclay R. The role of iron in infection. Med Lab Sci. 1985;42:166–177. [PubMed] [Google Scholar]
- 11.Eaton JW, Brandt P, Mahoney JR, et al. Haptoglobin: A natural bacteriostat. Science. 1982;215:691–693. doi: 10.1126/science.7036344. [DOI] [PubMed] [Google Scholar]
- 12.Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall SR, Tsoyi K, Ith B, et al. Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: The importance of neutrophils. Stem Cells. 2013;31:397–407. doi: 10.1002/stem.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krasnodembskaya A, Samarani G, Song Y, et al. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1003–L1013. doi: 10.1152/ajplung.00180.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadrzadeh SM, Bozorgmehr J. Haptoglobin phenotypes in health and disorders. Am J Clin Pathol. 2004;121(suppl):S97–S104. doi: 10.1309/8GLX5798Y5XHQ0VW. [DOI] [PubMed] [Google Scholar]
- 16.Huntoon KM, Wang Y, Eppolito CA, et al. The acute phase protein haptoglobin regulates host immunity. J Leukoc Biol. 2008;84:170–181. doi: 10.1189/jlb.0208100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin Z, Li X, Wan Y. Minireview: Nuclear receptor regulation of osteoclast and bone remodeling. Mol Endocrinol. 2015;29:172–186. doi: 10.1210/me.2014-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Blanc K, Rasmusson I, Götherström C, et al. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004;60:307–315. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- 19.Oshita K, Yamaoka K, Udagawa N, et al. Human mesenchymal stem cells inhibit osteoclastogenesis through osteoprotegerin production. Arthritis Rheum. 2011;63:1658–1667. doi: 10.1002/art.30309. [DOI] [PubMed] [Google Scholar]
- 20.Bostanci N, Ilgenli T, Emingil G, et al. Differential expression of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin mRNA in periodontal diseases. J Periodontal Res. 2007;42:287–293. doi: 10.1111/j.1600-0765.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 21.Mogi M, Otogoto J, Ota N, et al. Differential expression of RANKL and osteoprotegerin in gingival crevicular fluid of patients with periodontitis. J Dent Res. 2004;83:166–169. doi: 10.1177/154405910408300216. [DOI] [PubMed] [Google Scholar]
- 22.Liu D, Xu JK, Figliomeni L, et al. Expression of RANKL and OPG mRNA in periodontal disease: Possible involvement in bone destruction. Int J Mol Med. 2003;11:17–21. doi: 10.3892/ijmm.11.1.17. [DOI] [PubMed] [Google Scholar]
- 23.Stephens P, Davies KJ, al-Khateeb T, et al. A comparison of the ability of intra-oral and extra-oral fibroblasts to stimulate extracellular matrix reorganization in a model of wound contraction. J Dent Res. 1996;75:1358–1364. doi: 10.1177/00220345960750060601. [DOI] [PubMed] [Google Scholar]
- 24.Meisel R, Brockers S, Heseler K, et al. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia. 2011;25:648–654. doi: 10.1038/leu.2010.310. [DOI] [PubMed] [Google Scholar]
- 25.Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 26.Gupta N, Krasnodembskaya A, Kapetanaki M, et al. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67:533–539. doi: 10.1136/thoraxjnl-2011-201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zasloff M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J Am Soc Nephrol. 2007;18:2810–2816. doi: 10.1681/ASN.2007050611. [DOI] [PubMed] [Google Scholar]
- 28.Martin TJ. Historically significant events in the discovery of RANK/RANKL/OPG. World J Orthop. 2013;4:186–197. doi: 10.5312/wjo.v4.i4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slots J, Ting M. Systemic antibiotics in the treatment of periodontal disease. Periodontol 2000. 2002;28:106–176. doi: 10.1034/j.1600-0757.2002.280106.x. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi-Sakamoto M, Hirose K, Isogai E, et al. NF-kappaB-dependent induction of osteoprotegerin by Porphyromonas gingivalis in endothelial cells. Biochem Biophys Res Commun. 2004;315:107–112. doi: 10.1016/j.bbrc.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 31.Baek KJ, Choi Y, Ji S. Gingival fibroblasts from periodontitis patients exhibit inflammatory characteristics in vitro. Arch Oral Biol. 2013;58:1282–1292. doi: 10.1016/j.archoralbio.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cherayil BJ. The role of iron in the immune response to bacterial infection. Immunol Res. 2011;50:1–9. doi: 10.1007/s12026-010-8199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krewulak KD, Vogel HJ. Structural biology of bacterial iron uptake. Biochim Biophys Acta 2008;1778:1781–1804. [DOI] [PubMed] [Google Scholar]
- 35.Stephens P, Hiscox S, Cook H, et al. Phenotypic variation in the production of bioactive hepatocyte growth factor/scatter factor by oral mucosal and skin fibroblasts. Wound Repair Regen. 2001;9:34–43. doi: 10.1046/j.1524-475x.2001.00034.x. [DOI] [PubMed] [Google Scholar]
- 36.Stephens P, Davies KJ, Occleston N, et al. Skin and oral fibroblasts exhibit phenotypic differences in extracellular matrix reorganization and matrix metalloproteinase activity. Br J Dermatol. 2001;144:229–237. doi: 10.1046/j.1365-2133.2001.04006.x. [DOI] [PubMed] [Google Scholar]
- 37.Enoch S, Wall I, Peake M, et al. Increased oral fibroblast lifespan is telomerase-independent. J Dent Res. 2009;88:916–921. doi: 10.1177/0022034509342979. [DOI] [PubMed] [Google Scholar]
- 38.Enoch S, Peake MA, Wall I, et al. “Young” oral fibroblasts are geno/phenotypically distinct. J Dent Res. 2010;89:1407–1413. doi: 10.1177/0022034510377796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.