Abstract
The role of muscarinic receptors in several diseases including cancer has recently emerged. To evaluate the hypothesis that muscarinic acetylcholine receptors may play a role in bladder cancer as well as in other tumor types, we investigated their expression in bladder tumor specimens. All examined samples expressed the M1, M2 and M3 receptor subtypes. We also found that the level of M2 transcripts, but not those of M1 or M3, significantly increased with the tumor histologic grade. In view of these results, we proceeded to investigate whether the M2 agonist Arecaidine had any effect on in vitro cell growth and migration of T24 cells, a bladder tumor cell line expressing the muscarinic receptors, including the M2 subtype. We observed that Arecaidine significantly reduced T24 and 5637 cell proliferation and migration in a concentration dependent manner. The silencing of M2 receptor by siRNA in T24 and 5637 cell lines showed the inability of Arecaidine (100 μM) to inhibit cell proliferation after 48 hours, whereas the use of M1 and M3 antagonists in T24 appeared not to counteract the Arecaidine effect, suggesting that the inhibition of cell proliferation was directly dependent on M2 receptor activation. These data suggest that M2 muscarinic receptors may play a relevant role in bladder cancer and represent a new attractive therapeutic target.
Keywords: Arecaidine, bladder cancer, M2 muscarinic receptors, tumor grade, T24 cell line, Transitional cell carcinoma, urothelial cancer cells
Abbreviations
- 4-DAMP
1,1-dimethyl-4-diphenylacetoxypiperidinium iodide
- FFPE
formalin-fixed, paraffin-embedded
- mAChR
muscarinic acetylcholine receptor
- TCC
Transitional cell carcinoma
Introduction
Bladder cancer is the fifth most common tumor in western society, with a global incidence predicted to further increase in the future.1,2 Over 90% of the cases are transitional cell carcinomas (TCC) of the urothelium,1 a highly heterogeneous disease.3 Bladder urothelial cells normally express a variety of receptors that respond to signaling molecules and neurotransmitters.4-7 Among them, the muscarinic acetylcholine receptors (mAChRs), expressed both in detrusor muscle and in urothelial cells, are strongly involved in the biology of the normal human bladder.8,9 Specifically, the M2 and M3 receptors have been identified as the most abundant receptor subtypes, with M2 receptors mainly expressed in umbrella cells and in the bladder mucosa,10 and in a higher proportion (3:1) respect to M3 in the detrusor muscle.8,11 The main function of these receptors is the control of both contraction of detrusor cells and the release of substances such as ATP and NO on urothelium, thus affecting detrusor functions.8,12,13 The expression of muscarinic receptors in bladder is decreased in urothelial and suburothelial cells of patients affected by overactive human bladder.14,15 Functional alterations of mAChRs have also been detected in diabetic patients and with aging16,17 Although the activity of mAChRs has been investigated in normal bladder and in some pathological conditions, the role of these receptors in bladder cancer has been poorly investigated. Muscarinic receptors are also expressed in primary and metastatic colon, ovary, prostate, and lung carcinomas, breast cancer and melanoma.18-23 Interestingly, cell proliferation and migration appear modulated in these tumors mainly by the M3 receptor subtype.19,24-26 However, we have recently demonstrated that the M2 receptor subtype is able to inhibit cell growth and survival in human glioblastoma cells, highlighting a role for this receptor in the inhibition of cancer cell proliferation.27,28
In this study we attempt to investigate the expression of M2 muscarinic receptors in bladder cancer samples and to explore their biological effects on urothelial cancer cells as we already demonstrated in glioblastoma cells. Our results show that the M2 receptors are related to the tumor grade and that their selective stimulation strongly inhibits both in vitro proliferation and migration of T24 and 5637 cancer cells.
