Abstract
Posttranslational modifications present in the amino-terminal tails of histones play a pivotal role in the chromatin-mediated regulation of gene expression patterns that control plant developmental transitions. Therefore, the function of protein domains that specifically recognize these histone covalent modifications and recruit chromatin remodeling complexes and the transcriptional machinery to modulate gene expression is essential for a proper control of plant development. Plant HomeoDomain (PHD) motifs act as effectors that can specifically bind a number of histone modifications and mediate the activation or repression of underlying genes. In this review we summarize recent findings that emphasize the crucial role of this versatile family of chromatin “reader” domains in the transcriptional regulation of plant developmental processes such as meiosis and postmeiotic events during pollen maturation, embryo meristem initiation and root development, germination as well as flowering time.
Keywords: Arabidopsis, chromatin remodeling, flowering time, germination, meiosis, plant homeodomain, root development
Chromatin remodeling is central in the establishment and stable maintenance of gene expression patterns that control development in eukaryotic organisms, and particularly in plants.1,2 Among the different enzymatic activities involved in chromatin reorganization, those that mediate the covalent modification of histone tails have a significant impact in the regulation of transcriptional programs. A number of plant developmental transitions such as seed germination and the initiation of flowering are influenced by modifications like acetylation or methylation of specific residues in the N-terminal region of histones.3,4 These histone posttranslational modifications (PTM) that decorate nucleosomes are essential to orchestrate the recruitment of a variety of protein complexes that reorganize chromatin structure to promote changes in the transcription of underlying genes. In this way, specific recognition of histone marks by functional domains present in so called “reader” proteins and subsequent interaction with chromatin remodeling complexes and transcriptional machinery translate histone PTM into patterns of gene activation or repression that direct developmental transitions.5
The PHD Domains are Nucleosome Readers
The specific recognition of covalent histone modifications by “reader” modules has emerged in recent years as one of the key mechanisms that control gene expression. The first functional domain described to recognize a specific histone PTM was the bromodomain, a histone acetyl-lysine reader module.6 The discovery of additional “reader” pockets has been reported ever since. Among them, the Plant HomeoDomain (PHD) represents a large and versatile family of epigenomic effectors. The PHD domain is a Cys4-His-Cys3 double loop Zn finger motif organized in a cross-brace topology,7 described for the first time back in 1993 as a functional domain present in the Arabidopsis thaliana homeodomain protein HAT3.1.8 A wealth of members of this family has been identified in other eukaryotic organisms since then.9,10 However, functional data unveiling the mechanism of action of this motif had to wait until 2006, when several papers reported the ability of the PHDs present in the human BROMODOMAIN AND PHD FINGER TRANSCRIPTION FACTOR (BPTF) and INHIBITOR OF GROWTH2 (ING2) proteins to specifically bind trimethylated Lysine 4 in histone H3 (H3K4me3). These studies showed that binding of these PHD-containing proteins to H3K4me3, a landmark of active gene expression, could result in either transcriptional activation or repression, depending on the recruitment of histone acetyl transferase (HAT) or histone deacetylase (HDAC) complexes.11-16 Later reports have identified a number of PHD domains that interact with H3K4me2/3, but also new members of this family that bind other histone marks such as H3K9me3 or H3K36me3. In addition, the recognition of unmodified H3K4 and H3R2 by other PHD-containing proteins has also been reported.9,10 Acetylation of H3K14 and H4 can be also recognized by some PHDs such DPF3b,9 underscoring the versatility of this class of domain.
Besides the ability of PHD domains to recognize specific histone marks, these effector modules also target non-histone proteins. In fact, the simultaneous binding of PHD motifs to histone PTM and non-histone interactors has been reported.9 Furthermore, PHD-containing proteins often bear additional domains that can also recognize histone PTM. These features highlight the striking capacity of PHD domains to exert complex regulatory functions and to mediate the cross talk between the epigenomic status of the chromatin and additional cellular pathways.
PHD domains are widely conserved in eukaryotic organisms, and in plants these histone binding modules are involved in the control of a variety of biological mechanisms ranging from pathogen defense responses to a number of developmental processes. We recently reported the central role of 2 Arabidopsis homologous PHD-containing proteins, SHORT LIFE (SHL) and EARLY BOLTING IN SHORT DAYS (EBS), in the fine regulation of flowering time.17 Some other plant PHD proteins also participate in the control of flowering time or other developmental programs such as male gametophyte development, root development and germination. In this review we focus on the role of PHD containing proteins in the regulation of plant development.
PHD Containing Proteins in the Control of Meiosis and Post-meiotic Processes
Meiosis is a pivotal process in the developmental program of sporogenesis in plants.18 The Arabidopsis PHD containing proteins MALE MEIOCYTE DEATH1 (MMD1)/DUET and the adherin homolog AtSCC2/EMB2773 participate in the establishment of sister-chromatid cohesion during replication, and are essential for the proper segregation of chromosomes during mitosis and meiosis.19 Loss of MMD1 function triggers cell death in male meiocytes and causes defective progression through meiosis.20,21 AtSCC2 is also required for embryogenesis and sister-chromatid cohesion during the meiotic cell cycle.19 AtSCC2 knockdown lines showed sterility arising from defects in meiotic chromosome organization. The presence of a PHD finger suggests that these proteins may mediate interactions with chromatin in the context of the establishment of chromatid cohesion or the regulation of gene expression.
