Chemical approaches using small molecules to steer biological systems toward specific outcomes have been proven to be especially useful in studying stem cell biology. This study demonstrates the use of chemical approaches in maintaining human embryonic stem cell-derived hepatoblasts in a stable, self-renewing state. A similar strategy could be explored to enable self-renewal of other clinically relevant somatic stem cells originally derived from human pluripotent stem cells.
Keywords: Hepatoblasts, Human embryonic stem cells, Chemical approaches, Differentiation
Abstract
Somatic stem cells play crucial roles in organogenesis and tissue homeostasis and regeneration and may ultimately prove useful for cell therapy for a variety of degenerative diseases and injuries; however, isolation and expansion of most types of somatic stem cells from tissues are technically challenging. Human pluripotent stem cells are a renewable source for any adult cell types, including somatic stem cells. Generation of somatic stem cells from human pluripotent stem cells is a promising strategy to get these therapeutically valuable cells. Previously, we developed a chemically defined condition for mouse hepatoblast self-renewal through a reiterative screening strategy. In the present study, we efficiently generated hepatoblasts from human embryonic stem cells by a stepwise induction strategy. Importantly, these human embryonic stem cell-derived hepatoblasts can be captured and stably maintained using conditions previously established for mouse hepatoblast self-renewal, which includes basal media supplemented with insulin, transferrin, sodium selenite, epidermal growth factor, glycogen synthase kinase 3 inhibitor, transforming growth factor β receptor inhibitor, lysophosphatidic acid, and sphingosine 1-phosphate. The cells can stably retain hepatoblast phenotypes during prolonged culture and can differentiate into mature hepatocytes through in vitro provision of hepatocyte lineage developmental cues. After being embedded into three-dimensional Matrigel, these cells efficiently formed bile duct-like structures resembling native bile duct tissues. These human embryonic stem cell-derived hepatoblasts would be useful as a renewable source for cell therapy of liver diseases.
Significance
Somatic stem cells have been proposed as promising candidates for cell-based therapy; however, isolation of somatic stem cells from adult tissues is usually invasive and technically challenging. In the present study, hepatoblasts from human embryonic stem cells were efficiently generated. These human hepatoblasts were then stably captured and maintained by a growth factor and small molecule cocktail, which included epidermal growth factor, glycogen synthase kinase 3 inhibitor, transforming growth factor β receptor inhibitor, lysophosphatidic acid, and sphingosine 1-phosphate. These human embryonic stem cell-derived hepatoblasts would be useful as a renewable source for cell therapy of liver diseases.
Introduction
The liver is an important organ that performs essential functions related to digestion, metabolism, and detoxification. Viral diseases such as hepatitis and xenobiotic chemical exposure can cause serious liver damage. Liver transplantation remains the only viable treatment for end-stage liver diseases; however, this treatment is limited by a critical shortage of transplantable livers. Hepatocyte or hepatic stem/progenitor cell transplantation is being considered as an alternative to liver transplantation and has proved to be highly effective in preclinical studies using animal models [1, 2]. Unfortunately, extracting hepatocytes from liver tissues and expanding them in vitro is difficult. To realize cell therapy for liver diseases, a renewable cell source of hepatocytes has to be established, and stem cells harbor great promise in this context. Recently, significant progress has been made in generating hepatocytes from self-renewing human pluripotent stem cells [3–8]; however, it is still challenging to capture and maintain hepatic stem/progenitor cells that are generated during human pluripotent stem cell differentiation, ideally, under defined conditions. Recently, liver progenitors have been derived from human pluripotent stem cells and successfully cultured [9–11], but these cultured system included complex and undefined components such as fetal bovine serum or feeder cells that were not suitable for clinical development. Through a reiterative screening strategy, we recently identified a growth factor/small molecule cocktail capable of supporting murine hepatoblast self-renewal. This cocktail, hereinafter referred to as ECELS media, contains epidermal growth factor (EGF), glycogen synthase kinase 3 (GSK3) inhibitor (CHIR99021), transforming growth factor β (TGF-β) receptor inhibitor (E-616452 or SB431542), lysophosphatidic acid (LPA), and sphingosine 1-phosphate (S1P) [12]. It was unknown whether ECELS media could support the self-renewal of human hepatoblasts. In the current study, human embryonic stem cells (hESCs) were differentiated toward human hepatoblasts through a stepwise strategy. These hESC-derived hepatoblasts (hHBs) were then captured and successfully maintained in ECELS media. hHBs stably retain hepatoblast phenotypes during prolonged in vitro culture. When provided with hepatocyte lineage-commitment signals, the cells can be induced to further differentiate and mature. After being embedded into three-dimensional (3D) Matrigel, the cells efficiently form bile duct-like structures that resemble native bile duct tissues. Our study provides a framework to generate self-renewing human hepatoblasts from pluripotent stem cells, which could be useful as a fertile and renewable source of hepatocytes for cell replacement therapy of liver diseases.