Results
M2 receptor expression is proportional to the histological grading of the tumor in bladder carcinoma
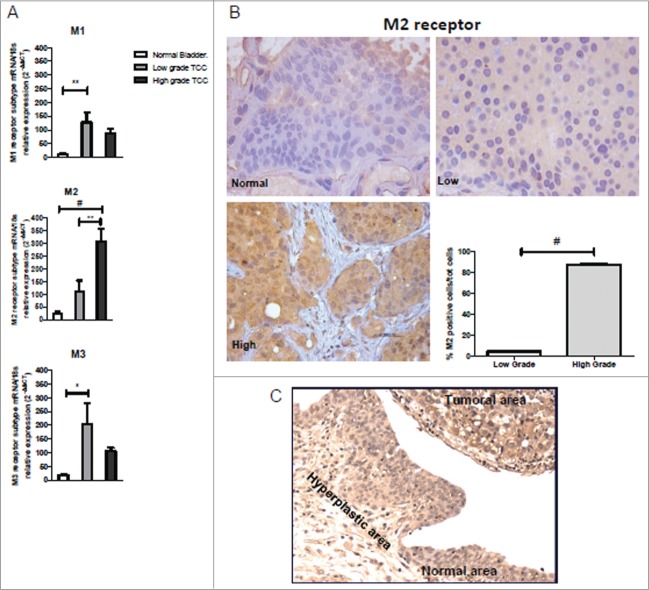
We have measured the mRNA levels for M1, M2 and M3 receptors by qPCR in formalin-fixed paraffin-embedded (FFPE) tissues of low (n = 8) and high (n = 8) grade cases of non-invasive TCC. Four samples of normal bladder tissue were used as comparison. As shown in Figure 1A, the mRNA levels for M1 and M3 receptor subtype were significantly upregulated only in low-grade tumor tissues compared to normal bladder (P < 0.01 and P < 0.05, respectively). The M2 receptor expression in the high-grade tumors was twelve times higher than in the normal tissue (P < 0.001) and almost fourfold increased respect to low-grade tumors (P < 0.01). No statistically significant difference in the mRNA expression levels of M2 receptors was found between normal and low-grade samples. The potential relation between M2 receptor expression and tumor grade was confirmed by quantifying the number of positive cells by immunohistochemistry on serial sections of FFPE samples using an antibody against the M2 receptor (Fig. 1B). The immunostaining (Fig. 1B) for M2 receptor appeared diffusely distributed within the heterogeneous cell populations that characterized this tumor type. As reported in the graph (Fig. 1B), there is a striking difference in the percentage of M2 positive cells between high and low grade samples (P < 0.001). The above results indicate that the expression of M2 receptor protein, as assessed by immunohistochemistry seems to correlate with the mRNA levels.
Figure 1.
Muscarinic receptor expression in bladder cancer biopsies. (A) M1, M2 and M3 mRNA expression levels in normal bladder and in low and high TCC grade. mRNA levels for M1 and M3 receptor subtype were significantly upregulated only in low-grade tumor tissues compared to controls, differently from mRNA levels for M2 subtype receptor whose expression in the high-grade tumors was statistically significant increased than both in normal tissue and low-grade tumors (B) Immunohistochemistry analysis for M2 receptor expression. M2 expression in normal, low, and high TCC grade (40×). The graph shows the quantification of the percentage of the M2 positive cells in high and low TCC grade. (C) Immunohistochemistry analysis showing the M2 receptor expression in the normal and transitional area nearby the tumoral region. Magnification 20×. *P < 0.05, **P < 0.01, #P < 0.001.
Moreover, the expression of M2 receptors was also observed both in the nearby normal tissue and in hyperplastic urothelial area in the same bioptic samples with TCC. We observed a progressive depth of staining for M2 expression from normal to tumoral area (Fig. 1C).
The M2 agonist arecaidine inhibits in vitro proliferation of the T24 and 5637 cell lines
We have recently shown that M2 receptors are highly present in tissues and cell lines derived from glial tumors and that the treatment with the M2 agonist Arecaidine inhibits cell growth and induces severe apoptosis.27,28 We thus investigated whether the M2 receptors could also mediate similar effects in a cell line derived from urothelial carcinoma (T24) after treatment with the M2 agonist Arecaidine. The T24 cells express all 3 muscarinic receptors analysed, yielding the following rank order of expression: M1≥ M2> M3 (Fig. 2A). With regard to the M2 subtype, the effect of its agonist Arecaidine on cell viability and proliferation was assessed by MTS assay. Results (Fig. 2B) showed that the stimulation with 100 μM of Arecaidine after 24 hours was able to significantly inhibit cell proliferation (P < 0.001 vs Control), whereas lower concentrations did not significantly affect T24 cell viability/proliferation. If the stimulation was extended up to 48 hours, all concentrations of Arecaidine (even the lowest) significantly decreased cell proliferation (all P < 0.001 vs Control).
Figure 2.
The M2 agonist Arecaidine inhibits in vitro cell proliferation of T24. (A) M1, M2 and M3 mRNA expression levels in T24 cell line. (B) MTS assay of T24 cell viability in absence (control) or in presence of (12.5, 25, 50, 100 μM) for 24 and 48 hrs. Cell survival was significantly decreased after both 24 and 48 hrs of treatment with 100 μM in presence of Arecaidine as well as at 48 hrs at lower concentrations. #P < 0.001. Ctl, control. O.D., optical density.
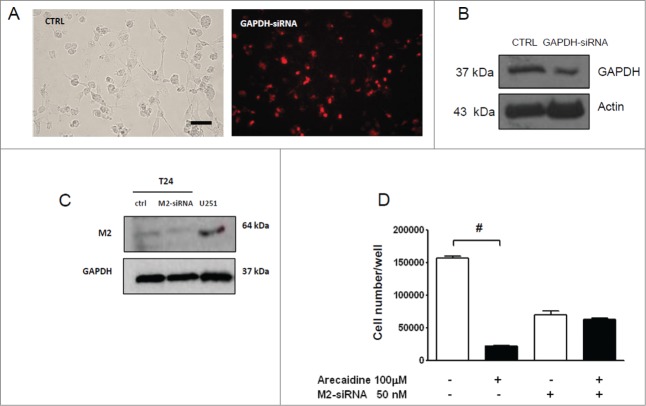
In order to confirm that the effect mediated by Arecaidine on T24 cell proliferation was dependent on M2 receptor activation, we treated the cell line with Arecaidine after M2 receptor silencing using a pool of 4 siRNAs targeting human M2 receptor. A 70% of transduction efficiency was obtained as indicated by Chromo-siRNA GAPDH transfection used as positive control (Fig. 3A-B). The use of siRNAs pool specific for CHRM2 entirely abolished the M2 expression in T24 cells after 48 hours of transfection (Fig. 3C, P < 0.05).