A number of MMD1-related genes, including MALE STERILITY1 (MS1) homologues, are crucial for pollen development, a postmeiotic process that drives mature pollen grain production from microspores.22 Arabidopsis ms1 mutants do not produce viable pollen, but these plants are phenotypically normal.23-25 Degeneration of pollen in ms1 mutants occurs after microspore release from the tetrads, time at which the tapetum appears abnormally vacuolated. The rice putative ortholog of AtMS1, PERSISTENT TAPETAL CELL1 (PTC1) also controls programmed tapetal development and pollen formation.26 In the same way, HvMS1 up- and down-regulation resulted in male sterility, a key target for selective breeding, as higher yield is commonly associated with hybrids in barley.27 Altogether, these observations suggest that MS1 function is conserved in both monocots and dicots, regulating the breakdown of the tapetum and the secretion of materials for pollen wall formation. The widely conserved MS1 function in higher plants plays an essential role in controlling transcription during specific stages of male gametogenesis and anther development. However, recognition of specific histone marks by MS1 homologues has not been demonstrated yet and putative MS1 target genes remain to be identified.
Besides, the Arabidopsis PHD-containing protein ASHR3 is involved in stamen development and interacts with the bHLH transcription factor ABORTED MICROSPORES (AMS). AMS can target ASHR3 to chromatin and then mediates the regulation of genes involved in stamen development and function.28
Regulation of Embryonic Meristem Initiation and Root Development by PHD-finger Protein Complexes
Plant growth depends on the activity of stem cells within meristems. One of the initial events in root meristem initiation is the specification of the hypophysis.29 MONOPTEROS/ AUXIN-RESPONSE FACTOR 5 (MP/ARF5) mediates this process through the promotion of auxin transport and by activating the expression of TARGET OF MP (TMO) transcription factors.30 PHD finger-containing proteins are also involved in this process; specifically, the OBERON1 (OBE1) and OBE2 proteins act downstream of auxin accumulation in specifying the vasculature and primary root meristem.31 obe1 obe2 double mutant seedlings resemble the mp mutant, showing an absence of root and defective development of the vasculature. However, and in contrast to mp, obe1 obe2 mutant embryos show auxin accumulation at the root pole and in the provascular region, and the auxin transduction pathway is fully active in these double mutants. OBE1 and 2 have been proposed to mediate through their PHD domains the recognition of H3K4 methylated marks in the chromatin of genes involved in the signaling pathways of auxin accumulation and translate these histone modifications into transcriptional outputs for the specification of root meristem and vasculature in the embryo.31 In fact, OBE1 has been shown to bind TMO7 chromatin, suggesting a role for OBE proteins in its MP-dependent activation.32
The PICKLE (PKL) CHD3 chromatin-remodeling factor also contains a PHD domain and is required to prevent the expression of embryonic traits in germinated seedlings.33 Although PKL promotes the accumulation of the H3K27me3 mark,34 it has been recently proposed that PKL and Polycomb Group (PcG) proteins antagonistically regulate root meristem activity in Arabidopsis.35 Alterations of meristematic activity in pkl and clf mutants are correlated with decreased or increased expression of root stem cell and meristem marker genes, respectively. Lower expression levels of these genes in pkl correlated with increased levels of H3K27me3, a mark deposited by PcG proteins, indicating that root meristem activity is controlled by the antagonistic activity of this complex and PKL, although this mechanism remains unknown. In addition to the repression of embryonic traits, PKL participates in other developmental processes and its histone binding properties are just beginning to be elucidated.35,36
PHD-containing proteins also mediate adaptive responses of the root to environmental factors. The Arabidopsis root system architecture is modified on Pi deprivation conditions, resulting in a reduction in primary root growth and in increased density and length of lateral roots. The per2 mutant, with strong defects in root hair elongation under low Pi conditions, is affected in the ALFIN LIKE 6 (AL6) gene.37 AL6 can bind H3K4me3 through its PHD domain,38 and controls the transcription of a number of key genes modulating root hair elongation under low Pi conditions. However, the exact mechanism by which AL6 triggers the activation of these genes with relevant functions in cellular Pi homeostasis remains elusive. It is tempting to speculate that under Pi starvation, H3K4me3 levels may increase at the promoter of the ENHANCER OF TRY AND CPC1 (ETC1) gene, which encodes a MYB transcription factor highly up-regulated during Pi deficiency and involved in trichome and root hair cell patterning.39 AL6 might be recruited to ETC1 by the recognition of the H3K4me3 mark and then positively regulate the downstream transcriptional events to promote root hair elongation during Pi deficiency.40
PHD-containing Proteins Mediate the Switch of Gene Expression Patterns During Seed Germination
Further to the role of PHD-containing proteins on gametophyte and root development, these effectors are also required for a proper regulation of gene expression patterns during seed germination. HIGH-LEVEL EXPRESSION OF SUGAR INDUCIBLE GENE2/VP1/ABI3-LIKE1 (HSI2/VAL1) and HSI2-LIKE1 (HSL1/VAL2) are 2 redundant B3-related transcriptional regulators that repress master genes of seed maturation during seedling development in Arabidopsis.41 Point mutations affecting the HSI2 PHD domain cause upregulation in Arabidopsis seedlings of a number of seed maturation genes such as AGAMOUS-LIKE15 (AGL15), FUSCA3 (FUS3), and other genes encoding seed specific proteins. Some of the genes that appeared differentially overexpressed in the hsi2 mutant plants are enriched in the repressive mark H3K27me3, suggesting that the repression activity of HSI2 might involve the deposition of this histone modification.42 The relationship of chromatin remodeling processes with the repression mechanism mediated by HSI2 remains to be elucidated, and in fact, the histone binding specificity of these PHD-containing proteins has not been analyzed yet.
