Abstract
Monoclonal antibody (mAb)-based treatment of cancer has a significant effect on current practice in medical oncology, and is considered now as one of the most successful therapeutic strategies for cancer treatment. MAbs are designed to initiate or enhance anti-tumor immune responses, which can be achieved by either blocking inhibitory immune checkpoint molecules or triggering activating receptors. TIM gene family members are type-I surface molecules expressed in immune cells, and play important roles in the regulation of both innate and adaptive arms of the immune system. Therapeutic strategies based on anti-TIMs mAbs have shown promising results in experimental tumor models, and synergistic combinations of anti-TIMs mAbs with cancer vaccines, adoptive T-cell therapy, radiotherapy and chemotherapy will have great impact on cancer treatment in future clinical development.
Keywords: antitumor immune response, cancer vaccines, chemotherapy, TIM family proteins, tumor immunity
Introduction
The use of monoclonal antibodies (mAbs) in cancer therapy has achieved considerable success in recent years as a result of the accumulating knowledge of cancerous conditions.1,2 Cancer cells build a microenvironment that includes in addition to cancer cells: immune cells, fibroblasts, endothelial cells, extracellular matrix, cytokines, and various receptors, and the interactions of these components have substantial effects on tumor suppression or progression, cancer cell stemness, and resistance to cancer therapy.3,4 The application of appropriate mAbs constitutes an attractive approach in cancer therapy that depends on in-depth understanding of the characteristics of each tumor.
MAbs used in the field of cancer immunotherapy can be divided into two types: (1) mAbs that directly bind to its targets on cancer cells; and (2) mAbs that are designed to manipulate immune responses against tumors by targeting specific molecules expressed in or by immune cells, such as the targeting of immune checkpoints on immune cells.5,6 Immune checkpoints are molecules expressed in immune cells, and play important roles in the maintenance of self-tolerance and control of immune response.7 However, accumulating evidence has shown that tumors can manipulate certain immune checkpoint signaling pathways, which results in tumor escape from immune surveillance and inhibition of antitumor immune response.7 On the other hand, because many of the immune checkpoints are expressed on the surface of immune cells, they can be easily targeted by mAbs, and, indeed the enhancement of antitumor immune responses using immunostimulatory mAbs against immune checkpoint molecules is now considered one of the powerful and promising strategies in cancer therapy.8 The first application of this strategy was the use of immunostimulatory mAbs against cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4).9 Anti-CTLA-4 mAbs are effective to rescue T cell dysfunction and trigger immune response against tumors in experimental models and preclinical testing, and have reached the clinic after successful testing in Phase 3 clinical trials for patients with malignant melanoma.10,11 These preliminary clinical findings encourage research to identify molecules involved in the regulation of tumor immunity and antitumor response, which are needed to enable development of new mAbs against immune-checkpoint molecules to enhance antitumor immunity and produce durable clinical responses.
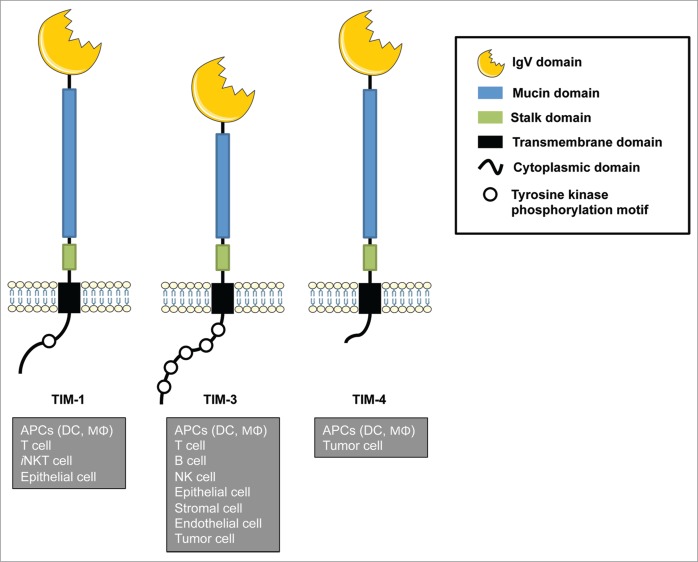
T cell immunoglobulin and mucin domain (TIM) proteins are type-I cell-surface molecules that share a unique structure, including an N-terminal immunoglobulin (Ig)-like domain, a mucin domain with O-linked and N-linked glycosylation, a single transmembrane domain, and a cytoplasmic region with tyrosine phosphorylation motifs, except in TIM-4 (Fig. 1).12,13 The TIM gene family consists of eight members (TIM-1–8) located on chromosome 11B1.1 in mouse, and three members (TIM-1, TIM-3, and TIM-4) located on chromosome 5q33.2 in human, in a region that links to asthma, allergy and autoimmune diseases.12,13 TIM family proteins (TIMs) are expressed on a wide variety of immune cells in addition to non-immune cells such as epithelial cells. However, the expression of TIMs has also been observed in other cell types, such as endothelial cells, stromal cells and transformed cells in chronic viral infections and cancer, suggesting that TIMs play important roles in the pathological process of these conditions.14 Additionally, several studies have clarified the involvement of TIMs in the regulation of a wide variety of immune conditions including allergy, asthma, transplant tolerance, autoimmunity, and antiviral immunity.15,16 Thus, these findings raise the possibility that TIMs serve as critical checkpoint machineries to regulate immune homeostasis and inflammation. More importantly, accumulating evidences have unveiled the critical roles of TIM members in the regulation of innate and adaptive immune cells at tumor microenvironment, which have substantial effects on tumor immunosurveillance and antitumor immune response, and consequently on the clinical outcome of cancer therapy.17 These advances have encouraged the generation of several mAbs to target TIMs that have been evaluated in various experimental models. In this review, we briefly introduce TIMs shared between mouse and human (TIM-1, TIM-3, and TIM-4), highlight the functions of each TIM in tumor microenvironment, and describe recent progress in studies that utilize anti-TIMs mAbs, their application and the therapeutic effects observed and expected in the field of cancer immunotherapy.
Figure 1.
Schematic structure and expression of TIM gene family members. TIM genes encode type-I cell-surface glycoproteins that consist of an extracellular IgV domain, a mucin-like domain, a stalk domain, a transmembrane domain, and an intracellular cytoplasmic tail with tyrosine phosphorylation sites. TIMs are expressed in a wide variety of cells, including both immune and non-immune cells. Figures were produced using Servier Medical Art.
