Platelet-rich plasma (PRP) as a treatment for male pattern hair loss was investigated in a randomized, placebo-controlled study in which 20 men received PRP on half of their scalp and placebo on the other. Patients received 3 treatments at 30-day intervals. Hair regrowth was quantified by a blinded evaluator using computerized trichograms. Patients were followed for 2 years; study endpoints were hair regrowth, hair dystrophy as measured by dermoscopy, burning or itching sensation, and cell proliferation as measured by Ki-67 evaluation. At the end of three treatment cycles, clinical improvement was seen in several parameters. Four of 20 patients experience continued hair loss and required retreatment.
Keywords: Autologous, Aging, Clinical translations, Clinical trials
Abstract
Platelet-rich plasma (PRP) has emerged as a new treatment modality in regenerative plastic surgery, and preliminary evidence suggests that it might have a beneficial role in hair regrowth. Here, we report the results of a randomized, evaluator-blinded, placebo-controlled, half-head group study to compare, with the aid of computerized trichograms, hair regrowth with PRP versus placebo. The safety and clinical efficacy of autologous PRP injections for pattern hair loss were investigated. PRP, prepared from a small volume of blood, was injected on half of the selected patients’ scalps with pattern hair loss. The other half was treated with placebo. Three treatments were administered to each patient at 30-day intervals. The endpoints were hair regrowth, hair dystrophy as measured by dermoscopy, burning or itching sensation, and cell proliferation as measured by Ki67 evaluation. Patients were followed for 2 years. Of the 23 patients enrolled, 3 were excluded. At the end of the 3 treatment cycles, the patients presented clinical improvement in the mean number of hairs, with a mean increase of 33.6 hairs in the target area, and a mean increase in total hair density of 45.9 hairs per cm2 compared with baseline values. No side effects were noted during treatment. Microscopic evaluation showed the increase of epidermis thickness and of the number of hair follicles 2 weeks after the last PRP treatment compared with baseline value (p < .05). We also observed an increase of Ki67+ keratinocytes in the epidermis and of hair follicular bulge cells, and a slight increase of small blood vessels around hair follicles in the treated skin compared with baseline (p < .05). Relapse of androgenic alopecia was not evaluated in all patients until 12 months after the last treatment. After 12 months, 4 patients reported progressive hair loss; this was more evident 16 months after the last treatment. Those four patients were re-treated. Our data clearly highlight the positive effects of PRP injections on male pattern hair loss and absence of major side effects. PRP may serve as a safe and effective treatment option against hair loss; more extensive controlled studies are needed.
Significance
Platelet-rich plasma (PRP) has emerged as a new treatment modality in regenerative plastic surgery, and preliminary evidence suggests that it might have a beneficial role in hair regrowth. Here, the results of a randomized, placebo-controlled, half-head group study to compare the hair regrowth with PRP versus placebo are reported. Hair regrowth was quantified by a blinded evaluator using computerized trichograms. The safety and clinical efficacy of autologous PRP injections for pattern hair loss were investigated. Of the 23 patients enrolled, 3 were excluded. At the end of the 3 treatment cycles, the patients presented clinical improvement in the mean number of hairs, with a mean increase of 33.6 hairs in the target area and a mean increase in total hair density of 45.9 hairs per cm2 compared with baseline values. No side effects were noted during treatment. The data clearly highlight the positive effects of PRP injections on male pattern hair loss and absence of major side effects.
Introduction
Androgenic alopecia is a common, chronic hair loss disorder. It is characterized by progressive hair loss, affecting both sexes. It affects up to 80% of white men and 40% of women. A number of products have been proposed as hair-loss therapies. Drug therapies specifically approved by the U.S. Food and Drug Administration (FDA) for treating androgenic alopecia are limited to minoxidil and finasteride. Both can be used alone or combined [1].
Proponents of platelet-rich plasma (PRP) technology suggest that its benefits include an increase in hard- and soft-tissue wound healing. In addition, the role of PRP for the treatment of alopecia (areata and androgenic) has been demonstrated in recent reports [2–4]. The activation of platelet α−granules releases numerous growth factors, including transforming growth factor (TGF), platelet-derived growth factor (PDGF),vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), insulin-like growth factor, and interleukin-1 [3]. It is proposed that growth factors released from platelets may act on stem cells in the bulge area of the follicles, stimulating the development of new follicles and promoting neovascularization [3]. In fact, in the bulge area, primitive stem cells of ectodermal origin are found, giving origin to epidermal cells and sebaceous glands. In matrix, germinative cells of mesenchymal origin are found at the dermal papilla. Interactions between these two kinds of cells as well as with binding growth factors (PDGF, TGF-β, and VEGF) activate the proliferative phase of the hair, giving rise to the future follicular unit [1]. For these reasons, Gkini et al. [1], and Khatu et al. [3] have reported in two different works that PRP could serve as a potential treatment for androgenic alopecia.