Materials and Methods
hESC Culture and Hepatic Differentiation
hESCs, H1 (passage 50) and Hues9 (passage 20) were cultured in Dulbecco’s modified Eagle’s medium (DMEM/F12), 1× N2, 1× B27, 1% penicillin/streptomycin, 20 ng/ml fibroblast growth factor 2 (FGF2), and 2 ng/ml TGF-β on dishes coated with 2% Matrigel (Growth Factor Reduced; BD Biosciences, San Jose, CA, http://www.bdbiosciences.com), as described previously [13]. The cells were regularly passaged using TrypLE. hESCs at approximately 50% confluence were cultured in differentiation medium (DMEM/F12, 1× B27 [minus insulin]) supplemented with 100 ng/ml activin A (R&D Systems, Minneapolis, MN, https://www.rndsystems.com/) and 2 mM GSK3 inhibitor CHIR99021 (Tocris Bioscience, Bristol, U.K., http://www.tocris.com) for 2 days and then treated by differentiation medium supplemented with 100 ng/ml activin A, 10 ng/ml vascular endothelial growth factor (VEGF), 10 ng/ml FGF2, and 0.25 ng/ml bone morphogenetic protein 4 (BMP4) for another 3 days to induce definitive endoderm differentiation. The definitive endoderm population was then treated with 2 μM TGF-β receptor inhibitor SB431542 (Tocris) and 10 ng/ml FGF7 for 2 days in differentiation medium to induce a primitive gut tube, which was subsequently differentiated into hepatoblasts by treatment with 10 ng/ml BMP4 and 10 ng/ml FGF2 for 4 days in DMEM/F12, 1× N2, and 1× B27. These hHBs were then maintained on a Matrigel-coated surface in ECELS media: DMEM/F12 supplemented with insulin-transferrin-sodium selenite (ITS; 5 μg/ml insulin, 10 μg/ml transferrin, and 6.7 ng/ml sodium selenite; Sigma-Aldrich, St. Louis, MO, https://www.sigmaaldrich.com), 5 mM nicotinamide (Sigma-Aldrich), 30 μg/ml 2-phospho-l-ascorbic acid (Sigma-Aldrich), and 50 μg/ml bovine serum albumin (BSA) or human recombinant albumin (Sigma-Aldrich), 1% penicillin/streptomycin, 10 ng/ml EGF, 3 μM CHIR99021, 2 μM SB431542, and 5 μM LPA and 0.5 μM S1P (Sigma-Aldrich). The culture was routinely split 1:6 using TrypLE. Overnight treatment with myosin II ATPase inhibitor (2 μM Blebbistatin; Tocris) was used to improve cell survival after cell passage during hepatic induction but was not required for routine hHB culture. To analyze cell proliferation, hHBs were seeded in Matrigel-coated 96-well plate at 3,000 cells/well. The growth curve of hHBs was generated using the CyQUANT NF Cell Proliferation Assay Kit (Invitrogen; Thermo Fisher Scientific, Waltham, MA, https://www.thermofisher.com), according to the product manual.
The derivation and long-term culture of hHBs were reproducible in both H1 and Hues9 cell lines. The results shown in the present study represented the data of experiments using H1 cells. All tissue culture reagents and growth factors were obtained from Invitrogen except as noted.
Immunocytochemistry and Flow Cytometry
Immunofluorescence assays and fluorescence-activated cell sorting (FACS) analyses were performed, as described previously [12]. Briefly, cells were fixed in 4% paraformaldehyde (Sigma-Aldrich), washed three times with phosphate-buffered saline (PBS) containing 0.1% Triton X-100 (Sigma-Aldrich), and incubated in blocking buffer, 0.1% Triton X-100, and 5% normal donkey serum (Jackson ImmunoResearch Inc, West Grove, PA, https://www.jacksonimmuno.com) in PBS for 30 minutes at room temperature. The cells were then incubated with primary antibody overnight at 4οC in blocking buffer. Cells were then stained with compatible Alexa Fluor-conjugated secondary antibodies (1,000×; Invitrogen) in PBS containing 0.1% Triton X-100 for 30 minutes at room temperature. The primary antibodies are described in supplemental online Table 1. For intracellular flow cytometry assay, cells were fixed and permeabilized using the BD Cytofix/Cytoperm kit (BD Biosciences). The cells were incubated with fluorescein isothiocyanate-conjugated mouse anti-epithelial cell adhesion molecule (EpCAM) (EBA-1; Santa Cruz Biotechnology, Dallas, TX, http://www.scbt.com), and Alexa Fluor 647-conjugated mouse anti-Ki-67 (B56; BD Biosciences) for 30 minutes at 4οC. Corresponding isotype antibodies were used as controls. For indirect flow cytometry, fixed cells were incubated with mouse anti-α1-fetoprotein (anti-AFP) for 30 minutes on ice and washed three times. Cells were then incubated with Alexa Fluor 488 F(ab′)2 fragment of donkey anti-mouse IgG (1:2,000; Invitrogen) for 30 minutes on ice and washed three times. Cells directly incubated with secondary antibody were used as control. Flow cytometry analysis was carried out with a Miltenyi cytometer (Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com).