TIM-1
In 2001, TIM-1, or kidney injury molecule-1 (KIM-1) as originally named, was the first member of the TIM gene family to be reported. It was identified after the screening of rat and human cDNAs to identify molecules involved in processes of injury and repair of the tubular epithelium of the kidney.18 TIM-1 expression was found to be dramatically increased in post-ischemic kidney, suggesting that TIM-1 may play an important role in the restoration of the morphological and functional integrity to post-ischemic kidney.18 Later studies identified the expression of TIM-1 on T cells, where it plays different roles in the regulation of T cell functions. Some reports suggest that TIM-1 acts as a co-stimulatory molecule for T cell activation, while others suggest a role for TIM-1 as an immune checkpoint which inhibits T cell activities.19,20
Several mAbs have been generated to target and manipulate TIM-1 functions in vitro and in vivo (Table 1). The therapeutic effects of these mAbs were tested in a variety of murine models of allergic and autoimmune diseases. Agonistic mAbs of TIM-1 such as 3B3 and 1H8.2 clones were found to increase lymphocytes proliferation and infiltration, enhance the production of pro-inflammatory cytokines, and suppress the production of anti-inflammatory cytokines.21-26 The administration of agonistic anti-TIM-1 mAbs in vivo results in enhanced inflammation, e.g., pulmonary inflammation,22 accelerates reject of transplanted xenografts,21,23 and worsen the severity of autoimmune diseases.24,25 On the other hand, antagonistic mAbs of TIM-1, such as RMT1–4,27 RMT1–10,24,25,27-34 3A2.5, and 4A2.226 clones, were found to limit the proliferation and infiltration of lymphocytes, decrease pro-inflammatory cytokines and increase anti-inflammatory cytokines.26 Other clones such as 222414 were found to elicit different immune responses depending on the model. 222414 clone has antagonistic effects in asthma35 and allergy model,26,36 while it shows agonistic effects in influenza infection.37 The administration of antagonistic anti-TIM-1 mAbs results in a significant decrease in inflammation such as asthma35 and allergy,26,36 and helps to attenuate inflammation in autoimmune disease24,25 and cisplatin-induced nephrotoxicity.31
Table 1.
Blocking monoclonal antibodies of TIM-1
| Clone | Host | Model | Effects | Ref. |
|---|---|---|---|---|
| 3B3 | C57BL/6 | Xenograft transplantation | Lymphocyte proliferation ↑ Tolerance ↓ | 21 |
| BALB/c | Ovalbumin-induced allergy | Lymphocyte proliferation ↑ IL-4, IL-10, IFN-γ production in CD4+ T cell ↑ |
22 | |
| BALB/c | Cardiac allograft | IL-17+ CD8+ T17 cell ↓ | 23 | |
| SJL | Autoimmune encephalomyelitis | Antigen specific T cell proliferation ↑ IL-4, IL-17, IFN-γ ↑ |
24 | |
| SJL | Autoimmune encephalomyelitis | Immunogenic DC ↑ Suppressive Treg ↓ IL-1β, IL-4, IL-6, IL-10, IL-23, IFN-γ, TNFα, TGFβ ↑ |
25 | |
| 1H8.2 | BALB/c | Ovalbumin-induced lung inflammation | Lymphocyte proliferation ↑ IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, TNFα, IFN-γ ↑ |
26 |
| RMT1-4 | C57BL/6 | Bleomycin-induced pulmonary fibrosis | IFN-γ production in NKT cell ↓ IL-4, IL-10, IL-13 ↑ |
27 |
| RMT1-10 | SJL | Autoimmune encephalomyelitis | Antigen specific T cell proliferation ↓ IL-17, IFN-γ ↓ IL-4, IL-10↑ |
24 |
| SJL | Autoimmune encephalomyelitis | IL-4, IL-10↑ IFN-γ ↓ | 25 | |
| C57BL/6 | Bleomycin-induced pulmonary fibrosis | IFN-γ production in NKT cell ↓ IL-10 ↑ | 27 | |
| C57BL/6 | Crescentic glomerulonephritis | Accumulation of Neutrophil, macrophage, CD4+ T cell ↓ Treg↑ IL-1β, IL-12, IL-17, TNFα, IFN-γ ↓ IL-4, IL-10↑ |
28 | |
| C57BL/6 | Islet allograft | IL-10 in B cell ↑ | 29 | |
| C57BL/6 | Lupus nephritis | CD4+ T cell proliferation ↓ Treg and B cell ↑ IL-2, IL-4, IL-17, IFN-γ ↓ TGFβ ↑ |
30 | |
| C57BL/6 | Cisplatin nephrotoxicity | Accumulation of CD4+ and CD8+ T cell ↓ IL-1β, IL-6, IFN-γ, TNFα ↓ IL-10 ↑ CXCL1, CXCL2, CXCL9, CXCL10, CCL2, CCL3, CCL5, ICAM1 ↓ |
31 | |
| C57BL/6 | Hepatic ischemia-reperfusion injury | Accumulation of Neutrophil, macrophage, T cell ↓ IL-6, TNFα, IL-1β, IFN-γ, CXCL-1, CXCL-2 ↓ |
32 | |
| BALB/c | Short ragweed pollen | Eosinophil infiltration ↓ IL-10, IL-13, and IFN-γ production ↑ |
33 | |
| BALB/c | Corneal allograft | CD4+ T cell proliferation ↓ Treg ↑ IL-4, IFN-γ ↓ TGFβ ↑ |
34 | |
| RMT1-17 | BALB/c | Immunization with ovalbumin | B cell proliferation ↑ IgG2b, IgG3 ↑ |
29 |
| 3A2.5 | BALB/c | Ovalbumin-induced lung inflammation | Lymphocyte proliferation ↓ IL-4, IL-5, IL-10↓ |
26 |
| 4A2.2 | BALB/c | Ovalbumin-induced lung inflammation | Lymphocyte proliferation ↓ IL-4, IL-5, IL-10, IL-13↓ |
26 |
| 222414 | BALB/c | Ovalbumin-induced asthma | Macrophage and eosinophils infiltration ↓ IL-4, IL-5, IL-10, IL-13 ↓ |
35 |
| BALB/c | Peanut-induced allergy | Lymphocyte proliferation ↓ IgE secretion from mast cells ↓ IL-4, IFN-γ ↓ |
36 | |
| C57BL/6 | Bleomycin-induced pulmonary fibrosis | IFN-γ production in NKT cell ↓ | 26 | |
| BALB/c | H1N1 influenza | Lymphocyte proliferation ↑ IL-4, IFN-γ production in CD4+ T cell ↑ |
37 |
As described above, the roles of TIM-1 in the regulation of immune responses have been particularly studied in inflammatory conditions. However, little is known about the role of TIM-1 in the tumor microenvironment or the application of anti-TIM-1 mAbs in cancer treatment. Growing evidence has unveiled the important roles of inflammation at different phases of tumor development, starting from initiation, promotion, conversion to malignancy, invasion, and metastasis.38 Inflammation is also involved in the regulation of immune surveillance and responses to cancer therapy.39 In this regard, it is of great interest to evaluate the involvement of TIM-1 in the regulation of tumor-associated inflammation for the appropriate application of anti-TIM-1 mAbs in cancer therapy. Agonistic mAbs of TIM-1 are expected to trigger or amplify antitumor immune responses through the enhancement of infiltration and proliferation by lymphocytes and the production of proinflammatory cytokines, but may increase the risks of worsening tumor-induced inflammation. On the other hand, antagonistic mAbs may be used to attenuate inflammation and prevent inflammation-related tumor progression. However, the therapeutic effects of anti-TIM-1 mAbs should be carefully evaluated in transplant and naturally occurring tumor models.