Rinaldi et al. [4] described the use of PRP in alopecia areata (AA). The results of this pilot study suggest that PRP may serve as a safe and effective treatment option in AA, and the authors call for more extensive, controlled studies with this method. Uebel et al. [5] showed that pretreatment of follicular units with PRP before transplantation resulted in improved hair growth and density. PRP has been reported to induce the proliferation of dermal papilla cells by upregulating fibroblast growth factor 7 (FGF-7) and β-catenin, as well as extracellular signal-related kinase (ERK) and Akt signaling [2]. Anagen-associated angiogenesis has been suggested as one of the important factors in active hair growth, because of the secretion of VEGF by the keratinocytes of the outer root sheath and fibroblasts of the dermal papilla [6, 7]. Increased secretion of VEGF influences growth of normal and pathological dermal structures [8]. Tobin et al. [9] reported the hair follicle mesenchyme exhibits significant hair cycle-associated plasticity. Modulation of these cell interchanges is likely to be important during clinically important hair follicle transformations (e.g., vellus to terminal and terminal to vellus during androgenetic alopecia [9]). Injection of PRP has been demonstrated to improve cutaneous ischemic conditions and to increase vascular structures around hair follicles [10]. Many of the current treatment modalities for pattern hair loss have been shown to modulate angiogenesis and enhance blood flow [11]. We aimed to clarify the effects of PRP scalp injection in humans affected by androgenic alopecia. It could improve hair regrowth, thereby making the PRP procedure an alternative to finasteride or minoxidil. The data we report demonstrate the clinical efficacy and histological safety of PRP treatment. Moreover, patients’ satisfaction and computerized trichogram analysis have confirmed the quality of the results.
Materials and Methods
Study Overview
This was a randomized, placebo-controlled, half-head group study conducted by plastic surgeons, biologists, pathologists of the University of Rome “Tor Vergata,” and a dermatologist of the Catholic University of the Sacred Heart of Rome. Evaluators of computerized trichograms were blinded to treatment. A follow-up period of 2 years was chosen because a reduction in the effect of PRP by 14 months after the last treatment has been observed.
The diagnosis of male pattern hair loss (MPHL) was established on the basis of a detailed medical history (any drugs causing hair loss), clinical examination, trichoscopic features (more than 20% variability in hair diameter between affected and uninvolved areas) and laboratory tests. Laboratory tests to exclude other hair loss causes, such as anemia, poor nutrition, thyroid dysfunction, and syphilis, included the following: complete blood cell count; measurement of serum levels of iron, serum ferritin, total iron-binding capacity, folic acid, T3, T4, thyroid-stimulating hormone, fT3, fT4, antithyroid peroxidase, and testosterone; and a Venereal Disease Research Laboratory blood test for syphilis.
The stage of alopecia was evaluated according to the Hamilton-Norwood scale. The study protocol complied with the Declaration of Helsinki. All patients provided written informed consent before participating in the study.
Patient Population and Randomization
The study enrolled 23 male patients. Inclusion criteria were age 19–63 years; MPHL from stage IIa to stage IV according to the Norwood-Hamilton classification (Table 1); and the participant was considered suitable for PRP injection from a surgical point of view as assessed by the expected cosmetic results and the transfusional service.
Table 1.
Summary of patients’ characteristics
Exclusion criteria were divided into two types: local and systemic. The systemic criteria included platelets disorders, thrombocytopenia, antiaggregating therapy, systemic treatments for MPHL (such as finasteride, dutasteride, and antiandrogens) in the previous 12 months, bone marrow aplasia, uncompensated diabetes, sepsis, and cancer. The local criteria included MPHL stages V–VII, topical treatments for MHPL (e.g., minoxidil, prostaglandin, analogs, retinoids, and corticosteroids) in the previous 12 months, and withdrawal of informed consent. Three patients with a propensity for keloids and patients who were immunosuppressed were excluded. In addition, the numbers of platelets in PRP obtained from all participants were microscopically counted. All participants included were assessed by two experts in plastic surgery.
The allocation sequence was generated using an online randomization generator and was concealed by a person unrelated to the trial management group. The participants, study personnel, and outcome assessors were all blinded to treatment allocation, and blinding was maintained until all data had been analyzed.
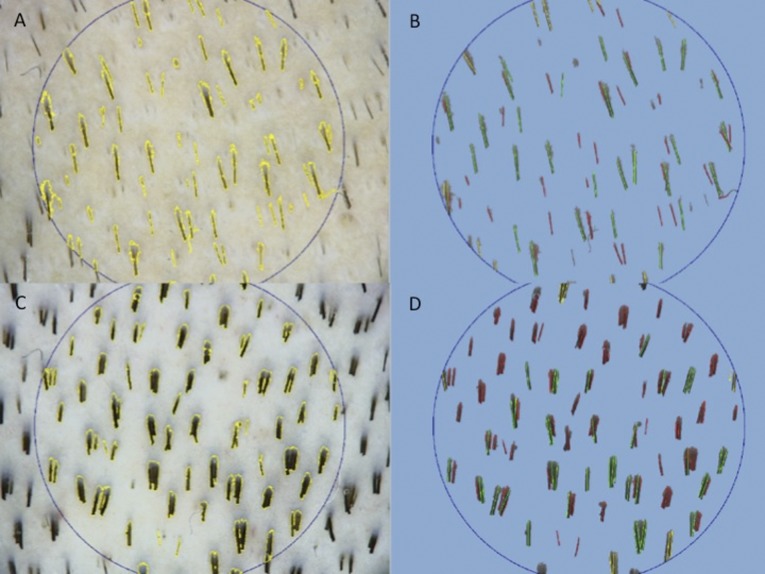
The primary outcome was residual hair count and hair density based on computerized trichogram (Fig. 1). The secondary outcomes were microscopic evaluation (Fig. 2) of the epidermis thickness in PRP-treated hair skin and increase in the number of follicles compared with baseline value and evaluation of safety and feasibility (Fig. 3).
Figure 1.
Trichoscan digital image analysis. (A, B): In these images, preoperative hair count was 67.5 hairs per cm2; density was 120.1 hairs per cm2; and proportions of anagen and telogen hairs were 52.2% and 47.8%, respectively. (C, D): In these images, postoperative hair count was 128.5 hairs per cm2; density was 228.6 hairs per cm2; and proportions of anagen and telogen hairs were 37.6% and 62.4%, respectively.