Members of the PHD-containing H3K4me2/3-binding AL family of proteins38 are also involved in the reprogramming of gene expression that takes place during seed germination in Arabidopsis.43 A number of AL proteins including AL6 interact with components of the Polycomb Repressor Complex 1 (PRC1) such as AtBMI1b and AtRING1a. Moreover, seeds of the double mutant al6 al7 show a germination delay under osmotic stress conditions similar to that displayed by PRC1 mutants Atbmi1a Atbmi1b, consistent with a role for these AL and PRC1 proteins in the promotion of germination. In addition, several seed developmental genes such as ABSCISIC ACID INSENSITIVE3 (ABI3), DELAY OF GERMINATION1 (DOG1), and CHOTTO1/AINTEGUMENTA-LIKE5 (CHO1/AIL5), known to repress seed germination, were derepressed in these double mutants. These genes also showed a delayed transition from the H3K4me3-enriched state associated to active transcription during seed development to an inactive transcriptional state characterized by accumulation of H3K27me3 during seed germination. Both AL6 and the PRC1 component LIKE HETEROCHROMATIN PROTEIN1 (LHP1), involved in the specific recognition of the H3K27me3 mark,44 can directly bind the chromatin of ABI3 and DOG1. Altogether, these observations suggest that the PHD-containing AL proteins may form complexes with PRC1 to positively regulate seed germination by promoting the switch from H3K4me3- to H3K27me3-enriched chromatin and subsequent silencing of seed developmental genes (Fig. 1A).43 These results emphasize the central role of PHD-containing proteins in the modulation of gene expression patterns that control developmental transitions such as germination in plants.
Figure 1.
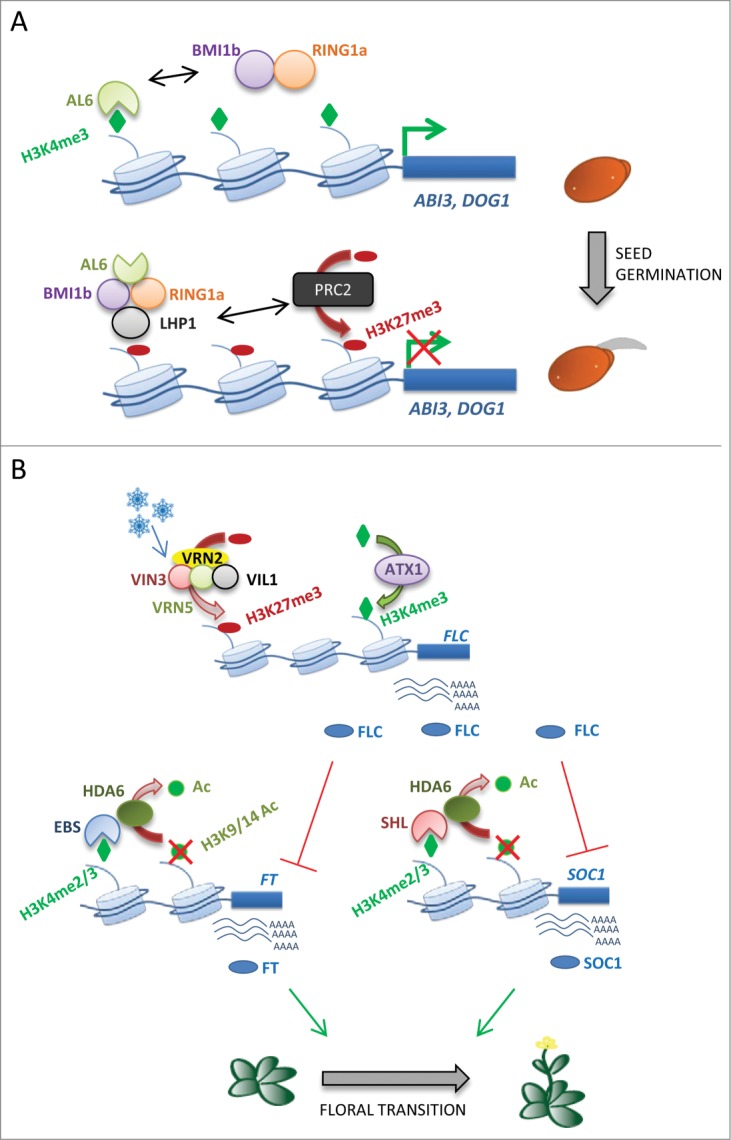
Hypothetical models for the functions of PHD-containing proteins in the control of plant developmental processes. (A) PHD-containing proteins of the AL family bind H3K4me3 and can interact with members of the PRC1 such as ATBMI1b and ATRING1a. Recruitment of PRC1 to regulatory regions of seed developmental genes results in the deposition of H3K27me3 and subsequent silencing of these genes. AL proteins mediate the transition from an H3K4me3- to an H3K27me3-enriched status in the chromatin of seed developmental genes, promoting seed germination. (B) PHD-containing proteins of the VEL family participate in the vernalization-dependent silencing of FLC, promoting the accumulation of the repressive mark H3K27me3. In contrast, ATX1 is required for the enrichment of H3K4me3 in the chromatin of FLC and its subsequent activation. FT and SOC1, 2 target genes of the FLC protein, are also regulated by the PHD proteins EBS and SHL, respectively. Both proteins are required to maintain low levels of histone H3 acetylation in the chromatin of these floral integrators. In addition, EBS and SHL interact in vivo with HDA6.
Regulation of Flowering Time by PHD-containing Proteins
The switch from vegetative to reproductive development is a crucial developmental transition because it determines the reproductive success of plants. For that reason the initiation of flowering is tightly regulated by several genetic pathways that integrate both developmental and environmental signals. Signaling from these regulatory pathways converge at the level of a few integrator genes that control the switch to flowering. The balance between floral promoting and repressing pathways finally determines the timing of reproductive development.4 Chromatin remodeling plays a key role in the regulation of these master genes of flowering time3 and many chromatin remodeling factors have been shown to regulate this process, including several PHD-containing proteins.