TIM-3
Following the discovery of TIM-1, in 2002 TIM-3 became the second member of TIM gene family to be identified as a molecule expressed selectively on interferon (IFN)-γ-secreting CD4+ T helper 1 and CD8+ T cytotoxic cells in mice and humans.40 However, TIM-3 was shown later to be expressed on a wide variety of immune cells, including dendritic cells, macrophages, natural killer (NK) cells, T cells, B cells, and non-immune cells such as epithelial cells, endothelial cells and stromal cells.13-16 Furthermore, recent studies have identified the expression of TIM-3 on cancer cells, especially those with stem-like properties.40
TIM-3 plays important roles in the regulation of both arms of innate and adaptive immunity.41,42 In tumors, TIM-3 expression is induced in innate immune cells, e.g., dendritic cells and macrophages, by tumor-released factors such as interleukin (IL)-10 and vascular endothelial growth factor, leading to a suppression of innate responses to nucleic acids released from apoptotic tumor cells by interacting with HMGB1.44 Additionally, TIM-3 serves as an exhaustion marker for T cells in tumors. TIM-3 interacts with galectin-9 and causes exhaustion and apoptosis of antigen-specific CTLs, which correlates with impaired antitumor immune responses.45,46 TIM-3 expression was also observed on Foxp3+ regulatory T cells; however, the function of TIM-3 in this population should be explored in future studies.47
Several blocking mAbs have been generated to target mouse and human TIM-3 (Table 2). RMT3–23 is a blocking mAb that targets mouse TIM-3, and has been widely used and tested in various murine tumor models including melanoma,44,48-50 colon adenocarcinoma,44,48,49 sarcoma,48 prostate carcinoma,48 and ovarian cancer.51 Monotherapy with RMT3–23 clone was effective to increase tumor-infiltrating IFN-γ-producing CD4+ and CD8+ T cells and suppress Treg functions in sarcoma and colon adenocarcinoma models.48,49 RMT3–23 clone has also shown synergistic effects when combined with anti-CD137 mAb, resulting in increased tumor-infiltrating CD4+ and CD8+ T cells, decreased Treg and MDSCs, and suppressed tumor growth in ovarian cancer model.51 RMT3–23 mAb was also used in combination with other cancer therapy. In combination with cancer vaccines, such as DNA vaccines encoding melanoma-derived antigens (e.g., TRP2, gp100), or tumor cell vaccine FVAX (irradiated B16 melanoma cells engineered to continuously secrete Flt3 ligand), RMT3–23 mAb triggers an effective antitumor immune response against established B16 melanomas. This response was mediated by enhanced production of IFNβ1 and IL-12 and increases in the frequencies of active NK and T cells.44,50 These effects were maximized when RMT3–23 clone was used in combination with RMT4–53 (anti-TIM-4 mAb).50 In combination with chemotherapy, RMT3–23 clone induced an effective immune response against MC38 colon adenocarcinomas that was accompanied by increased levels of pro-inflammatory cytokines such as IFNβ1 and IL-12.44 Monotherapy with other mAbs targeting TIM-3, such as 8B.2C12 clone, has also induced a potent antitumor immune response against colon adenocarcinomas with elevated levels of IFN-γ production.49 MAbs of human TIM-3 have shown similar results observed in mouse. 2E2 and AF2365 clones were effective to enhance proliferation and cytotoxic activities of NK cells, and to expand antigen-specific CD8+ T cells with increased IFN-γ production when combined with peptide vaccination of melanoma.45,46,53 ATIK2a clone, another mAb that targets human TIM-3, was found to induce antibody-dependent cell-mediated cytotoxicity (ADCC), and was effective in eradicating TIM-3-expressing leukemia stem cells in an acute myeloid leukemia model.54,55 On the other hand, 334823 and 344801 clones may act as antagonist mAb because the cross-linking of these mAb with its targets on NK cells has suppressed NK cell-mediated cytotoxicity in in vitro experiments.56
Table 2.