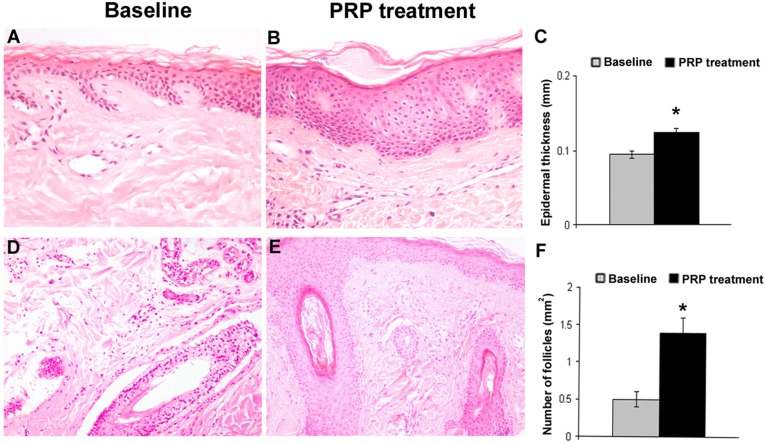
Figure 2.
Epidermis thickness and the number of follicles of hair skin are increased after PRP treatment. (A, B): Representative photomicrographs of hair skin epidermis at baseline (A) and 2 weeks after PRP treatment (B) (hematoxylin and eosin stain; original magnification: ×200). (C): Bar graph of epidermis thickness. (D, E): Representative photomicrographs of dermal hair follicles at baseline (D) and after PRP treatment (E) (hematoxylin and eosin stain; original magnification: ×100). (F): Bar graph of the number of hair follicles per mm2 at baseline and after PRP treatment. ∗, p < .05. Abbreviation: PRP, platelet-rich plasma.
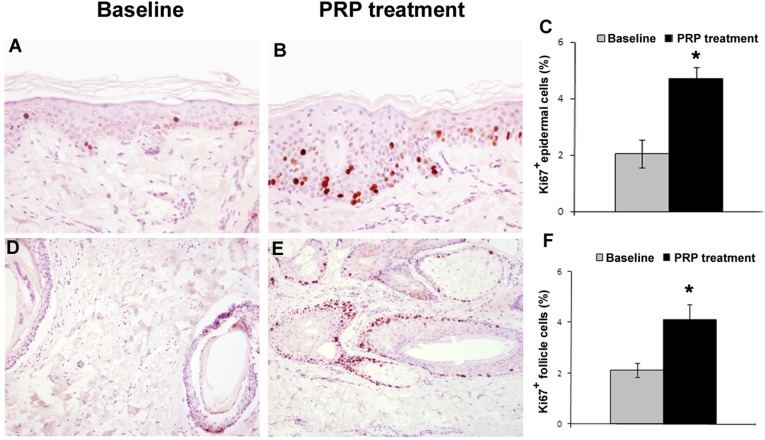
Figure 3.
Proliferation of epidermis basal cells and hair follicular bulge cells is increased after PRP treatment. (A, B): Representative photomicrographs of Ki67+ proliferating cells by immunohistochemistry of hair skin epidermis at baseline (A) and after PRP treatment (B) (hematoxylin and eosin stain; original magnification: ×200). (C): Morphometric analysis of Ki67+ cells of hair skin epidermis at baseline and after PRP treatment. (D, E): Representative photomicrographs of Ki67+ proliferating cells by immunohistochemistry of hair follicles at baseline (D) and after PRP treatment (E) (hematoxylin and eosin stain; original magnification: ×100). (F): Morphometric analysis of the percentage of Ki67+ nuclei in hair follicles at baseline and after PRP treatment. ∗, p < .05. Abbreviation: PRP, platelet-rich plasma.
Procedures
PRP was prepared from a small volume of blood (18 ml) according to the method of the Cascade-Selphyl-Esforax system (Aesthetic Factors, LLC, Wayne, NJ, http://www.selphyl.com), with modifications [12–14], and from 60 ml of blood according to the P.R.L. Platelet Rich Lipotransfert system (CORIOS Soc. Coop, San Giuliano Milanese, Italy, http://www.corios.it), with modifications, using PRP alone (C-punt; Biomed Device, Modena, Itlay, http://www.biomeddevice.it) without the combined use of stromal vascular fraction cells. Briefly, blood was taken from a peripheral vein using sodium citrate as an anticoagulant. The current systems for preparing platelet concentrations use various centrifuges (in the Cascade-Selphyl-Esforax procedure, we used 1,100g for 10 minutes; in the P.R.L. Platelet Rich Lipotransfert system, we used 1,200 rpm for 10 minutes). PRP was prepared in all cases with approval of the transfusional service. Although the method of preparation is not selective and may include leukocytes, the final aim is to obtain a platelet pellet. Growth factors are secreted only once platelet activation begins, which, in turn, is stimulated by calcium (Ca2+). To optimize the secretion process, the optimum concentration of Ca2+ was previously determined [12, 13]. Then, autologous PRP, not activated obtained after centrifugation (9 ml), was switched with 10-ml tubes containing Ca2+. Autologous PRP, not activated, obtained by the P.R.L. Platelet Rich Lipotransfert procedure after centrifugation (20 ml), was inserted in a light selector device. At the end of the procedure, 9 ml of PRP was harvested.