Hepatocyte Differentiation and Hepatocyte Functional Analysis
hHBs were cultured on Matrigel-coated six-well plates. When cells reached 50% confluence, hepatocyte differentiation was induced by switching the cultures to Williams medium supplemented with 20 ng/ml hepatocyte growth factor (HGF; PeproTech, Rocky Hill, NJ, https://www.peprotech.com), 20 ng/ml oncostatin M (OSM; PeproTech), 0.1 μM dexamethasone (Sigma-Aldrich), 2 μM TGF-β receptor inhibitor (SB431542), and 1 μM γ-secretase inhibitor (RO4929097; Selleck Chemicals, Houston, TX, http://www.selleckchem.com) for 2 weeks. Glycogen storage was revealed using a periodic acid-Schiff staining kit (Sigma-Aldrich), according to the product instructions. Dil-labeled low-density lipoprotein (Dil-LDL; Invitrogen) and cholyl-l-lysyl-fluorescein (BD Bioscience) were used according to the manufacturers’ instructions. Albumin secretion was analyzed by using a human albumin enzyme-linked immunosorbent assay (ELISA) kit (Bethyl Laboratories, Montgomery, TX, https://www.bethyl.com), as specified in the product insert. An ethoxyresorufin-O-deethylase (EROD) assay was performed, as previously described [14, 15].
Bile Duct Induction
Bile duct induction was performed, as described previously [12]. Briefly, hHBs were dissociated into single cells using TrypLE, suspended in cold DMEM/F12 supplemented with ITS and 20 ng/ml EGF (2,000 cells/ml), and mixed 1:1 with growth factor-reduced Matrigel. The cold mixture was then aliquotted into a 24-well plate at 0.5 ml/well. The plate was incubated in an incubator at 37οC for 2 hours to allow the formation of gel. Thereafter, 0.5 ml DMEM/F12 supplemented with ITS and 20 ng/ml EGF was overlaid on the gel (0.5 ml/well). The cells formed bile duct-like structures after 1-week culture. F-actin was revealed using Alexa Fluor 555-conjugated phalloidin (Invitrogen). Immunostaining was performed as described. The primary antibodies are shown in supplemental online Table 1.
Polymerase Chain Reaction Analyses
For the semiquantitative reverse transcription polymerase chain reaction (RT-PCR) analyses, RNA was extracted using Trizol reagent (Invitrogen). Reverse transcription was performed with 1 μg of RNA using the High-Capacity cDNA Reverse Transcription Kit (Invitrogen). The primers used are shown in supplemental online Table 2. The RT-PCR was performed in 30 cycles (94°C for 30 seconds, annealing temperature for 30 seconds, and 72°C for 30 seconds) using the Taq PCR Mastermix (Tiangen Biotech (Beijing) Co., Ltd., People’s Republic of China, http://www.tiangen.com).
Results
Generation of Hepatoblasts From Human Embryonic Stem Cells
hESCs (H1 and Hues9 lines) were cultured under feeder-free conditions on Matrigel [13]. The cells homogeneously expressed pluripotent markers, including octamer-binding transcription factor 4 (Oct4), stage-specific embryonic antigen-3 (SSEA3), SRY (sex determining region Y)-box 2 (Sox2), and SSEA4 (Fig. 1). The pluripotency of hESCs was further confirmed by the standard embryoid body differentiation methods, as reported [16]. Immunocytochemisty assays confirmed that hESCs cultured under feeder-free conditions can differentiate into endoderm (Sox17), mesoderm (brachyury), and neuroectoderm (βIII-tubulin) derivatives (supplemental online Fig. 1).
Figure 1.
Feeder-free culture of human embryonic stem cells. The cells homogeneously expressed pluripotent markers, including octamer-binding transcription factor 4 (Oct4) (A), stage-specific embryonic antigen-3 (SSEA3) (B), SRY (sex determining region Y)-box 2 (Sox2) (C), and SSEA4 (D). Magnification: ×100. Abbreviation: DAPI, 4′,6-diamidino-2-phenylindole.