Blocking monoclonal antibodies of TIM-3
| Clone | Host | Model | Therapy | Effects | Ref. |
|---|---|---|---|---|---|
| RMT3-23 | C57BL/6 | Melanoma (B16-F10) | Antibody therapy | Tumor growth↓ | 48 |
| C57BL/6 | Melanoma (B16-F10) | Antibody therapy | Treg suppressive functions↓ | 49 | |
| C57BL/6 | Melanoma (B16-F10) | Tumor vaccination (FVAX) | Inhibition of tumor growth NK cell infiltration ↑ NK cell activation ↑ |
50 | |
| C57BL/6 | Melanoma (B16-F10) | DNA vaccine (gp100) | Inhibition of tumor growth NK cell infiltration ↑ NK cell activation ↑ |
50 | |
| C57BL/6 | Melanoma (B16-F10) | DNA vaccine (TRP2) | Tumor growth↓ Interferons↑ IL-12↑ NK cell activation ↑ |
44 | |
| C57BL/6 | Colon cancer (MC38) | Chemotherapy (Cisplatin) | Tumor growth↓ | 44 | |
| C57BL/6 | Colon cancer (MC38) | Antibody therapy | Tumor growth↓ CD4+ and CD8+ T cell ↑ IFN-γ production ↑ |
48 | |
| BALB/c | Colon cancer (CT26) | Antibody therapy | Tumor growth↓ CD4+ and CD8+ T cell ↑ | 48 | |
| BALB/c | Colon cancer (CT26) | Antibody therapy | Treg suppressive functions ↓ | 49 | |
| C57BL/6 | Colon cancer (CT26) | Antibody therapy | Treg suppressive functions ↓ | 49 | |
| C57BL/6 | Sarcoma (WT3) | Antibody therapy | Tumor growth↓ IFN-γ production ↑ | 48 | |
| C57BL/6 | Prostate carcinoma (TRAMP-C1) | Antibody therapy | Tumor growth↓ | 48 | |
| C57BL/6 | Ovarian cancer (ID8) | Combination with anti-CD137 | Tumor growth↓ CD4+ and CD8+ T cell ↑ CD4+FoxP3+ Treg ↓ CD11b+Gr-1+MDSC cell ↓ |
51 | |
| C57BL/6 | Phagocytosis of apoptotic cells | Antibody therapy | Phagocytosis / cross presentation by CD8+ DC↓ Auto-antibody production↑ | 52 | |
| 8B.2C12 | BALB/c | Colon cancer (CT26) | Antibody therapy | tumor growth ↓ IFN-γ production ↑ | 49 |
| 2E2 | PBMC | Melanoma | Peptide vaccination | Proliferation of NY-ESO-1+ CD8+ T cell ↑ IFN-γ production ↑ |
45 |
| PBMC | Melanoma | Peptide vaccination | Proliferation of NY-ESO-1+ CD8+ T cell ↑ IFN-γ, TNFα↑ |
46 | |
| PBMC | Melanoma | In vitro blockade | NK cell-mediated cytotoxicity ↑ IFN-γ production ↑ NK cell proliferation ↑ |
53 | |
| AF2365 | PBMC | Melanoma | In vitro blockade | NK cell-mediated cytotoxicity ↑ | 53 |
| ATIK2a | BALB/c | Acute myeloid leukemia (AML) | Antibody-dependent cell-mediated cytotoxicity | Elimination of TIM-3 expressing leukemia stem cell (LSC) | 54, 55 |
| 344823 | Human NK cell | Cytotoxic assay | In vitro blockade | NK cell-mediated cytotoxicity ↓ | 56 |
| 344801 | Human NK cell | Cytotoxic assay | In vitro blockade | NK cell-mediated cytotoxicity ↓ | 56 |
Together, the expression of TIM-3 in innate and adaptive immune cells, in addition to tumor cells, makes it an attractive target to enhance immune responses against tumors or to eradicate TIM-3-expressing cancer stem cells.
TIM-4
To identify genes regulated by lymphotoxin signaling pathway, cDNA expression microarrays were used to compare profiles of splenic gene expression in mice with single lymphotoxin-α (LTα), tumor necrosis factor (TNF), or combined LTα/TNF deficiencies with wild-type C57BL/6 controls. This led to the identification of a gene that is selectively downregulated in spleens of LTα- or LTβ-deficient mice, and was originally named SMUCKLER (spleen, mucin-containing, and knockout of lymphotoxin).57 Sequential and structural analysis of this gene revealed similar characteristics of TIM gene family, and thus it was renamed TIM-4.57 TIM-4 shares the same structure with other TIM family members, but it also has unique features.13-17 In contrast to other TIMs, TIM-4 lacks the putative tyrosine phosphorylation sequence in its intracellular domain that is implicated in signaling by other TIMs, which suggests a role for TIM-4 as a binding receptor more than a signaling receptor.58 TIM-4 is expressed exclusively in antigen presenting cells (APCs) such as dendritic cells and macrophages,59 where it serves as a binding receptor of phosphatidylserine (PS) and enhances the engulfment of apoptotic cells.60 However, the function of TIM-4 as a PS receptor seems to be dependent on the subset of APCs. For example, TIM-4 serves as a critical phagocytosis receptor in peritoneal macrophages, since the deficiency of TIM-4 results in a significant decrease in the ability of peritoneal macrophages to engulf apoptotic cells.61 However, splenic and tumor-associated macrophages were still able to engulf apoptotic cells even in the absence of TIM-4, suggesting the existence of other phagocytosis receptors expressed in these subsets of APCs.61,62
Recent studies have identified a novel role of TIM-4 in the regulation of tumor immunity and antitumor immune responses.62 TIM-4 plays an important role in controlling tumor-specific responses through regulation of processing and presentation of antigens derived from phagocytized apoptotic tumor cells. TIM-4 was found to interact with metabolic sensor AMPKα1 (adenosine monophosphate-activated protein kinase) in tumor-associated macrophages after the engulfment of apoptotic tumor cells.62 This interaction led to excessive degradation of tumor-derived antigens via autophagy-mediated mechanisms.62 Additionally, TIM-4 may be involved in the removal of tumor antigen-specific T cells, which was previously shown in an influenza infection model.63
The therapeutic effects of TIM-4 blockade in cancer therapy were confirmed by RMT4–53 clone against mouse TIM-4.50,62 This clone was effective in triggering a potent antitumor immune response against tumors such as melanoma and colon adenocarcinoma.50,62 These studies combined RMT4–53 clone with cancer vaccines, such as DNA vaccines or the tumor cell vaccine (FVAX) against B16 melanoma,50 or in combination with chemotherapy cytotoxic agents such as cisplatin or oxaliplatin against colon adenocarcinoma.62 RMT4–53 clone has increased the frequencies of IFN-γ-producing CD8+ T cells and inhibited tumor growth.50 Additionally, as mentioned previously, RMT4–53 synergized with RMT3–23 to maximize the therapeutic effects of cancer vaccines against B16 melanomas through NK and T cell-mediated mechanisms.50 Although no reports about mAbs that target human TIM-4 are available, one study suggests that the targeting of IgV domain of TIM-4 in a region thought to be critical for TIM-1 binding by specific shRNA may enhance the therapeutic effects of vaccination against gastric cancer.64 The generation of blocking mAbs that target human TIM-4 is thus a promising area of research for identification of therapeutics that may enhance antitumor immune responses against tumors.