For each patient, the scalp affected by hair loss was divided into four areas (frontal, parietal, vertex, and occipital) and cleansed with 70% alcohol; local anesthesia was not injected in the treated areas. PRP (0.1 ml/cm2) was injected in selected areas of the scalp. PRP injections were performed with a 30-gauge, 1-ml Luer-lock syringe (Fig. 4). Interfollicular injection of PRP was performed 3 times in each patient at intervals of 30 days. Patients with hair loss localized to the frontal and parietal areas were injected with the PRP only on the frontal areas; the parietal area was treated with placebo (injection of physiological solution). Patients with hair loss in the parietal and vertex parts were injected with the PRP only in the parietal part of the scalp; the vertex area was treated with placebo (injection of physiological solution). The same number of injections was repeated in the scalp half treated with PRP and in the half treated with placebo. Photographs of the areas of a sample scalp treated with PRP are shown in Figure 1.
Figure 4.
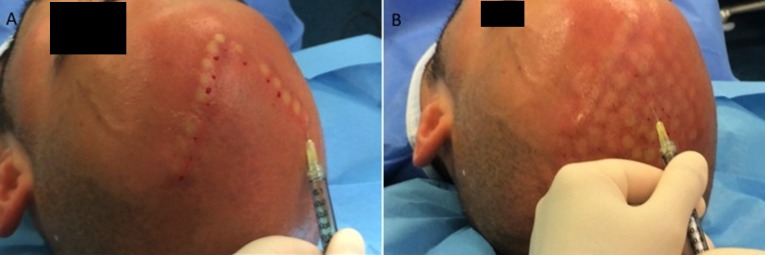
Intraoperative injection with the platelet-rich plasma (PRP) at 0.1 ml/cm2. (A): Intraoperative injection with PRP at 0.1 ml/cm2 in the frontal line. (B): At the end of injection with PRP (0.1 ml/cm2).
All patients were evaluated in 6 stages: T0, beginning of study; T1, 2 months; T2, 6 months; T3, 12 months, T4, 16 months; and T5, 24 months. The effects of the treatment on hair growth were assessed in all patients with the help of global photography, physician’s and patient’s global assessment scale, and standardized phototrichograms.
Phototrichograms were taken of all scalps by a trained evaluator using Fotofinder video-epiluminescence microscopy (FotoFinder Systems; http://www.fotofinder.de) in combination with the Trichoscan digital image analysis (Tricholog GmbH and Datinf GmbH; http://trichoscan.com). TrichoScan is a digital software-supported epiluminescence technique for measuring hair count (number of hairs per 0.65 cm2), hair density (number of hairs per cm2), hair diameter, anagen-to-telogen ratio, and vellus hair-to-terminal hair ratio. To determine the quality of hair leading to an increased hair density, it is important to differentiate the number of terminal and vellus hairs. In TrichoScan analysis, all hairs with a diameter >40 µm are categorized as terminal hairs; those with lesser diameter are categorized as vellus hairs. In all patients, in both the treatment and control half-heads, two transitional areas of hair loss were defined and marked with a semipermanent tattoo for the subsequent trichogram. In the target area, hairs were clipped and dyed brown for 10 minutes to improve the hair contrast for the analytic software. The evaluator of the computerized trichogram analysis was blinded regarding the treatment and control areas of the scalp, and was not involved in administration of treatment.
Histopathological Evaluation
Incisional punch biopsies (diameter: 3 mm) of the hair skin were obtained at baseline and after 2 months from the last PRP treatment, and fixed in buffered formalin. Morphometric analysis [15] was performed on hematoxylin-and eosin-stained paraffin serial sections by evaluating the thickness of epidermis and the number of follicles per mm2, according to the method [16]. All samples were cut perpendicularly at the skin surface and embedded, paying attention to the correct orientation.
Immunohistochemistry
Immunohistochemistry was performed using mouse monoclonal anti-Ki67 (DakoCytomation; Dako; http://www.dako.com) [17–19]. The percentage of Ki67+ cells in the basal layer of the epidermis and in the outer root sheath of hair follicles, and the number of vessels per mm2 were calculated according to morphometric criteria [18].
Statistical Analysis
Statistical analyses of the data were performed using the Statistical Package for the Social Sciences (SPSS), version 19.0 (IBM; http://www-01.ibm.com). The normality of quantitative variables was tested by the Kolmogorov-Smirnov test. Hair density was expressed as mean plus or minus the SD. Hair density differences between the different time points were assessed by one-way repeated measures analysis of variance; post hoc analysis was performed using the Sidak test. All tests were 2-tailed and statistical significance was considered for p < .05.
Results
The various hair-growth parameters measured after 3 months of the first treatment were compared with the baseline study before treatment and between both treatment and control areas. Mean total hair counts, hair density, and terminal and vellus hair densities for the treatment and control areas are listed in Table 2. At baseline, there were no statistical differences in hair count, hair density, and terminal and anagen hair densities between the treatment and control area of the scalp.
Table 2.