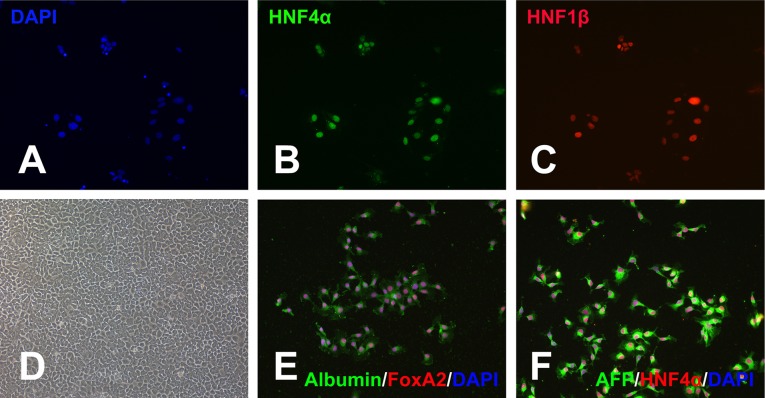
When the hESCs reached 50% confluence, hepatic differentiation was induced by a stepwise strategy, as reported, with modification (Fig. 2A) [17]. hESCs were treated with 100 ng/ml activin A and 2 μM CHIR99021 for 2 days in insulin-free differentiation medium to induce primitive streak, which was then patterned toward definitive endoderm by treatment with 100 ng/ml activin A, 10 ng/ml VEGF, 10 ng/ml FGF2, and 0.25 ng/ml BMP4 for another 3 days. Definitive endoderm was positive for Sox17 and forkhead box protein A2 (FoxA2) (Fig. 2B, 2C) but negative for hepatocyte nuclear factor 4α (HNF4α). FACS analysis confirmed that more than 80% of cells coexpressed definitive endoderm markers Sox17/C-X-C chemokine receptor type 4 (CXCR-4) (Fig. 2D). In contrast, endoderm differentiation was less efficient when using B27 supplements with insulin (∼50% cells were positive for FoxA2, and Oct4 expression was prevalent after induction) (Fig. 2E). The definitive endoderm population was further patterned toward primitive gut tube, which was positive for HNF4α and HNF1β (Fig. 3A–3C), by treating with 2 μM SB431542 and 10 ng/ml FGF7 for 2 days in differentiation medium [18, 19]. Primitive gut tube was subsequently treated with 10 ng/ml BMP4 and 10 ng/ml FGF2 for 4 days in DMEM/F12 supplemented with N2 and B27 to induce hepatoblasts. This strategy can generate human hepatoblasts with very high efficiency. Figure 3D shows the phase-contrast microscope image of hepatoblasts soon after hepatic induction. Immunocytochemistry assay demonstrated that more than 95% cells were double-positive for albumin/FoxA2 or AFP/HNF4α (Fig. 3E, 3F).
Figure 2.
The induction of endoderm from hESCs. (A): Schematic representation of the hepatic induction process. hESCs were sequentially induced into primitive streak and definitive endoderm. Definitive endoderm was positive for forkhead box protein A2 (FoxA2) (B) and SRY (sex determining region Y)-box 17 (Sox17) (C). Fluorescence-activated cell sorting analysis confirmed that more than 80% of cells coexpressed definitive endoderm markers Sox17/C-X-C chemokine receptor type 4 (CXCR-4) (D); however, endoderm induction became less efficient when using B27 supplements with insulin (E). Magnification (B, C, E): ×200. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; DMEM/F12, Dulbecco’s modified Eagle’s medium, nutrient mixture F-12; FITC, fluorescein isothiocyanate; hESC, human embryonic stem cell; PE, phycoerythrin.
Figure 3.
The induction of hepatoblasts. Definitive endoderm was treated with SB431542 and FGF7 to induce primitive gut tube, which was positive for HNF4α and HNF1β (A–C). Primitive gut tube was then induced into hepatoblasts by treatment with BMP4 and FGF2 (D–F). (D): Phase-contrast microscope image of human embryonic stem cell-derived hepatoblasts. These cells were analyzed for the expression of albumin/forkhead box protein A2 (FoxA2) (E) or α1-fetoprotein (AFP)/ HNF4α (F). Magnifications: ×200. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; HNF4α, hepatocyte nuclear factor 4α.