A Group of PHD Proteins Regulate a Family of Floral Repressors in Arabidopsis
The Arabidopsis FLOWERING LOCUS C (FLC) gene encodes a MADS box protein with a central role in the repression of flowering and specifically in the vernalization response.45 Vernalization refers to the acceleration of flowering observed in plants in response to a prolonged exposure to the cold temperatures of winter. The molecular mechanism underlying vernalization in Arabidopsis relies on the mitotically stable repression of FLC in response to low temperatures.46,47 This stable repression depends on the chromatin-mediated silencing of FLC through the deposition of repressive histone marks, mainly H3K27me3, by a PRC2 complex and the maintenance of the repressed state through subsequent cell divisions. The VERNALIZATION5/VIN3-LIKE (VEL) family of proteins containing PHD domains has been shown to play a fundamental role in the vernalization-mediated repression of FLC (Fig. 1B). The VEL family includes VERNALIZATION INSENSITIVE3 (VIN3), VIN3-LIKE/VERNALIZATION5 (VIL1/VRN5), VIL2/VEL1, VIL3/VEL2 and VIL4/VEL3. All the members except VIL4/VEL3, which appears to be a pseudogene, bear a PHD domain.48 Early studies showed that VIN3 and VRN5 are required for a correct vernalization response and for the establishment of the repressed state in the chromatin of FLC through the enrichment of H3K9 and H3K27 methylation and histone deacetylation. Whereas VIN3 is only expressed upon cold exposure, VRN5 is constitutively expressed, and both proteins interact during vernalization.49 The function of these PHD finger proteins in vernalization has been linked to the PRC2 activity.46,50 Exposure to cold triggers the association of the core PRC2 component VRN2 with the PHD-containing proteins VIN3, VRN5 and VEL1 to form a PHD-PRC2 complex. In the early stages of cold exposure, VRN5 binds to a discrete region of FLC intron 1 in a VIN3-dependent manner. As vernalization progresses, VRN5 distribution spreads across the FLC locus and results in an increase of H3K27me3. The interaction between these PHD proteins and the PRC2 complex enhances the activity of PRC2 at FLC and contributes to the increase of H3K27me3 and the silencing of the gene.
Recent results indicate that all the members of the VEL family mediate the vernalization response in Arabidopsis by repressing the expression of the FLC and its relatives MADS AFFECTING FLOWERING1/FLOWERING LOCUS M (MAF1/FLM) and MAF2-5, referred to as the FLC clade, but they have different functions and targets among the FLC family.46 Also, in addition to their function in the vernalization response, VIL1/VRN5 and VIL2/VEL1 play a role in the photoperiodic repression of MAF1/FLM and MAF5, respectively, by increasing the levels of H3K27me3 and H3K9me2.48,51
Despite their divergent roles in the regulation of these floral repressors, the members of the VEL family have some common features. Apart from sharing the same domain architecture,48 they all seem to preferentially recognize in vitro the H3K9me2 histone mark, although they can also bind H3K4me2.46,51 Furthermore, all these proteins are required for the enrichment of H3K27me3 and H3K9me2 in their target loci, suggesting that the binding of their PHD domains to H3K9me2 triggers the increase of these repressive marks that eventually leads to the silencing of target genes.
VIL proteins are conserved in crop species with a vernalization requirement, such as wheat. Three VIL genes, TmVIL1-3, have been identified in einkorn wheat (Triticum monococcum).52 Their expression is upregulated by vernalization and non-inductive photoperiods, suggesting that the role of VIL proteins in vernalization is conserved in this species. VIL orthologues named as AetVIL1-3 are also present in wild wheat (Aegilops tauschii).53 However, their transcriptional regulation by low temperatures is not as clear, and further research will be needed to clarify their involvement in the vernalization response.
Other species without vernalization requirements, like rice (Oryza sativa), also carry VIL homologues. OsVIL2 acts as an activator of flowering in rice by inhibiting the expression of the floral repressor LEC2 AND FUSCA3 Like 1 (OsLFL1), a putative B3 DNA-binding domain-containing transcription factor.54 OsVIL2 binds histone H3 through its PHD domain and mediates the enrichment of H3K27me3 in the OsLFL1 chromatin. Furthermore, OsVIL2 physically interacts with the rice PRC2 component EMBRYONIC FLOWER 2a (OsEMF2a), suggesting that both proteins might act together in a PHD-PRC2 complex similar to the one present in Arabidopsis to participate in the chromatin-mediated repression of OsLFL1 and thus promote flowering in rice.
Besides these members of the VIL family, ARABIDOPSIS TRITHORAX1 (ATX1), the homolog of Drosophila trxG protein Trithorax (TRX), bears 2 PHD domains.55 ATX1 regulates flowering time by activating the expression of FLC (Fig. 1B)56 and binds dynamically to the chromatin of this floral repressor, mediating the deposition of H3K4me3. Loss of ATX1 activity is accompanied by a reduction in H3K4me3 and an increase in H3K27me2 levels in FLC chromatin and leads to early flowering.
The rice Early Heading Date 3 (Ehd3) PHD-containing protein is involved in the photoperiodic regulation of flowering time.57 Under non-inductive long days, Ehd3 negatively regulates the expression of the floral repressor Grain Number, Plant Height, and Heading Date7 (Ghd7), leading to the upregulation of the downstream floral activator Ehd1 and the promotion of flowering. Under inductive short day conditions, Ehd3 controls the expression of Ehd1 independently of Ghd7. Interestingly, the rice ortholog of Arabidopsis ATX1 (OsTxr1) also downregulates the expression of Ghd7 under LD and physically interacts with Ehd3.58 OsTxr1 is able to bind histone H3 through its PHD domain and has histone methyltransferase activity in vivo. It is possible that the interaction between these 2 PHD-finger proteins is involved in the chromatin-mediated regulation of the floral repressor Gdh7 in rice.