Taken together, these observations suggest that blockade by anti-TIM-4 mAbs serves as a promising strategy in cancer therapy, in particular to enhance tumor antigen-specific T cell responses. In this regard it is also of great interest to evaluate the effects of anti-TIM-4 mAbs on the functions of central and effector memory T cells in future studies.
Conclusion
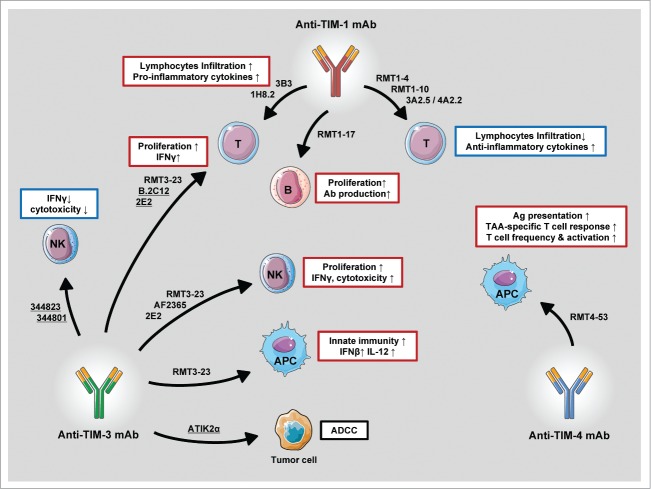
Activating an effective immune response against tumor is the main goal in cancer immunotherapy, which requires an orchestrated actions mediated by various types of immune cells, including APCs such as dendritic cells and macrophages, NK cells, and T lymphocytes.65 On the other hand, the immunosuppressive microenvironment of tumors strongly interferes with immune functions of these cells by various mechanisms.66,67 Recent studies have identified TIM family molecules as key players for the regulation of innate and adaptive immune response in the tumor microenvironment.17 TIM-3 and TIM-4 expression are highly induced in dendritic cells and macrophages in tumors.44,62 TIM-3 inhibits innate immune responses mediated by DAMPs,44 while TIM-4 suppresses antigen-specific responses by repressing presentation of immunogenic tumor-associated antigens and establishing tolerated tumor environments.62 Additionally, TIM-3 is highly expressed on effector immune cells such as T cells and NK cells, and serves as an exhaustion marker for T cells and NK cells.45,46,53 Together, these results suggest that the manipulation of TIMs functions by mAbs is a promising and powerful strategy to initiate or amplify an existing immune response against tumors (Fig. 2). Indeed, anti-TIMs mAbs have been shown to improve the performance of APCs by enhancing secretion of cytokines and antigen presentation to T cells,44,62 recovery of exhausting T cell, and NK cells.45,46,53 Monotherapies with anti-TIMs mAbs have been evaluated in experimental mouse tumor models, and anti-TIMs mAbs have also shown preclinical synergistic effects with several forms of cancer vaccines and chemotherapy (Tables 1–3). Another interesting area that is still largely unexplored is the combination of anti-TIMs mAbs with adoptive transfer of T cells. However, when thinking about combining treatments with anti-TIMs mAbs, one has to be mindful that the simultaneous or sequential manipulation of TIMs could trigger untreatable autoimmunity68,69 as a result of simultaneously inhibiting important immunological brakes. Another obstacle to the application of anti-TIMs mAbs is the possibility of inducing adverse toxicity, most commonly reversible autoimmunity or systemic inflammatory reactions.52,61
Figure 2.
Primary immune effects mediated by anti-TIMs mAb. Anti-TIMs mAbs are a powerful tool to manipulate the functions of innate and adaptive immune cells and thus orchestrate immune response against tumors. Underlined clones refer to human mAbs. Figures were produced using Servier Medical Art.
Table 3.
Blocking monoclonal antibodies of TIM-4
| Clone | Host | Model | Therapy | Effects | Ref. |
|---|---|---|---|---|---|
| RMT4-53 | C57BL/6 | Melanoma (B16-F10) | DNA vaccine (gp100) | Tumor growth↓ T cell infiltration ↑ T cell activation ↑ |
50 |
| C57BL/6 | Melanoma (B16-F10) | Tumor vaccination (FVAX) | Tumor growth↓ T cell infiltration ↑ T cell activation ↑ |
50 | |
| C57BL/6 | Melanoma (B16-F10) | Chemotherapy (Cisplatin) | Tumor growth↓ Antigen presentation ↑ IFN-γ-producing T cells ↑ |
62 | |
| C57BL/6 | Colon cancer (MC38) | Chemotherapy (Cisplatin) | Tumor growth↓ Antigen presentation ↑ IFN-γ-producing T cells ↑ |
62 | |
| C57BL/6 | Colon cancer (MC38) | Chemotherapy (Oxaliplatin) | Tumor growth↓ Antigen presentation ↑ IFN-γ-producing T cells ↑ |
62 |
In conclusion, TIM molecules play critical roles in the regulation of tumor immunosurveillance and anti-tumor immunity, and anti-TIMs mAbs can be utilized as potent tools to manipulate the functions of various immune cells, and thus orchestrate immune responses against tumors. The molecules have the potential to greatly impact the therapeutic effects of cancer regimens.70
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 2012; 12:278-87; PMID:22437872; http://dx.doi.org/ 10.1038/nrc3236 [DOI] [PubMed] [Google Scholar]
- 2. Adler MJ, Dimitrov DS. Therapeutic antibodies against cancer. Hematol Oncol Clin North Am 2012; 26:447-81, vii; PMID:22520975; http://dx.doi.org/ 10.1016/j.hoc.2012.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mbeunkui F, Johann DJ, Jr. Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol 2009; 63:571-82; PMID:19083000; http://dx.doi.org/ 10.1007/s00280-008-0881-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14:1014-22; PMID:24048123; http://dx.doi.org/ 10.1038/ni.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vacchelli E, Eggermont A, Galon J, Sautès-Fridman C, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Monoclonal antibodies in cancer therapy. Oncoimmunology 2013; 2:e22789; PMID:23482847; http://dx.doi.org/ 10.4161/onci.22789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 2010; 10:317-27; PMID:20414205; http://dx.doi.org/ 10.1038/nri2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ileana E, Champiat S, Soria J-C. [Immune-checkpoints: the new anti-cancer immunotherapies]. Bull Cancer 2013; 100:601-10; PMID:23735730 [DOI] [PubMed] [Google Scholar]