hESC-Derived Hepatoblasts Can Self-Renew Stably Under Serum-Free Conditions
We attempted to capture and maintain these hHBs in ECELS media that we recently established and found that it was sufficient to sustain hHB self-renewal. hHBs were routinely split 1:6 using TrypLE and were stably cultured for more than 20 passages on Matrigel coated plates. Long-term cultured hHBs exhibited characteristic phenotypes of hepatoblasts/liver progenitor cells. Immunocytochemistry showed that hHBs (at passage 10) were a highly homogeneous cell population and expressed typical hepatoblast markers and liver-enriched transcriptional factors (Fig. 4). hHBs homogeneously coexpressed AFP/HNF4α, EpCAM/HNF4α, and pan-cytokeratin/FoxA2 (Fig. 4A–4C). They were positive for GATA binding protein 4 (GATA4) (Fig. 4D), a critical transcriptional factor for liver development [20], and coexpressed hepatoblast markers intercellular adhesion molecule 1 (ICAM-1) and delta-like 1 homolog (DLK1) [21, 22] (Fig. 4E). hHBs also coexpressed epithelial genes E-cadherin and cell proliferation marker Ki-67 (Fig. 4F). In addition, they were positive for β-catenin (Fig. 4G), hairy and enhancer of split-1 (Hes1) (Fig. 4H), and Yes-associated protein 1 (YAP1; with primary nuclear localization) (Fig. 4I); however, the cells were negative for pluripotent markers such as Oct4 and Sox2 (Fig. 4J). FACS analysis confirmed the homogeneous expression of AFP (99% positive at passage 10) (Fig. 4K). Nonetheless, hHBs were negative for CD133, kinase insert domain receptor (KDR), and leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5), all established markers of hepatoblasts and adult liver progenitors [22–24].
Figure 4.
Self-renewing human embryonic stem cell-derived hepatoblasts are a homogeneous cell population that expressed typical hepatoblast markers and liver-enriched transcriptional factors but did not express the pluripotent markers (A–J). Fluorescence-activated cell sorting analysis confirmed the expression of AFP (K). Magnification: ×200. Abbreviations: AFP, α1-fetoprotein; DAPI, 4′,6-diamidino-2-phenylindole; HNF4α, hepatocyte nuclear factor 4α; pan-CK, pan-cytokeratin; FoxA2, forkhead box protein A2; GATA4, GATA binding protein 4; ICAM-1, intercellular adhesion molecule 1; DLK1, delta-like 1 homolog; E-Cad, E-Cadherin; Hes1, hairy and enhancer of split-1; YAP1, Yes-associated protein 1.
To confirm the self-renewal of hHBs, the expression of EpCAM and Ki-67 was analyzed by FACS in both early and late-passage hHBs. FACS analyses showed that 84.4% and 99.2% of cells were double-positive for EpCAM and Ki-67 at passages 5 and 18, respectively (Fig. 5A–5C). A small percentage of early passage hHBs were negative for EpCAM. EpCAM expression in hepatoblasts is highly dynamic during liver development [25]. These cells may represent hepatocyte-restricted, late-stage hepatoblasts, which have limited self-renewal capacity in vitro. Despite long-term expansion, hHBs retained normal chromosome numbers (passage 20, n = 10) (Fig. 5D) and robust proliferation capacity. A typical cell growth curve of hHBs (passage 18) is shown in Figure 5E.
Figure 5.
Human embryonic stem cell-derived hepatoblasts (hHBs) maintain phenotypic and genetic stability after long-term cultures. The expression of EpCAM and Ki-67 by both early and late passage of hHBs was analyzed by fluorescence-activated cell sorting (A–C). The chromosomes of hHBs were analyzed at passage 20. (D): Representative chromosome spreads are shown. Scale bar = 12 μm. (E): A typical cell growth curve of hHBs at passage 18. Abbreviations: APC, allophycocyanin; EpCAM, epithelial cell adhesion molecule; FITC, fluorescein isothiocyanate.