Relevant hair-growth parameters assessed by Trichoscan analysis for the treatment and control half-head areas at baseline and after 14 weeks
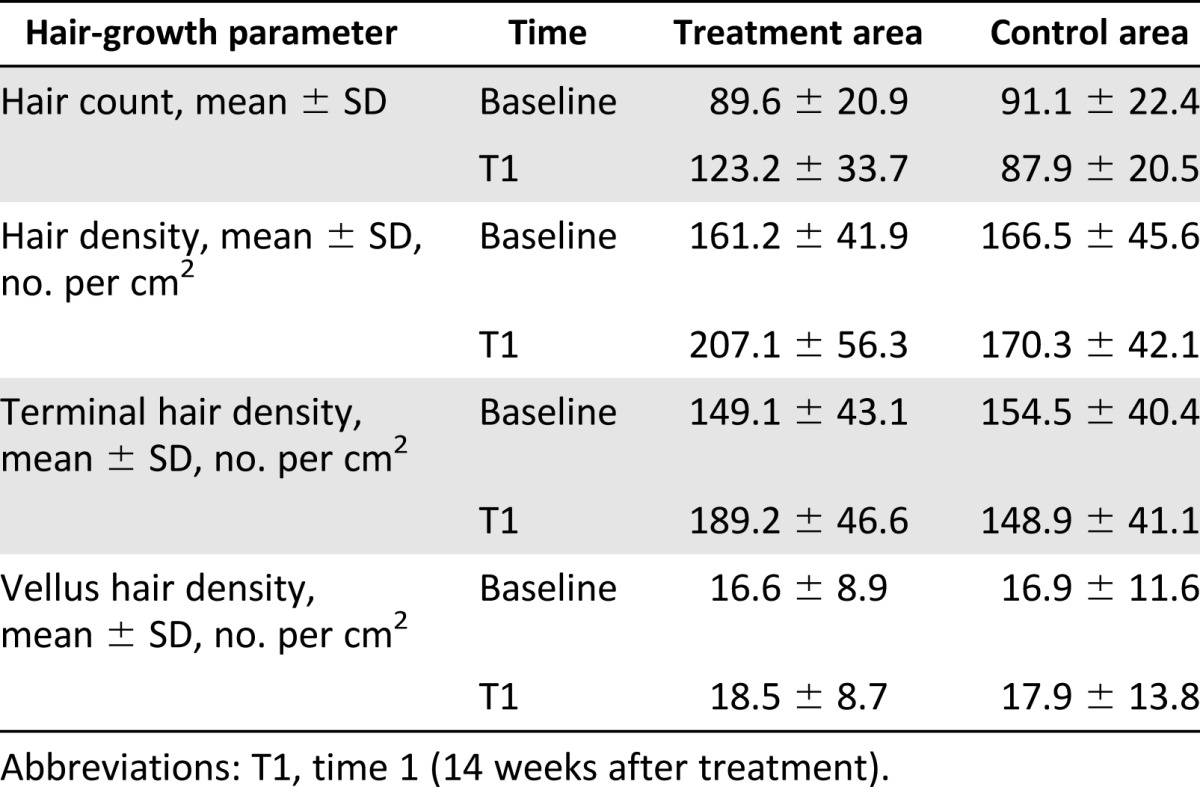
The results of this study showed a significant increase in the mean hair count for the treatment area after 3 months (3 months vs. 0 month), with a mean increase of 33.6 hairs in the target area compared with baseline, while the control area showed a mean decrease of 3.2 hairs (control vs. treatment: p < .0001). Accordingly, in the treatment area, a mean increase in total hair density of 45.9 hairs per cm2 compared with baseline was observed after 3 months, and the control area displayed a mean decrease of 3.8 hairs per cm2) in hair density at the same time (control vs. treatment: p < .0001). In addition, terminal hair density improved significantly by 40.1 hairs per cm2 in the treatment area compared with baseline, while it decreased by 5.6 hairs per cm2 in the control area of the scalp (control vs. treatment: p = .0003). There were no statistically significant differences in vellus hair density between the study and the control area after 3 months.
Microscopic evaluation showed the increase of epidermis thickness in PRP-treated hair skin after 2 weeks from the last PRP treatment compared with baseline value (p < .05). Two-week PRP treatment was also accompanied by an increase in the number of follicles compared with baseline value (p < .05) (Fig. 2).
To better report the effects of PRP, we investigated the proliferation of epidermal and hair follicular bulge cells. After 2 weeks from the last treatment, we observed an increase of Ki67+ basal keratinocytes of epidermis and of hair follicular bulge cells compared with baseline (p < .05) (Fig. 3). PRP treatment also was associated with an increase in small blood vessels around hair follicles in the treated skin treated compared with baseline (p < .05). The clinical result of the areas of one patient’s scalp treated with PRP is shown in Figure 5.
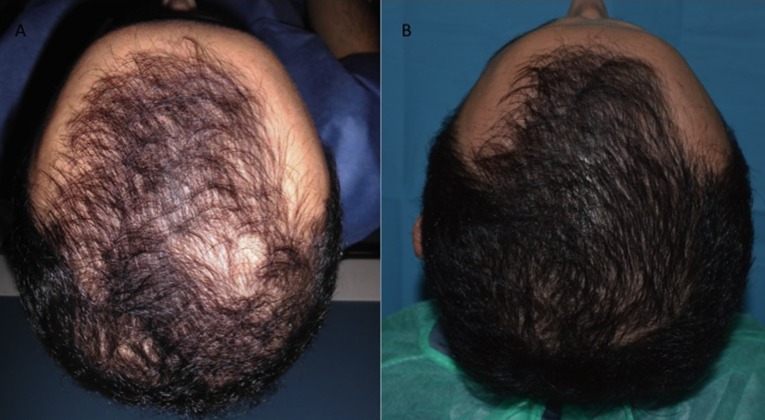
Figure 5.
A 29-year-old male patient affected by hair loss. (A): Preoperative situation of the frontal line and parietal area. (B): Postoperative situation of the frontal line and parietal area 2 weeks after the last treatment with increase in the hair count and hair density.
The relapse of androgenic alopecia in all patients was not evaluated until 12 months after the last treatment. Four patients reported progressive hair loss that was more evident 16 months after the last treatment. Those four patients were treated again with three PRP injections.