Regulation of the Arabidopsis Floral Integrators by the PHD Containing Proteins EBS and SHL
In Arabidopsis, the floral promotion pathways converge in the regulation of 2 master genes, the so-called floral integrators FLOWERING LOCUS T (FT) and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1). The transcriptional activation of these genes determines the transition from vegetative to reproductive development.59 Previous studies implicated EBS in the repression of flowering, and showed that this PHD-containing protein is necessary for the repression of FT.60,61 EBS also encompasses a BROMO ADJACENT HOMOLOGY (BAH) motif, frequently found in chromatin remodeling proteins. Proteins with this modular architecture are present in all plant species but not in other eukaryotic organisms, suggesting their specificity for the plant kingdom. At least one homolog of EBS is present in the Arabidopsis genome, SHL, which had been proposed to be required for proper development and fertility62 and to regulate the expression of SOC1 and SEPALLATA3 (SEP3) genes.63
A recent study shows that EBS and SHL participate in the chromatin-mediated repression of flowering,17 and have independent roles in this process. While EBS is necessary for the repression of FT, SHL negatively regulates the expression of SOC1. The PHD domains of EBS and SHL bind H3K4me2/3, suggesting that the recognition by these proteins of this specific histone marks may mediate their role as chromatin effectors. EBS and SHL were shown to bind discrete regions in the chromatin of FT and SOC1, respectively. Moreover, EBS and SHL are required to maintain low levels of acetylation in their target genes, as evidenced by the hyperacetylation of H3K9K14 observed in the chromatin of FT and SOC1 in ebs and shl mutants, respectively. In addition, HISTONE DEACETYLASE6 (HDA6) is able to interact in planta with EBS and SHL (Fig. 1B). The data derived from this study are consistent with a model in which EBS and SHL could bind the chromatin of their target genes FT and SOC1 through the PHD-mediated recognition of H3K4me2/3, subsequently recruiting HDA6, which leads to histone deacetylation and repression of these floral integrators.17
The observations described above highlight the pivotal role of PHD proteins in mediating the cross talk between different histone modifications and the important implications of this signaling pathway in the regulation of gene expression patterns that control developmental transitions in plants and particularly flowering time. Future studies are likely to provide a deeper understanding of the chromatin remodeling complexes that mediate the action of PHD-containing proteins in a variety of cellular pathways regulating plant development and differentiation.
Funding
This work was funded by projects of the Spanish Ministry of Science and Innovation (BIO2010-15589, CSD2007-00057, BIO2013-43098-R) and EU MC-ITN (EpiTRAITS) to JAJ and MP. AM was funded by an FPU fellowship from the Ministry of Education.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Jarillo JA, Pineiro M, Cubas P, Martinez-Zapater JM. Chromatin remodeling in plant development. Int J Dev Biol 2009; 53:1581-96; PMID:19247973; http://dx.doi.org/ 10.1387/ijdb.072460jj [DOI] [PubMed] [Google Scholar]
- 2. He G, Elling AA, Deng XW. The epigenome and plant development. Annu Rev Plant Biol 2011; 62:411-35; PMID:21438682; http://dx.doi.org/ 10.1146/annurev-arplant-042110-103806 [DOI] [PubMed] [Google Scholar]
- 3. He Y. Chromatin regulation of flowering. Trends Plant Sci 2012; 17:556-62; PMID:22658650; http://dx.doi.org/ 10.1016/j.tplants.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 4. Jarillo JA, Piñeiro M. Timing is everything in plant development. The central role of floral repressors. Plant Sci 2011; 181:364-78; PMID:21889042; http://dx.doi.org/ 10.1016/j.plantsci.2011.06.011 [DOI] [PubMed] [Google Scholar]
- 5. Suganuma T, Workman JL. Signals and combinatorial functions of histone modifications. Ann Rev Biochem 2011; 80:473-99; PMID:21529160; http://dx.doi.org/ 10.1146/annurev-biochem-061809-175347 [DOI] [PubMed] [Google Scholar]
- 6. Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM. Structure and ligand of a histone acetyltransferase bromodomain. Nature 1999; 399:491-6; PMID:10365964; http://dx.doi.org/ 10.1038/20974 [DOI] [PubMed] [Google Scholar]
- 7. Sanchez R, Zhou M-M. The PHD finger: a versatile epigenome reader. Trends Biochem Sci 2011; 36:364-72; PMID:21514168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schindler U, Beckmann H, Cashmore AR. HAT3.1, a novel Arabidopsis homeodomain protein containing a conserved cysteine-rich region. Plant J 1993; 4:137-50; PMID:8106082; http://dx.doi.org/ 10.1046/j.1365-313X.1993.04010137.x [DOI] [PubMed] [Google Scholar]
- 9. Li Y, Li H. Many keys to push: diversifying the 'readership' of plant homeodomain fingers. Acta Biochim Biophys Sin (Shanghai) 2012; 44:28-39; PMID:22194011; http://dx.doi.org/ 10.1093/abbs/gmr117 [DOI] [PubMed] [Google Scholar]