Self-Renewing hHBs Are Bipotent
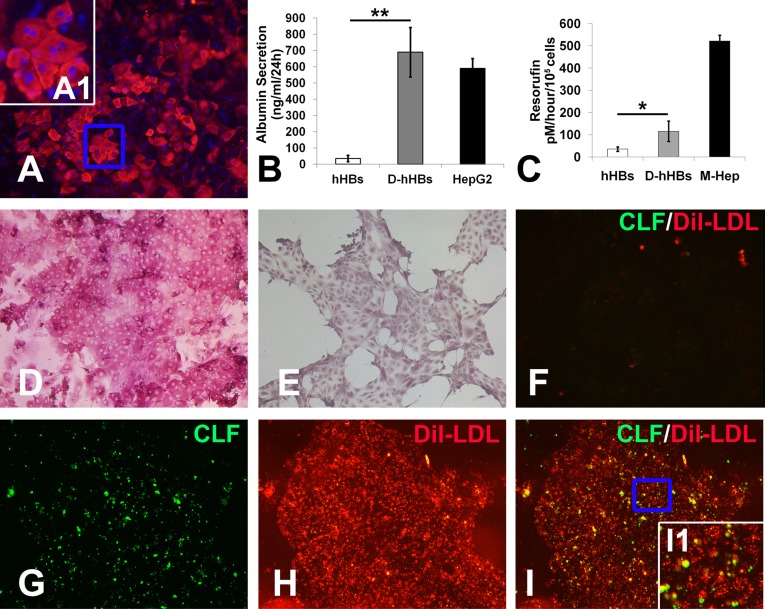
During liver development, hepatoblasts act as bipotent liver progenitors that can give rise to both hepatocytes and cholangiocytes; therefore, we investigated whether hHBs were also bipotent. In fetal liver, HGF acts in concert with OSM and glucocorticoid hormones to stimulate hepatocyte lineage specification of hepatoblasts [26, 27]. In our previous study, we confirmed that the combination of HGF, OSM, and dexamethasone, hereinafter referred to as HOD media, can induce hepatocyte differentiation of mouse hepatoblasts [12]. In addition, blocking Notch and TGF-β signaling (both are important for cholangiocyte specification) using small molecules can further enhance hepatocyte maturation in HOD media [12]. Accordingly, hHBs can rapidly differentiate into functional hepatocytes on treatment with 2 μM TGF-β receptor inhibitor (SB431542) and 1 μM γ-secretase inhibitor (RO4929097) in HOD media for 2 weeks on a Matrigel-coated surface (Fig. 6). The cells expressed albumin with ∼50% efficiency (Fig. 6A). Higher magnification of the boxed areas in Figure 6A showed mature hepatic binuclear cells (Fig. 6A, inset A1). Accordingly, hHB-derived hepatocytes secrete human albumin, as measured by a human albumin ELISA (Fig. 6B). The metabolic capacities of hHB-derived hepatocytes were demonstrated by cytochrome P4501A1 isoenzyme activity assessed with the EROD assay (Fig. 6C). Periodic acid-Schiff staining revealed abundant cytoplasmic glycogen storage in the differentiated cells (Fig. 6D), although this was largely absent in undifferentiated hHBs (Fig. 6E). After incubated with Dil-LDL and cholyl-l-lysyl-fluorescein, hHB-derived hepatocytes, but not untreated hHBs (Fig. 6F), demonstrated a capacity for LDL uptake and formation of bile canaliculi in between adjacent cells (Fig. 6G–6I). Inset I1 of Figure 6I shows the boxed area at higher magnification. Taken together, these data suggest that hHBs possess the intrinsic properties of hepatoblasts; they can respond to fetal hepatocyte lineage commitment signals and efficiently generate functional hepatocytes.
Figure 6.
Hepatocyte differentiation of hHBs. hHBs can differentiate into albumin-expressing hepatocytes on treatment with SB431542 and RO4929097 in HOD media for 2 weeks on a Matrigel-coated surface (A). Inset A1 shows the boxed area in (A). (B): Enzyme-linked immunosorbent assays were performed to measure human albumin secretion. Cytochrome P4501A1 isoenzyme activity of hHB-derived hepatocytes was examined by ethoxyresorufin-O-deethylase assay (results are mean ± SEM, n = 3). Cytoplasmic glycogen storage was revealed by periodic acid-Schiff staining in hHB-derived hepatocytes (D) and untreated hHBs (E). LDL uptake and bile canaliculi were revealed by incubating the hHBs (F) or hHB-derived hepatocytes (G–I) with Dil-LDL and cholyl-l-lysyl-fluorescein. Inset I1 depicts the boxed area in (I). ∗, p < .05, ∗∗, p < .01. Magnification (A, D–I): ×200. Abbreviations: CLF, cholyl-l-lysyl-fluorescein; Dil, Dil-labeled; hHBs, human embryonic stem cell-derived hepatoblasts; HOD, hepatocyte growth factor, oncostatin M, and dexamethasone; LDL, low-density lipoprotein.
Next, we tested whether hHBs can form bile duct-like structures in 3D culture. Dissociated single hHBs were embedded in 50% Matrigel diluted in DMEM/F12 supplemented with ITS and EGF. After 1-week culture, hHBs efficiently formed duct-like structures (Fig. 7A, 7B). Hematoxylin and eosin staining showed that the bile duct-like structures were lined by a single layer of epithelium (Fig. 7B). These duct-like structures demonstrated a polarized distribution of bile duct genes similar to native bile ducts with CD49 and cytokeratin on the basolateral region and F-actin and aquaporin-1 (Aqp-1) at the apical region (Fig. 7C, 7D). RT-PCR analyses confirmed the upregulation of cholangocyte genes such as cytokeratin 7 (CK7), HNF6α, and cystic fibrosis transmembrane conductance regulator (CFTR) after induction (Fig. 7E). These data confirmed that hHBs can recapitulate bile duct morphogenesis in a 3D Matrigel culture. Taken together, the above data suggest that hHBs are bipotent human hepatoblasts.