- 8. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:22437870; http://dx.doi.org/ 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer 2007; 7:95-106; PMID:17251916; http://dx.doi.org/ 10.1038/nrc2051 [DOI] [PubMed] [Google Scholar]
- 10. Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immun 2013; 13:5; PMID:23390376 [PMC free article] [PubMed] [Google Scholar]
- 11. Ascierto PA, Marincola FM, Ribas A. Anti-CTLA4 monoclonal antibodies: the past and the future in clinical application. J Transl Med 2011; 9:196; PMID:22077981; http://dx.doi.org/ 10.1186/1479-5876-9-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McIntire JJ, Umetsu SE, Akbari O, Potter M, Kuchroo VK, Barsh GS, Freeman GJ, Umetsu DT, DeKruyff RH. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol 2001; 2:1109-16; PMID:11725301; http://dx.doi.org/ 10.1038/ni739 [DOI] [PubMed] [Google Scholar]
- 13. Kane LP. T cell Ig and mucin domain proteins and immunity. J Immunol 2010; 184:2743-9; PMID:20200285; http://dx.doi.org/ 10.4049/jimmunol.0902937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuchroo VK, Dardalhon V, Xiao S, Anderson AC. New roles for TIM family members in immune regulation. Nat Rev Immunol 2008; 8:577-80; PMID:18617884; http://dx.doi.org/ 10.1038/nri2366 [DOI] [PubMed] [Google Scholar]
- 15. Kuchroo VK, Meyers JH, Umetsu DT, DeKruyff RH. TIM family of genes in immunity and tolerance. Adv Immunol 2006; 91:227-49; PMID:16938542; http://dx.doi.org/ 10.1016/S0065-2776(06)91006-2 [DOI] [PubMed] [Google Scholar]
- 16. Meyers JH, Sabatos CA, Chakravarti S, Kuchroo VK. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol Med 2005; 11:362-9; PMID:16002337; http://dx.doi.org/ 10.1016/j.molmed.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 17. Baghdadi M, Jinushi M. The impact of the TIM gene family on tumor immunity and immunosuppression. Cell Mol Immunol 2014; 11:41-8; PMID:24336162; http://dx.doi.org/ 10.1038/cmi.2013.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 1998; 273:4135-42; PMID:9461608; http://dx.doi.org/ 10.1074/jbc.273.7.4135 [DOI] [PubMed] [Google Scholar]
- 19. de Souza AJ, Oriss TB, O’malley KJ, Ray A, Kane LP. T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation. Proc Natl Acad Sci U S A 2005; 102:17113-8; PMID:16284246; http://dx.doi.org/ 10.1073/pnas.0508643102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Encinas JA, Janssen EM, Weiner DB, Calarota SA, Nieto D, Moll T, Carlo DJ, Moss RB. Anti-T-cell Ig and mucin domain-containing protein 1 antibody decreases TH2 airway inflammation in a mouse model of asthma. J Allergy Clin Immunol 2005; 116:1343-9; PMID:16337469; http://dx.doi.org/ 10.1016/j.jaci.2005.08.031 [DOI] [PubMed] [Google Scholar]
- 21. Mariat C, Degauque N, Balasubramanian S, Kenny J, DeKruyff RH, Umetsu DT, Kuchroo V, Zheng XX, Strom TB. Tim-1 signaling substitutes for conventional signal 1 and requires costimulation to induce T cell proliferation. J Immunol 2009; 182:1379-85; PMID:19155484; http://dx.doi.org/ 10.4049/jimmunol.182.3.1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Umetsu SE, Lee W-L, McIntire JJ, Downey L, Sanjanwala B, Akbari O, Berry GJ, Nagumo H, Freeman GJ, Umetsu DT, et al. . TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol 2005; 6:447-54; PMID:15793575; http://dx.doi.org/ 10.1038/ni1186 [DOI] [PubMed] [Google Scholar]
- 23. Yuan X, Ansari MJ, D’Addio F, Paez-Cortez J, Schmitt I, Donnarumma M, Boenisch O, Zhao X, Popoola J, Clarkson MR, et al. . Targeting Tim-1 to overcome resistance to transplantation tolerance mediated by CD8 T17 cells. Proc Natl Acad Sci U S A 2009; 106:10734-9; PMID:19528638; http://dx.doi.org/ 10.1073/pnas.0812538106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xiao S, Najafian N, Reddy J, Albin M, Zhu C, Jensen E, Imitola J, Korn T, Anderson AC, Zhang Z, et al. . Differential engagement of Tim-1 during activation can positively or negatively costimulate T cell expansion and effector function. J Exp Med 2007; 204:1691-702; PMID:17606630; http://dx.doi.org/ 10.1084/jem.20062498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xiao S, Zhu B, Jin H, Zhu C, Umetsu DT, DeKruyff RH, Kuchroo VK. Tim-1 stimulation of dendritic cells regulates the balance between effector and regulatory T cells. Eur J Immunol 2011; 41:1539-49; PMID:21469101; http://dx.doi.org/ 10.1002/eji.201040993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sizing ID, Bailly V, McCoon P, Chang W, Rao S, Pablo L, Rennard R, Walsh M, Li Z, Zafari M, et al. . Epitope-dependent effect of anti-murine TIM-1 monoclonal antibodies on T cell activity and lung immune responses. J Immunol 2007; 178:2249-61; PMID:17277130; http://dx.doi.org/ 10.4049/jimmunol.178.4.2249 [DOI] [PubMed] [Google Scholar]
- 27. Kim HS, Kim HS, Lee CW, Chung DH. T cell Ig domain and mucin domain 1 engagement on invariant NKT cells in the presence of TCR stimulation enhances IL-4 production but inhibits IFN-gamma production. J Immunol 2010; 184:4095-106; PMID:20220086; http://dx.doi.org/ 10.4049/jimmunol.0901991 [DOI] [PubMed] [Google Scholar]
- 28. Nozaki Y, Nikolic-Paterson DJ, Snelgrove SL, Akiba H, Yagita H, Holdsworth SR, Kitching AR. Endogenous Tim-1 (Kim-1) promotes T-cell responses and cell-mediated injury in experimental crescentic glomerulonephritis. Kidney Int 2012; 81:844-55; PMID:22205357; http://dx.doi.org/ 10.1038/ki.2011.424 [DOI] [PubMed] [Google Scholar]