Discussion
Androgenic alopecia remains the most common hair disorder without satisfactory treatment. Since androgenetic alopecia is characterized by a shortened anagen phase and miniaturization of terminal to vellus hair, current therapeutic strategies target cellular proliferation and differentiation during the hair cycle [1].
Hair transplant and a number of products have been proposed as hair-loss therapies. Drug therapies approved by the FDA were limited to minoxidil and finasteride used alone or combined [1]. Minoxidil prolongs anagen and increases hair follicle size through stimulation of potassium channels and prostaglandin endoperoxide synthase-1, which increases the level of prostaglandin E2 [11]. Minoxidil promotes the survival of dermal papilla cells by increasing Bcl-2/Bax ratio and by activating ERK and Akt [20]. Oral finasteride also induces the prolongation of anagen hairs, which results in gradual thickening and elongation of the hairs [21]. In addition, finasteride has been shown to reduce the pattern hair loss associated with increased expression of caspases and apoptosis inhibitors; therefore, it is ultimately suggested to activate anagen hair growth [22, 23].
There are new data regarding PRP’s potential effect on hair. Antiapoptotic effects of activated PRP have been suggested as one of the major contributing factors stimulating hair growth [2, 24]. PRP-induced activation of antiapoptotic regulators, such as the Bcl-2 protein and Akt signaling, prolongs the survival of dermal papilla cells during the hair cycle [2, 25]. In addition, the upregulation of FGF-7/β-catenin signaling pathways with PRP treatment is suggested to stimulate hair growth by inducing follicular stem cell differentiation as well as prolonging the anagen phase of the hair growth cycle [2, 26]. It also appears to increase the perifollicular vascular plexus through the increase of VEGF and PDGF levels, which have angiogenic potential [1].
Gkini et al. [1] reported in a nonrandomized trial the efficacy of PRP injection in 22 patients affected by androgenic alopecia. This prospective cohort study was based on 3 treatment sessions with an interval of 3 weeks. At 6 months from the beginning of the treatment, a booster session was also performed. The patients presented hair density significantly increased at 6 weeks (154.80 ± 34.39 hairs per cm2), at 3 months (170.70 ± 37.81 hairs per cm2), at 6 months (156.23 ± 37.75 hairs per cm2), and at 1 year (153.70 ± 39.92 hairs per cm2) compared with the onset of therapy (p < .001).
Kang et al. [27] reported the clinical efficacy of injection of CD34+ cell-containing PRP preparations for male and female pattern hair loss. In this study, 3 months after the first treatment, the 13 patients presented clinical improvement in the mean number of hairs (20.5% ± 17.0%), mean hair thickness (31.3% ± 30.1%, and mean 2-point score (84.4% ± 51.7%) compared with baseline values. At 6 months, the patients presented clinical improvement in mean hair count (29.2% ± 17.8%), mean hair thickness (46.4% ± 37.5%), and mean 2-point score (121.3% ± 66.8%) compared with baseline.
Khatu et al. [3] reported a significant reduction in hair loss between first and fourth PRP injection in a group of 11 male patients affected by androgenic alopecia. In this study, hair count increased from average number of 71 hair follicular units to 93 hair follicular units. Therefore, the average mean gain was 22.09 follicular units per cm2.
In a study conducted with 64 patients affected by androgenic alopecia, Schiavone et al. [28] reported the results obtained after two injections of leukocyte platelet-rich plasma (L-PRP) with the addition of concentrated plasmatic proteins, at baseline and after 3 months (single spin at baseline and double-spin centrifugation at 3 months). Macrophotographs were taken at baseline and after 6 months, and 2 independent evaluators rated them using the Jaeschke rating of clinical change. The mean change in clinical rating was 3.2 (95% confidence interval [CI]: 2.9–3.5) and 3.9 (95% CI: 3.5–4.3), respectively, and the proportion of patients reaching a clinically important difference was 40.6% and 54.7%, respectively, according to the 2 evaluators.
Rinaldi et al. [4] described the efficacy and safety of PRP in the first report on alopecia areata in a randomized, double-blind, placebo- and active-controlled, half-head, parallel-group study. A total of 45 patients with alopecia areata were randomized to receive intralesional injections of PRP, triamcinolone acetonide (TrA), or placebo on half of their scalp. The other half was not treated. Three treatments were administered to each patient at intervals of 1 month. Patients treated with PRP had significantly increased hair regrowth compared with those treated with TrA; 27% of patients treated with TrA achieved complete remission at 12 months, compared with 60% of patients treated with PRP, which is significantly higher than that seen in TrA- and placebo-treated patients. At 6 months, 38% of the patients in the TrA group had relapse of disease, while no patients from the PRP group had relapse at this time point. At 12 months, 71% of the patients in the TrA group experienced relapse of disease, while only 31% of the patients in the PRP group had a relapse. Whereas 96% of the patients in the PRP group had regrowth of fully pigmented hair from the beginning of hair growth, only 25% in the TrA group had pigmented hair at the beginning of hair regrowth.
In accordance with these results, Rinaldi et al. [4] reported that both PRP and TrA decreased the number of dystrophic hairs as assessed by dermoscopic photomicrographs, and also decreased the itching or burning sensation of the patients. Both PRP and TrA significantly increased the levels of Ki67 in alopecia areata patches compared with placebo, and levels were significantly higher after PRP treatment compared with TrA. The effect of PRP on Ki67 levels was evident already after 2 months, and was sustained throughout the study period (1 year) [4].
Now the questions are as follows: What is the quantity of PRP to inject in the selected areas? What is the diameter of selected areas in which to inject PRP? What is the better method to prepare PRP? What is the better method to inject PRP? What is the best concentration of platelets?