Figure 7.
hHBs formed bile duct-like structures in three-dimensional Matrigel. (A): hHBs can form duct-like structures after being embedded in Matrigel for 1 week. Scale bar = 200 μm. (B): Hematoxylin and eosin staining showed that the bile duct-like structures were lined by a single layer of epithelium. Scale bar = 25 μm. (C, D): These duct-like structures exhibited a polarized distribution of bile duct genes similar to native bile ducts. Scale bars = 200 μm. (E): Reverse transcription polymerase chain reaction analysis confirmed the upregulation of cholangiocyte markers. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; hHBs, human embryonic stem cell-derived hepatoblasts; RT, reverse transcription.
Discussion
Somatic stem cells are essential to tissue homeostasis and regeneration following damage and have been proposed as promising candidates for cell-based therapy. Hematopoietic stem cell transplantation, for example, has been used for more than 50 years for the management of malignant and nonmalignant hematological disorders; however, isolation of somatic stem cells from adult tissues is usually invasive and technically challenging. In addition, in vitro expansion of most types of somatic stem cells is still not possible and represents a major obstacle to obtaining such cells in numbers sufficient for therapeutic application. Human pluripotent stem cells, including both induced pluripotent stem cells and hESCs, are theoretically a renewable source for any adult cell type, including somatic stem cells. In recent years, there has been tremendous progress in differentiating hESCs toward terminal differentiated cells [28]; however, the generation of somatic stem cells from hESCs has been less successful, largely due to the difficulty of maintaining hESC-derived somatic stem cells in a stable, self-renewing state. In our recent studies, we used a reiterative screening strategy to establish chemically defined conditions for mouse hepatoblast self-renewal [12]. Using similar conditions, we successfully isolated and maintained human hepatoblasts during hepatic differentiation of hESCs. Although previous efforts had generated liver progenitors from hESCs [9–11], these studies used undefined media components (i.e., animal serum). Our study provides a strategy for generating clinically viable hepatoblasts from human pluripotent stem cells; hHBs could be a renewable source of functional hepatocytes for various applications such as drug screening and cell therapy. It will be interesting to test their in vivo potential in suitable animal models in the future.
The activation of the phosphoinositide 3-kinase signaling pathway is detrimental to endoderm induction of hESCs [29], and small-molecule inhibitors of this pathway have been widely used to enhance endoderm differentiation. In our study, we found that inhibition of phosphoinositide 3-kinases by small molecule LY294002 during endoderm induction caused substantial cell death; however, using basal medium with insulin-free B27 supplements can efficiently induce Sox17/FoxA2 double-positive definitive endoderm without causing significant cell death. The definitive endoderm population was further converted to primitive gut tube by treatment with KGF and S431542 [18, 19]. Although many differentiation protocols directly initiate hepatic induction from definitive endoderm by BMP/FGF treatment, we found it was important to specify the primitive gut tube before hepatic induction. In our experiments, AFP/HNF4α-positive hepatoblast-like cells could be generated by treating the definitive endoderm population with FGF2 and BMP4 and could be maintained successfully by ECELS media. Consistent with a previous study, however, these cells were refractory to hepatocyte induction [19] and gave rise to albumin-positive hepatocytes with low efficiency (less than 10% after induction in HOD media supplemented with SB431542 and RO4929097).
Conclusion
Chemical approaches using small molecules to steer biological systems toward specific outcomes have been proven to be especially useful in studying stem cell biology [30]. Our current study once again demonstrates the power of chemical approaches in manipulating stem cell fates. A similar strategy could be explored to enable self-renewal of other clinically relevant somatic stem cells originally derived from human pluripotent stem cells.
Supplementary Material
Acknowledgments
This study was supported by the Shanghai Pujiang Program (Grant 12PJ1411000), China National Natural Science Foundation (Grants 81322016, 31071298, and 30800567), and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant XDA01040302). We thank Jem Efe (Genomics Institute of the Novartis Research Foundation, San Diego, CA) for editing this article.