- 10. Musselman CA, Kutateladze TG. Handpicking epigenetic marks with PHD fingers. Nucleic Acids Res 2011; 39:9061-71; PMID:21813457; http://dx.doi.org/ 10.1093/nar/gkr613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Becker PB. Gene regulation: a finger on the mark. Nature 2006; 442:31-2; PMID:16823438; http://dx.doi.org/ 10.1038/442031a [DOI] [PubMed] [Google Scholar]
- 12. Mellor J. It takes a PHD to read the histone code. Cell 2006; 126:22-4; PMID:16839870; http://dx.doi.org/ 10.1016/j.cell.2006.06.028 [DOI] [PubMed] [Google Scholar]
- 13. Wysocka J, Swigut T, Xiao H, Milne TA, Kwon SY, Landry J, Kauer M, Tackett AJ, Chait BT, Badenhorst P, et al. . A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature 2006; 442:86-90; PMID:16728976 [DOI] [PubMed] [Google Scholar]
- 14. Shi X, Hong T, Walter KL, Ewalt M, Michishita E, Hung T, Carney D, Peña P, Lan F, Kaadige MR, et al. . ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature 2006; 442:96-9; PMID:16728974; http://dx.doi.org/ 10.1038/nature05140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pena PV, Davrazou F, Shi X, Walter KL, Verkhusha VV, Gozani O, Zhao R, Kutateladze TG. Molecular mechanism of histone H3K4me3 recognition by plant homeodomain of ING2. Nature 2006; 442:100-3; PMID:16728977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li H, Ilin S, Wang W, Duncan EM, Wysocka J, Allis CD, Patel DJ. Molecular basis for site-specific read-out of histone H3K4me3 by the BPTF PHD finger of NURF. Nature 2006; 442:91-5; PMID:16728978; http://dx.doi.org/ 10.1038/nature05020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lopez-Gonzalez L, Mouriz A, Narro-Diego L, Bustos R, Martinez-Zapater JM, Jarillo JA, Piñeiro M. Chromatin-dependent repression of the Arabidopsis floral integrator genes involves plant specific PHD-containing proteins. Plant Cell 2014; 26:3922-38; PMID:25281686; http://dx.doi.org/ 10.1105/tpc.114.130781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang CJ, Tseng CC. Recent advances in understanding of meiosis initiation and the apomictic pathway in plants. Front Plant Sci 2014; 5:497; PMID:25295051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sebastian J, Ravi M, Andreuzza S, Panoli AP, Marimuthu MP, Siddiqi I. The plant adherin AtSCC2 is required for embryogenesis and sister-chromatid cohesion during meiosis in Arabidopsis. Plant J 2009; 59:1-13; PMID:19228337; http://dx.doi.org/ 10.1111/j.1365-313X.2009.03845.x [DOI] [PubMed] [Google Scholar]
- 20. Reddy TV, Kaur J, Agashe B, Sundaresan V, Siddiqi I. The DUET gene is necessary for chromosome organization and progression during male meiosis in Arabidopsis and encodes a PHD finger protein. Development 2003; 130:5975-87; PMID:14573517; http://dx.doi.org/ 10.1242/dev.00827 [DOI] [PubMed] [Google Scholar]
- 21. Yang X, Makaroff CA, Ma H. The Arabidopsis MALE MEIOCYTE DEATH1 gene encodes a PHD-finger protein that is required for male meiosis. Plant Cell 2003; 15:1281-95; PMID:12782723; http://dx.doi.org/ 10.1105/tpc.010447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borg M, Twell D. Life after meiosis: patterning the angiosperm male gametophyte. Biochem Soc Trans 2010; 38:577-82; PMID:20298224; http://dx.doi.org/ 10.1042/BST0380577 [DOI] [PubMed] [Google Scholar]
- 23. Wilson ZA, Morroll SM, Dawson J, Swarup R, Tighe PJ. The Arabidopsis MALE STERILITY1 (MS1) gene is a transcriptional regulator of male gametogenesis, with homology to the PHD-finger family of transcription factors. Plant J 2001; 28:27-39; PMID:11696184; http://dx.doi.org/ 10.1046/j.1365-313X.2001.01125.x [DOI] [PubMed] [Google Scholar]
- 24. Ito T, Shinozaki K. The MALE STERILITY1 gene of Arabidopsis, encoding a nuclear protein with a PHD-finger motif, is expressed in tapetal cells and is required for pollen maturation. Plant Cell Physiol 2002; 43:1285-92; PMID:12461128; http://dx.doi.org/ 10.1093/pcp/pcf154 [DOI] [PubMed] [Google Scholar]
- 25. Ito T, Nagata N, Yoshiba Y, Ohme-Takagi M, Ma H, Shinozaki K. Arabidopsis MALE STERILITY1 encodes a PHD-type transcription factor and regulates pollen and tapetum development. Plant Cell 2007; 19:3549-62; PMID:18032630; http://dx.doi.org/ 10.1105/tpc.107.054536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li H, Yuan Z, Vizcay-Barrena G, Yang C, Liang W, Zong J, Wilson ZA, Zhang D. Persistent tapetal cell1 encodes a PHD-finger protein that is required for tapetal cell death and pollen development in rice. Plant Physiol 2011; 156:615-30; PMID:21515697; http://dx.doi.org/ 10.1104/pp.111.175760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fernandez Gomez J, Wilson ZA. A barley PHD finger transcription factor that confers male sterility by affecting tapetal development. Plant Biotechnol J 2014; 12:765-77; PMID:24684666; http://dx.doi.org/ 10.1111/pbi.12181 [DOI] [PubMed] [Google Scholar]
- 28. Thorstensen T, Grini PE, Mercy IS, Alm V, Erdal S, Aasland R, Aalen RB. The Arabidopsis SET-domain protein ASHR3 is involved in stamen development and interacts with the bHLH transcription factor ABORTED MICROSPORES (AMS). Plant Mol Biol 2008; 66:47-59; PMID:17978851; http://dx.doi.org/ 10.1007/s11103-007-9251-y [DOI] [PubMed] [Google Scholar]