Gkini et al. [1] injected PRP at a dose of 0.05–0.1 ml/cm2, with a 27-gauge needle using BD-Luer Lok 1-ml syringes (Becton, Dickinson, and Company; https://www.bd.com). Nappage technique was performed at a depth of 1.5–2.5 mm. The blood harvested (16 ml) was introduced into two tubes (Regen-BCT; Regen Lab; http://www.regenlab.com) and centrifuged for 5 minutes at 1,500g. Then, 2 ml of upper supernatant plasma (platelet-poor plasma [PPP]) was removed from each tube; 3 ml of PRP was yielded, which was resuspended by gentle inversions of the tube. Total amount of yielded PRP was approximately 6 ml. The activation process included the addition of calcium gluconate in a 1:9 ratio (0.1 ml calcium gluconate per 0.9 ml of PRP). The platelets in whole blood and PRP were microscopically counted and mean platelet counts were 1.9 × 105 and 1.102 × 106 platelets/μl, respectively. The concentration of platelets in PRP was approximately 5.8 times as great as that in whole blood.
Khatu et al. [3] prepared PRP by collecting 20 ml of fresh blood. The tubes were subjected at first centrifugation to 1,500 revolutions per minute for 6 minutes. This tube underwent a second centrifugation, which was longer and faster than the first, called a “hard spin”, at 2,500 revolutions per minute for 15 minutes. The upper layer containing PPP was discarded and the lower layer of PRP was loaded in an insulin syringe containing calcium chloride (one part calcium chloride and nine parts PRP) as an activator. Nappage technique was performed at a depth of a 1 cm × 1 cm over the right parietal area in the midpupillary line, 10 cm proximal to the right eyebrow in each patient. A total volume of 2–3 ml was injected.
Rinaldi et al. [4] prepared PRP by collecting 36 ml of peripheral blood. The tubes were centrifuged for 8 minutes at 70g. The PRP fraction was separated and suspended with calcium gluconate.
In our study, PRP was prepared from a small volume of blood (18 ml) according to the method of the Cascade-Selphyl-Esforax system [12, 13]. We centrifuged the blood harvested in two tubes at 1,100g for 10 minutes. PRP was prepared in all cases with approval of the transfusional service. Then, autologous PRP that was not activated after centrifugation (9 ml) was switched with 10-ml tubes containing Ca2+ extracted by the Cascade-Selphyl-Esforax Kit. The PRP (0.1 ml/cm2) was injected in selected areas of the scalp in the amount. It is suggested that a sufficient number of platelets could be obtained in all patients by using an automated PRP preparation system.
Conclusion
Giusti et al. [29] demonstrated that the optimal platelet concentration for the induction of angiogenesis in human endothelial cells was 1.5 million platelets per microliter, whereas excessively high concentrations of platelets were suggested to decrease the angiogenic potential [29]. In this study, a mean of 1,484,555.6 platelets per microliter in the PRP preparation could effectively stimulate follicular and perifollicular angiogenesis, which is suggested to be one of the major factors in active hair growth [5, 11]. Our data and data published by us [19] and other authors since 2011 suggest that the injection of PRP preparations has a positive therapeutic effect on male androgenic alopecia without major side effects.
Author Contributions
P.G. and V.C.: conception and design, manuscript writing, final approval of the manuscript; S.G.: data analysis and interpretation; A.B., A.O., and M.G.S.: collection and/or assembly of data.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213–219. doi: 10.4103/0974-2077.150743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li ZJ, Choi HI, Choi DK, et al. Autologous platelet-rich plasma: A potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012;38:1040–1046. doi: 10.1111/j.1524-4725.2012.02394.x. [DOI] [PubMed] [Google Scholar]
- 3.Khatu SS, More YE, Gokhale NR, et al. Platelet-rich plasma in androgenic alopecia: myth or an effective tool. J Cutan Aesthet Surg. 2014;7:107–110. doi: 10.4103/0974-2077.138352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trink A, Sorbellini E, Bezzola P, et al. A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br J Dermatol. 2013;169:690–694. doi: 10.1111/bjd.12397. [DOI] [PubMed] [Google Scholar]
- 5.Uebel CO, da Silva JB, Cantarelli D, et al. The role of platelet plasma growth factors in male pattern baldness surgery. Plast Reconstr Surg. 2006;118:1458–1466; discussion 1467. doi: 10.1097/01.prs.0000239560.29172.33. [DOI] [PubMed] [Google Scholar]
- 6.Lachgar S, Moukadiri H, Jonca F, et al. Vascular endothelial growth factor is an autocrine growth factor for hair dermal papilla cells. J Invest Dermatol. 1996;106:17–23. doi: 10.1111/1523-1747.ep12326964. [DOI] [PubMed] [Google Scholar]
- 7.Kozlowska U, Blume-Peytavi U, Kodelja V, et al. Expression of vascular endothelial growth factor (VEGF) in various compartments of the human hair follicle. Arch Dermatol Res. 1998;290:661–668. doi: 10.1007/s004030050370. [DOI] [PubMed] [Google Scholar]
- 8.Tarallo V, Vesci L, Capasso O, et al. A placental growth factor variant unable to recognize vascular endothelial growth factor (VEGF) receptor-1 inhibits VEGF-dependent tumor angiogenesis via heterodimerization. Cancer Res. 2010;70:1804–1813. doi: 10.1158/0008-5472.CAN-09-2609. [DOI] [PubMed] [Google Scholar]