Author Contributions
M.Z., P.S., Y.W., J.C., L.L.: collection and/or assembly of data; W.W.: provision of chemical compounds, collection and/or assembly of data; C.J. and W.L.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Mizuguchi T, Mitaka T, Katsuramaki T, et al. Hepatocyte transplantation for total liver repopulation. J Hepatobiliary Pancreat Surg. 2005;12:378–385. doi: 10.1007/s00534-005-0986-z. [DOI] [PubMed] [Google Scholar]
- 2.Shin S, Kaestner KH. The origin, biology, and therapeutic potential of facultative adult hepatic progenitor cells. Curr Top Dev Biol. 2014;107:269–292. doi: 10.1016/B978-0-12-416022-4.00010-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behbahan IS, Duan Y, Lam A, et al. New approaches in the differentiation of human embryonic stem cells and induced pluripotent stem cells toward hepatocytes. Stem Cell Rev. 2011;7:748–759. doi: 10.1007/s12015-010-9216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Touboul T, Hannan NRF, Corbineau S, et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology. 2010;51:1754–1765. doi: 10.1002/hep.23506. [DOI] [PubMed] [Google Scholar]
- 5.Cai J, Zhao Y, Liu Y, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–1239. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- 6.Basma H, Soto-Gutiérrez A, Yannam GR, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26:1117–1127. doi: 10.1634/stemcells.2007-1102. [DOI] [PubMed] [Google Scholar]
- 8.Hay DC, Zhao D, Fletcher J, et al. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells. 2008;26:894–902. doi: 10.1634/stemcells.2007-0718. [DOI] [PubMed] [Google Scholar]
- 9.Zhao D, Chen S, Cai J, et al. Derivation and characterization of hepatic progenitor cells from human embryonic stem cells. PLoS One. 2009;4:e6468. doi: 10.1371/journal.pone.0006468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takayama K, Nagamoto Y, Mimura N, et al. Long-term self-renewal of human ES/iPS-derived hepatoblast-like cells on human laminin 111-coated dishes. Stem Cell Rep. 2013;1:322–335. doi: 10.1016/j.stemcr.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanagida A, Ito K, Chikada H, et al. An in vitro expansion system for generation of human iPS cell-derived hepatic progenitor-like cells exhibiting a bipotent differentiation potential. PLoS One. 2013;8:e67541. doi: 10.1371/journal.pone.0067541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lv L, Han Q, Chu Y, et al. Self-renewal of hepatoblasts under chemically defined conditions by iterative growth factor and chemical screening. Hepatology. 2015;61:337–347. doi: 10.1002/hep.27421. [DOI] [PubMed] [Google Scholar]
- 13.Yao S, Chen S, Clark J, et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci USA. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidambi S, Yarmush RS, Novik E, et al. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc Natl Acad Sci USA. 2009;106:15714–15719. doi: 10.1073/pnas.0906820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkening S, Stahl F, Bader A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab Dispos. 2003;31:1035–1042. doi: 10.1124/dmd.31.8.1035. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Zhou H, Abujarour R, et al. Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells. 2009;27:2992–3000. doi: 10.1002/stem.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng X, Ying L, Lu L, et al. StemBook. Cambridge, MA: Harvard Stem Cell Institute; 2008. Monolayer endoderm differentiation from human ESCs. [Internet], Available at http://www.ncbi.nlm.nih.gov/books/NBK133280. [PubMed] [Google Scholar]
- 18.Schulz TC, Young HY, Agulnick AD, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS One. 2012;7:e37004. doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao D, Chen S, Duo S, et al. Promotion of the efficient metabolic maturation of human pluripotent stem cell-derived hepatocytes by correcting specification defects. Cell Res. 2013;23:157–161. doi: 10.1038/cr.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Tanimizu N, Nishikawa M, Saito H, et al. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J Cell Sci. 2003;116:1775–1786. doi: 10.1242/jcs.00388. [DOI] [PubMed] [Google Scholar]
- 22.Turner R, Lozoya O, Wang Y, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology. 2011;53:1035–1045. doi: 10.1002/hep.24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huch M, Dorrell C, Boj SF, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldman O, Han S, Sourisseau M, et al. KDR identifies a conserved human and murine hepatic progenitor and instructs early liver development. Cell Stem Cell. 2013;12:748–760. doi: 10.1016/j.stem.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka M, Okabe M, Suzuki K, et al. Mouse hepatoblasts at distinct developmental stages are characterized by expression of EpCAM and DLK1: Drastic change of EpCAM expression during liver development. Mech Dev. 2009;126:665–676. doi: 10.1016/j.mod.2009.06.939. [DOI] [PubMed] [Google Scholar]
- 26.Kamiya A, Kinoshita T, Ito Y, et al. Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J. 1999;18:2127–2136. doi: 10.1093/emboj/18.8.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki A, Iwama A, Miyashita H, et al. Role for growth factors and extracellular matrix in controlling differentiation of prospectively isolated hepatic stem cells. Development. 2003;130:2513–2524. doi: 10.1242/dev.00459. [DOI] [PubMed] [Google Scholar]
- 28.Wobus AM, Boheler KR. Embryonic stem cells: Prospects for developmental biology and cell therapy. Physiol Rev. 2005;85:635–678. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- 29.McLean AB, D’Amour KA, Jones KL, et al. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. doi: 10.1634/stemcells.2006-0219. [DOI] [PubMed] [Google Scholar]
- 30.Li W, Li K, Wei W, et al. Chemical approaches to stem cell biology and therapeutics. Cell Stem Cell. 2013;13:270–283. doi: 10.1016/j.stem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.