- 29. Ma J, Usui Y, Takeda K, Harada N, Yagita H, Okumura K, Akiba H. TIM-1 signaling in B cells regulates antibody production. Biochem Biophys Res Commun 2011; 406:223-8; PMID:21303660; http://dx.doi.org/ 10.1016/j.bbrc.2011.02.021 [DOI] [PubMed] [Google Scholar]
- 30. Nozaki Y, Kitching AR, Akiba H, Yagita H, Kinoshita K, Funauchi M, Matsumura I. Endogenous Tim-1 promotes severe systemic autoimmunity and renal disease MRL-Fas(lpr) mice. Am J Physiol Renal Physiol 2014; 306:F1210-21; PMID:24623145; http://dx.doi.org/ 10.1152/ajprenal.00570.2013 [DOI] [PubMed] [Google Scholar]
- 31. Nozaki Y, Nikolic-Paterson DJ, Yagita H, Akiba H, Holdsworth SR, Kitching AR. Tim-1 promotes cisplatin nephrotoxicity. Am J Physiol Renal Physiol 2011; 301:F1098-104; PMID:21835770; http://dx.doi.org/ 10.1152/ajprenal.00193.2011 [DOI] [PubMed] [Google Scholar]
- 32. Uchida Y, Ke B, Freitas MCS, Ji H, Zhao D, Benjamin ER, Najafian N, Yagita H, Akiba H, Busuttil RW, et al. . The emerging role of T cell immunoglobulin mucin-1 in the mechanism of liver ischemia and reperfusion injury in the mouse. Hepatology 2010; 51:1363-72; PMID:20091883; http://dx.doi.org/ 10.1002/hep.23442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fukushima A, Sumi T, Fukuda K, Kumagai N, Nishida T, Akiba H, Okumura K, Yagita H, Ueno H. Antibodies to T-cell Ig and mucin domain-containing proteins (Tim)-1 and -3 suppress the induction and progression of murine allergic conjunctivitis. Biochem Biophys Res Commun 2007; 353:211-6; PMID:17174273; http://dx.doi.org/ 10.1016/j.bbrc.2006.12.023 [DOI] [PubMed] [Google Scholar]
- 34. Tan X, Jie Y, Zhang Y, Qin Y, Xu Q, Pan Z. Tim-1 blockade with RMT1-10 increases T regulatory cells and prolongs the survival of high-risk corneal allografts in mice. Exp Eye Res 2014; 122:86-93; PMID:24613782; http://dx.doi.org/ 10.1016/j.exer.2014.02.019 [DOI] [PubMed] [Google Scholar]
- 35. Encinas JA, Janssen EM, Weiner DB, Calarota SA, Nieto D, Moll T, Carlo DJ, Moss RB. Anti-T-cell Ig and mucin domain-containing protein 1 antibody decreases TH2 airway inflammation in a mouse model of asthma. J Allergy Clin Immunol 2005; 116:1343-9; PMID:16337469; http://dx.doi.org/ 10.1016/j.jaci.2005.08.031 [DOI] [PubMed] [Google Scholar]
- 36. Feng B-S, Chen X, He S-H, Zheng P-Y, Foster J, Xing Z, Bienenstock J, Yang PC. Disruption of T-cell immunoglobulin and mucin domain molecule (TIM)-1/TIM4 interaction as a therapeutic strategy in a dendritic cell-induced peanut allergy model. J Allergy Clin Immunol 2008; 122:55-61, e1-7; PMID:18547633; http://dx.doi.org/ 10.1016/j.jaci.2008.04.036 [DOI] [PubMed] [Google Scholar]
- 37. Soo Hoo W, Jensen ER, Saadat A, Nieto D, Moss RB, Carlo DJ, Moll T. Vaccination with cell immunoglobulin mucin-1 antibodies and inactivated influenza enhances vaccine-specific lymphocyte proliferation, interferon-gamma production and cross-strain reactivity. Clin Exp Immunol 2006; 145:123-9; PMID:16792682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140:883-99; PMID:20303878; http://dx.doi.org/ 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860-7; PMID:12490959; http://dx.doi.org/ 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, et al. . Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002; 415:536-41; PMID:11823861; http://dx.doi.org/ 10.1038/415536a [DOI] [PubMed] [Google Scholar]
- 41. Kikushige Y, Shima T, Takayanagi S, Urata S, Miyamoto T, Iwasaki H, Takenaka K, Teshima T, Tanaka T, Inagaki Y, et al. . TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell 2010; 7:708-17; PMID:21112565; http://dx.doi.org/ 10.1016/j.stem.2010.11.014 [DOI] [PubMed] [Google Scholar]
- 42. Han G, Chen G, Shen B, Li Y. Tim-3: an activation marker and activation limiter of innate immune cells. Front Immunol 2013; 4:449; PMID:24339828; http://dx.doi.org/ 10.3389/fimmu.2013.00449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev 2010; 235:172-89; PMID:20536563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD, et al. . Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol 2012; 13:832-42; PMID:22842346; http://dx.doi.org/ 10.1038/ni.2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 2010; 207:2175-86; PMID:20819923; http://dx.doi.org/ 10.1084/jem.20100637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fourcade J, Sun Z, Pagliano O, Chauvin J-M, Sander C, Janjic B, Tarhini AA, Tawbi HA, Kirkwood JM, Moschos S, et al. . PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8⁺ T cells induced by melanoma vaccines. Cancer Res 2014; 74:1045-55; PMID:24343228; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sakuishi K, Ngiow SF, Sullivan JM, Teng MWL, Kuchroo VK, Smyth MJ, Anderson AC. TIM3(+)FOXP3(+) regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology 2013; 2:e23849; PMID:23734331; http://dx.doi.org/ 10.4161/onci.23849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MWL, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res 2011; 71:3540-51; PMID:21430066; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-0096 [DOI] [PubMed] [Google Scholar]