- 29. Bennett T, Scheres B. Root development-two meristems for the price of one? Curr Top Dev Biol 2010; 91:67-102; PMID:20705179; http://dx.doi.org/ 10.1016/S0070-2153(10)91003-X [DOI] [PubMed] [Google Scholar]
- 30. Schlereth A, Moller B, Liu W, Kientz M, Flipse J, Rademacher EH, Schmid M, Jürgens G, Weijers D. MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 2010; 464:913-6; PMID:20220754; http://dx.doi.org/ 10.1038/nature08836 [DOI] [PubMed] [Google Scholar]
- 31. Thomas CL, Schmidt D, Bayer EM, Dreos R, Maule AJ. Arabidopsis plant homeodomain finger proteins operate downstream of auxin accumulation in specifying the vasculature and primary root meristem. Plant J 2009; 59:426-36; PMID:19392692; http://dx.doi.org/ 10.1111/j.1365-313X.2009.03874.x [DOI] [PubMed] [Google Scholar]
- 32. Saiga S, Moller B, Watanabe-Taneda A, Abe M, Weijers D, Komeda Y. Control of embryonic meristem initiation in Arabidopsis by PHD-finger protein complexes. Development 2012; 139:1391-8; PMID:22378640; http://dx.doi.org/ 10.1242/dev.074492 [DOI] [PubMed] [Google Scholar]
- 33. Ogas J, Kaufmann S, Henderson J, Somerville C. PICKLE is a CHD3 chromatin-remodeling factor that regulates the transition from embryonic to vegetative development in Arabidopsis. Proc Natl Acad Sci USA 1999; 96:13839-44; PMID:10570159; http://dx.doi.org/ 10.1073/pnas.96.24.13839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang H, Rider SD, Jr, Henderson JT, Fountain M, Chuang K, Kandachar V, Simons A, Edenberg HJ, Romero-Severson J, Muir WM, et al. . The CHD3 remodeler PICKLE promotes trimethylation of histone H3 lysine 27. J Biol Chem 2008; 283:22637-48; PMID:18539592; http://dx.doi.org/ 10.1074/jbc.M802129200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aichinger E, Villar CB, Di Mambro R, Sabatini S, Kohler C. The CHD3 chromatin remodeler PICKLE and polycomb group proteins antagonistically regulate meristem activity in the Arabidopsis root. Plant Cell 2011; 23:1047-60; PMID:21441433; http://dx.doi.org/ 10.1105/tpc.111.083352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang H, Bishop B, Ringenberg W, Muir WM, Ogas J. The CHD3 remodeler PICKLE associates with genes enriched for trimethylation of histone H3 lysine 27. Plant Physiol 2012; 159:418-32; PMID:22452853; http://dx.doi.org/ 10.1104/pp.112.194878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chandrika NN, Sundaravelpandian K, Yu SM, Schmidt W. ALFIN-LIKE 6 is involved in root hair elongation during phosphate deficiency in Arabidopsis. New Phytol 2013; 198:709-20; PMID:23432399; http://dx.doi.org/ 10.1111/nph.12194 [DOI] [PubMed] [Google Scholar]
- 38. Lee WY, Lee D, Chung WI, Kwon CS. Arabidopsis ING and Alfin1-like protein families localize to the nucleus and bind to H3K4me3/2 via plant homeodomain fingers. Plant J 2009; 58:511-24; PMID:19154204; http://dx.doi.org/ 10.1111/j.1365-313X.2009.03795.x [DOI] [PubMed] [Google Scholar]
- 39. Kirik V, Simon M, Huelskamp M, Schiefelbein J. The ENHANCER OF TRY AND CPC1 gene acts redundantly with TRIPTYCHON and CAPRICE in trichome and root hair cell patterning in Arabidopsis. Dev Biol 2004; 268:506-13; PMID:15063185; http://dx.doi.org/ 10.1016/j.ydbio.2003.12.037 [DOI] [PubMed] [Google Scholar]
- 40. Chandrika NN, Sundaravelpandian K, Schmidt W. A PHD in histone language: on the role of histone methylation in plant responses to phosphate deficiency. Plant Signal Behav 2013; 8:e24381; PMID:23531693; http://dx.doi.org/ 10.4161/psb.24381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsukagoshi H, Saijo T, Shibata D, Morikami A, Nakamura K. Analysis of a sugar response mutant of Arabidopsis identified a novel B3 domain protein that functions as an active transcriptional repressor. Plant Physiol 2005; 138:675-85; PMID:15894743; http://dx.doi.org/ 10.1104/pp.104.057752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Veerappan V, Wang J, Kang M, Lee J, Tang Y, Jha AK, Shi H, Palanivelu R, Allen RD. A novel HSI2 mutation in Arabidopsis affects the PHD-like domain and leads to derepression of seed-specific gene expression. Planta 2012; 236:1-17; PMID:22476218; http://dx.doi.org/ 10.1007/s00425-012-1630-1 [DOI] [PubMed] [Google Scholar]
- 43. Molitor AM, Bu Z, Yu Y, Shen WH. Arabidopsis AL PHD-PRC1 complexes promote seed germination through H3K4me3-to-H3K27me3 chromatin state switch in repression of seed developmental genes. PLoS Genet 2014; 10:e1004091; PMID:24465219; http://dx.doi.org/ 10.1371/journal.pgen.1004091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Turck F, Roudier F, Farrona S, Martin-Magniette ML, Guillaume E, Buisine N, Gagnot S, Martienssen RA, Coupland G, Colot V. Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of Histone H3 Lysine 27. PLoS Genet 2007; 3:e86; PMID:17542647; http://dx.doi.org/ 10.1371/journal.pgen.0030086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Michaels SD, Amasino RM. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 1999; 11:949-56; PMID:10330478; http://dx.doi.org/ 10.1105/tpc.11.5.949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim D-H, Sung S. Coordination of the vernalization response through a VIN3 and FLC gene family regulatory network in Arabidopsis. Plant Cell 2013; 25:454-69; PMID:23417034; http://dx.doi.org/ 10.1105/tpc.112.104760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Song J, Irwin J, Dean C. Remembering the prolonged cold of winter. Curr Biol 2013; 23:R807-11; PMID:24028964; http://dx.doi.org/ 10.1016/j.cub.2013.07.027 [DOI] [PubMed] [Google Scholar]