- 9.Tobin DJ, Gunin A, Magerl M, et al. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme: Implications for hair growth control. J Invest Dermatol. 2003;120:895–904. doi: 10.1046/j.1523-1747.2003.12237.x. [DOI] [PubMed] [Google Scholar]
- 10.Li W, Enomoto M, Ukegawa M, et al. Subcutaneous injections of platelet-rich plasma into skin flaps modulate proangiogenic gene expression and improve survival rates. Plast Reconstr Surg. 2012;129:858–866. doi: 10.1097/PRS.0b013e3182450ac9. [DOI] [PubMed] [Google Scholar]
- 11.Semalty M, Semalty A, Joshi GP, et al. Hair growth and rejuvenation: An overview. J Dermatolog Treat. 2011;22:123–132. doi: 10.3109/09546630903578574. [DOI] [PubMed] [Google Scholar]
- 12.Cervelli V, Scioli MG, Gentile P, et al. Platelet-rich plasma greatly potentiates insulin-induced adipogenic differentiation of human adipose-derived stem cells through a serine/threonine kinase Akt-dependent mechanism and promotes clinical fat graft maintenance. Stem Cells Translational Medicine. 2012;1:206–220. doi: 10.5966/sctm.2011-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cervelli V, Gentile P, Scioli MG, et al. Application of platelet-rich plasma in plastic surgery: clinical and in vitro evaluation. Tissue Eng Part C Methods. 2009;15:625–634. doi: 10.1089/ten.TEC.2008.0518. [DOI] [PubMed] [Google Scholar]
- 14.Cervelli V, Gentile P, De Angelis B, et al. Application of enhanced stromal vascular fraction and fat grafting mixed with PRP in post-traumatic lower extremity ulcers. Stem Cell Res (Amst) 2011;6:103–111. doi: 10.1016/j.scr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Campagnolo L, Costanza G, Francesconi A, et al. Sortilin expression is essential for pro-nerve growth factor-induced apoptosis of rat vascular smooth muscle cells. PLoS One. 2014;9:e84969. doi: 10.1371/journal.pone.0084969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takikawa M, Nakamura S, Nakamura S, et al. Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol Surg. 2011;37:1721–1729. doi: 10.1111/j.1524-4725.2011.02123.x. [DOI] [PubMed] [Google Scholar]
- 17.Ferlosio A, Arcuri G, Doldo E, et al. Age-related increase of stem marker expression influences vascular smooth muscle cell properties. Atherosclerosis. 2012;224:51–57. doi: 10.1016/j.atherosclerosis.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Stasi MA, Scioli MG, Arcuri G, et al. Propionyl-L-carnitine improves postischemic blood flow recovery and arteriogenetic revascularization and reduces endothelial NADPH-oxidase 4-mediated superoxide production. Arterioscler Thromb Vasc Biol. 2010;30:426–435. doi: 10.1161/ATVBAHA.109.201533. [DOI] [PubMed] [Google Scholar]
- 19.Cervelli V, Garcovich S, Bielli A, Cervelli G, Curcio BC, Scioli MG, Orlandi A, Gentile P. The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: Clinical and histomorphometric evaluation. Biomed Res Int 2014; 2014:760709. doi: 10.1155/2014/760709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han JH, Kwon OS, Chung JH, et al. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J Dermatol Sci. 2004;34:91–98. doi: 10.1016/j.jdermsci.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Tosti A, Piraccini BM. Finasteride and the hair cycle. J Am Acad Dermatol. 2000;42:848–849. doi: 10.1067/mjd.2000.103272. [DOI] [PubMed] [Google Scholar]
- 22.Sawaya ME, Blume-Peytavi U, Mullins DL, et al. Effects of finasteride on apoptosis and regulation of the human hair cycle. J Cutan Med Surg. 2002;6:1–9. doi: 10.1007/s10227-001-0024-y. [DOI] [PubMed] [Google Scholar]
- 23.de Rivero Vaccari JP, Sawaya ME, Brand F, 3rd, et al. Caspase-1 level is higher in the scalp in androgenetic alopecia. Dermatol Surg. 2012;38:1033–1039. doi: 10.1111/j.1524-4725.2012.02378.x. [DOI] [PubMed] [Google Scholar]
- 24.Ferraris C, Cooklis M, Polakowska RR, et al. Induction of apoptosis through the PKC pathway in cultured dermal papilla fibroblasts. Exp Cell Res. 1997;234:37–46. doi: 10.1006/excr.1997.3601. [DOI] [PubMed] [Google Scholar]
- 25.Kwon OS, Pyo HK, Oh YJ, et al. Promotive effect of minoxidil combined with all-trans retinoic acid (tretinoin) on human hair growth in vitro. J Korean Med Sci. 2007;22:283–289. doi: 10.3346/jkms.2007.22.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sohn KC, Shi G, Jang S, et al. Pitx2, a beta-catenin-regulated transcription factor, regulates the differentiation of outer root sheath cells cultured in vitro. J Dermatol Sci. 2009;54:6–11. doi: 10.1016/j.jdermsci.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: A preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72–79. doi: 10.1111/jdv.12062. [DOI] [PubMed] [Google Scholar]
- 28.Schiavone G, Raskovic D, Greco J, et al. Platelet-rich plasma for androgenetic alopecia: A pilot study. Dermatol Surg. 2014;40:1010–1019. doi: 10.1097/01.DSS.0000452629.76339.2b. [DOI] [PubMed] [Google Scholar]
- 29.Giusti I, Rughetti A, D’Ascenzo S, et al. Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Transfusion. 2009;49:771–778. doi: 10.1111/j.1537-2995.2008.02033.x. [DOI] [PubMed] [Google Scholar]