- 49. Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 2010; 207:2187-94; PMID:20819927; http://dx.doi.org/ 10.1084/jem.20100643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baghdadi M, Nagao H, Yoshiyama H, Akiba H, Yagita H, Dosaka-Akita H, Jinushi M. Combined blockade of TIM-3 and TIM-4 augments cancer vaccine efficacy against established melanomas. Cancer Immunol Immunother 2013; 62:629-37; PMID:23143694; http://dx.doi.org/ 10.1007/s00262-012-1371-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guo Z, Cheng D, Xia Z, Luan M, Wu L, Wang G, Zhang S. Combined TIM-3 blockade and CD137 activation affords the long-term protection in a murine model of ovarian cancer. J Transl Med 2013; 11:215; PMID:24044888; http://dx.doi.org/ 10.1186/1479-5876-11-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nakayama M, Akiba H, Takeda K, Kojima Y, Hashiguchi M, Azuma M, Yagita H, Okumura K. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood 2009; 113:3821-30; PMID:19224762; http://dx.doi.org/ 10.1182/blood-2008-10-185884 [DOI] [PubMed] [Google Scholar]
- 53. da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK, Osman I, Bhardwaj N. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res 2014; 2:410-22; PMID:24795354; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kikushige Y, Miyamoto T. TIM-3 as a novel therapeutic target for eradicating acute myelogenous leukemia stem cells. Int J Hematol 2013; 98:627-33; PMID:24046178; http://dx.doi.org/ 10.1007/s12185-013-1433-6 [DOI] [PubMed] [Google Scholar]
- 55. Kikushige Y, Akashi K. TIM-3 as a therapeutic target for malignant stem cells in acute myelogenous leukemia. Ann N Y Acad Sci 2012; 1266:118-23; PMID:22901263; http://dx.doi.org/ 10.1111/j.1749-6632.2012.06550.x [DOI] [PubMed] [Google Scholar]
- 56. Ndhlovu LC, Lopez-Vergès S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012; 119:3734-43; PMID:22383801; http://dx.doi.org/ 10.1182/blood-2011-11-392951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shakhov AN, Rybtsov S, Tumanov AV, Shulenin S, Dean M, Kuprash DV, Nedospasov SA. SMUCKLER/TIM4 is a distinct member of TIM family expressed by stromal cells of secondary lymphoid tissues and associated with lymphotoxin signaling. Eur J Immunol 2004; 34:494-503; PMID:14768054; http://dx.doi.org/ 10.1002/eji.200324590 [DOI] [PubMed] [Google Scholar]
- 58. Park D, Hochreiter-Hufford A, Ravichandran KS. The phosphatidylserine receptor TIM-4 does not mediate direct signaling. Curr Biol 2009; 19:346-51; PMID:19217291; http://dx.doi.org/ 10.1016/j.cub.2009.01.042 [DOI] [PubMed] [Google Scholar]
- 59. Wong K, Valdez PA, Tan C, Yeh S, Hongo J-A, Ouyang W. Phosphatidylserine receptor Tim-4 is essential for the maintenance of the homeostatic state of resident peritoneal macrophages. Proc Natl Acad Sci U S A 2010; 107:8712-7; PMID:20421466; http://dx.doi.org/ 10.1073/pnas.0910929107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature 2007; 450:435-9; PMID:17960135; http://dx.doi.org/ 10.1038/nature06307 [DOI] [PubMed] [Google Scholar]
- 61. Rodriguez-Manzanet R, Sanjuan MA, Wu HY, Quintana FJ, Xiao S, Anderson AC, Weiner HL, Green DR, Kuchroo VK. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proc Natl Acad Sci U S A 2010; 107:8706-11; PMID:20368430; http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Baghdadi M, Yoneda A, Yamashina T, Nagao H, Komohara Y, Nagai S, Akiba H, Foretz M, Yoshiyama H, Kinoshita I, et al. . TIM-4 glycoprotein-mediated degradation of dying tumor cells by autophagy leads to reduced antigen presentation and increased immune tolerance. Immunity 2013; 39:1070-81; PMID:24315994; http://dx.doi.org/ 10.1016/j.immuni.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 63. Albacker LA, Yu S, Bedoret D, Lee W-L, Umetsu SE, Monahan S, Freeman GJ, Umetsu DT, DeKruyff RH. TIM-4, expressed by medullary macrophages, regulates respiratory tolerance by mediating phagocytosis of antigen-specific T cells. Mucosal Immunol 2013; 6:580-90; PMID:23149665; http://dx.doi.org/ 10.1038/mi.2012.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sun H-W, Wu C, Tan H-Y, Wang Q-S. A new development of FG-CC’ siRNA blocking interaction of Tim-1 and Tim-4 can enhance DC vaccine against gastric cancer. Hepatogastroenterology 2012; 59:2677-82; PMID:22709877 [DOI] [PubMed] [Google Scholar]
- 65. Snook AE, Waldman SA. Advances in cancer immunotherapy. Discov Med 2013; 15:120-5; PMID:23449114 [PMC free article] [PubMed] [Google Scholar]
- 66. Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol 2006; 16:3-15; PMID:16153857; http://dx.doi.org/ 10.1016/j.semcancer.2005.07.008 [DOI] [PubMed] [Google Scholar]
- 67. Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother 2006; 29:233-40; PMID:16699366; http://dx.doi.org/ 10.1097/01.cji.0000199193.29048.56 [DOI] [PubMed] [Google Scholar]
- 68. Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJT. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov 2010; 9:325-38; PMID:20305665; http://dx.doi.org/ 10.1038/nrd3003 [DOI] [PubMed] [Google Scholar]
- 69. Chung CH. Managing premedications and the risk for reactions to infusional monoclonal antibody therapy. Oncologist 2008; 13:725-32; PMID:18586928; http://dx.doi.org/ 10.1634/theoncologist.2008-0012 [DOI] [PubMed] [Google Scholar]
- 70. O’Shea JJ, Kanno Y, Chan AC. In search of magic bullets: the golden age of immunotherapeutics. Cell 2014; 157:227-40; PMID:24679538; http://dx.doi.org/ 10.1016/j.cell.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]