- 48. Sung S, Schmitz RJ, Amasino RM. A PHD finger protein involved in both the vernalization and photoperiod pathways in Arabidopsis. Genes Dev 2006; 20:3244-8; PMID:17114575; http://dx.doi.org/ 10.1101/gad.1493306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Greb T, Mylne JS, Crevillen P, Geraldo N, An H, Gendall AR, Dean C. The PHD finger protein VRN5 functions in the epigenetic silencing of Arabidopsis FLC. Curr Biol 2007; 17:73-8; PMID:17174094; http://dx.doi.org/ 10.1016/j.cub.2006.11.052 [DOI] [PubMed] [Google Scholar]
- 50. De Lucia F, Crevillen P, Jones AM, Greb T, Dean C. A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc Natl Acad Sci USA 2008; 105:16831-6; PMID:18854416; http://dx.doi.org/ 10.1073/pnas.0808687105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim D-H, Sung S. The plant homeo domain finger protein, VIN3-LIKE 2, is necessary for photoperiod-mediated epigenetic regulation of the floral repressor, MAF5. Proc Natl Acad Sci USA 2010; 107:17029-34; PMID:20837520; http://dx.doi.org/ 10.1073/pnas.1010834107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fu D, Dunbar M, Dubcovsky J. Wheat VIN3-like PHD finger genes are up-regulated by vernalization. Mol Genet Genomics 2007; 277:301-13; PMID:17123111; http://dx.doi.org/ 10.1007/s00438-006-0189-6 [DOI] [PubMed] [Google Scholar]
- 53. Koyama K, Hatano H, Nakamura J, Takumi S. Characterization of three VERNALIZATION INSENSITIVE3-like (VIL) homologs in wild wheat, Aegilops tauschii Coss. Hereditas 2012; 149:62-71; PMID:22568701; http://dx.doi.org/ 10.1111/j.1601-5223.2012.02255.x [DOI] [PubMed] [Google Scholar]
- 54. Yang J, Lee S, Hang R, Kim SR, Lee YS, Cao X, Amasino R, An G. OsVIL2 functions with PRC2 to induce flowering by repressing OsLFL1 in rice. Plant J 2013; 73:566-78; PMID:23083333; http://dx.doi.org/ 10.1111/tpj.12057 [DOI] [PubMed] [Google Scholar]
- 55. Fromm M, Avramova Z. ATX1/AtCOMPASS and the H3K4me3 marks: how do they activate Arabidopsis genes? Curr Opin Plant Biol 2014; 21C:75-82; PMID:25047977; http://dx.doi.org/ 10.1016/j.pbi.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 56. Pien S, Fleury D, Mylne JS, Crevillen P, Inze D, Avramova Z, Dean C, Grossniklaus U. ARABIDOPSIS TRITHORAX1 dynamically regulates FLOWERING LOCUS C activation via Histone 3 Lysine 4 trimethylation. Plant Cell 2008; 20:580-8; PMID:18375656; http://dx.doi.org/ 10.1105/tpc.108.058172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Matsubara K, Yamanouchi U, Nonoue Y, Sugimoto K, Wang ZX, Minobe Y, Yano M. Ehd3, encoding a plant homeodomain finger-containing protein, is a critical promoter of rice flowering. Plant J 2011; 66:603-12; PMID:21284756; http://dx.doi.org/ 10.1111/j.1365-313X.2011.04517.x [DOI] [PubMed] [Google Scholar]
- 58. Choi SC, Lee S, Kim SR, Lee YS, Liu C, Cao X, An G. Trithorax group protein Oryza sativa Trithorax1 controls flowering time in rice via interaction with early heading date3. Plant Physiol 2014; 164:1326-37; PMID:24420930; http://dx.doi.org/ 10.1104/pp.113.228049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fornara F, de Montaigu A, Coupland G. Snapshot: control of flowering in Arabidopsis. Cell 2010; 141:550-2; PMID:20434991; http://dx.doi.org/ 10.1016/j.cell.2010.04.024 [DOI] [PubMed] [Google Scholar]
- 60. Gomez-Mena C, Pineiro M, Franco-Zorrilla JM, Salinas J, Coupland G, Martinez-Zapater JM. Early bolting in short days: an Arabidopsis mutation that causes early flowering and partially suppresses the floral phenotype of leafy. Plant Cell 2001; 13:1011-24; PMID:11340178; http://dx.doi.org/ 10.1105/tpc.13.5.1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Piñeiro M, Gomez-Mena C, Schaffer R, Martinez-Zapater JM, Coupland G. Early bolting in short days is related to chromatin remodeling factors and regulates flowering in Arabidopsis by repressing FT. Plant Cell 2003; 15:1552-62; PMID:12837946; http://dx.doi.org/ 10.1105/tpc.012153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mussig C, Kauschmann A, Clouse SD, Altmann T. The Arabidopsis PHD-finger protein SHL is required for proper development and fertility. Mol Gen Genet 2000; 264:363-70; PMID:11129039; http://dx.doi.org/ 10.1007/s004380000313 [DOI] [PubMed] [Google Scholar]
- 63. Mussig C, Altmann T. Changes in gene expression in response to altered SHL transcript levels. Plant Mol Biol 2003; 53:805-20; PMID:15082927; http://dx.doi.org/ 10.1023/B:PLAN.0000023661.65248.4b [DOI] [PubMed] [Google Scholar]