Figure 3.
Effect of M2-silencing on T24 cell growth. (A) T24 transfected with Chromo GAPDH siRNA for 48 hrs (red) used as control of transfection (bar = 50 μm). (B) Western blot of the GAPDH expression in T24 cells in absence (CTRL) and in presence of Chromo-GAPDH siRNA (after 48 hrs of transfection). Actin and GAPDH were used to normalize the bands. (C) Representative image of Western blot of M2 receptor expression in T24 cells in absence and in presence of M2-siRNA pool (after 48 hrs of transfection). U251 cells were used as positive control of M2 receptor expression (see Ref 28) and actin to normalize the bands. (D) MTS analysis performed in T24 cell lines after 48 hrs of siRNA transfection and additional 48 hrs of Arecaidine (100 μM) treatment. The values are the mean ± SEM of 2 independent experiments performed in triplicate. ***P < 0.001. CTRL, control; O.D., optical density.
As indicated by MTS assay (Fig. 3D), although the silencing step caused per se an inevitable decrease in cell number (see Control bar), possibly due to the lipofectamine toxicity, however the additional treatment with (100 μM) Arecaidine after M2 receptor silencing, was ineffective to inhibit T24 cell viability compared with control (P > 0.05).
To further confirm the involvement of M2 receptors in the decrease of cell proliferation, we treated the T24 cell line with the M1 or M3 antagonists (10−6M pirenzepine and 10−8M 1,1-dimethyl-4-diphenylacetoxypiperidinium iodide [4-DAMP], respectively). The antagonists alone were not able to affect cell proliferation as well as the M1 antagonist pirenzepine did not counteract the decrease in cell proliferation-Arecaidine induced (Fig. 4). Considering that the increase in cancer cell proliferation is normally mediated via the stimulation of the M3 receptor subtype,19 we also verified that the specific inhibition of M3 receptor with 4-DAMP enhanced the M2-mediated effects. Results (Fig. 4) showed that the combined use of 100μM of Arecaidine and 4-DAMP significantly strengthened a decrease in T24 cell proliferation (P < 0.001), suggesting that Arecadine could partially bind the M3 receptors, producing an antagonist effects respect to M2 receptor on T24 cell proliferation.
Figure 4.
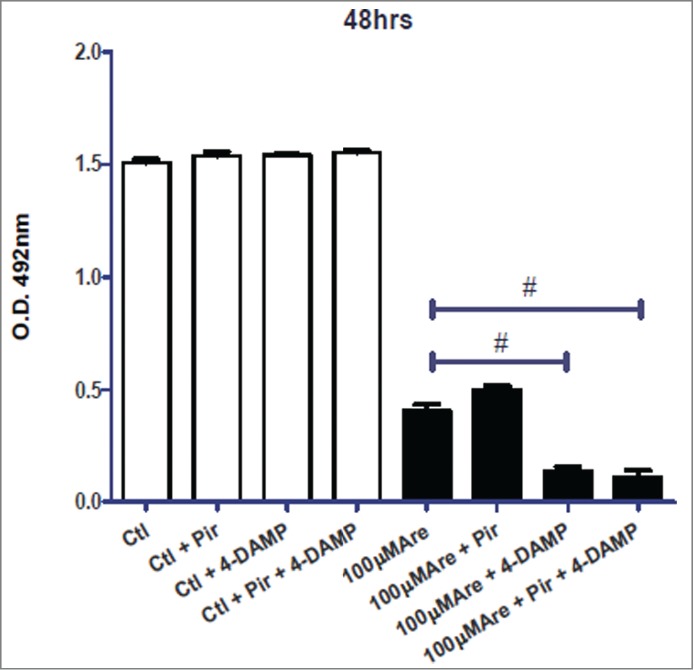
MTS analysis in absence or in presence of Arecaidine alone or in combination with M1 (pirenzepine) and M3 antagonist (4-DAMP). In particular, the M3 antagonist further enhances the decrease in T24 cell viability/proliferation mediated by Arecaidine. #P < 0.001. Ctl, control, Are, Arecaidine, Pir, Pirenzepine, O.D., optical density.
In order to rule out the potential Arecaidine toxicity, we tested its effect in human dermal fibroblasts, used as control. M2 receptors appeared faintly expressed in human fibroblasts (Fig. 5A). Moreover, neither the Arecaidine treatment nor the presence of M2 antagonist gallamine (10−6M) affected cell viability of fibroblasts at all concentrations analysed (Fig. 5B).
Figure 5.
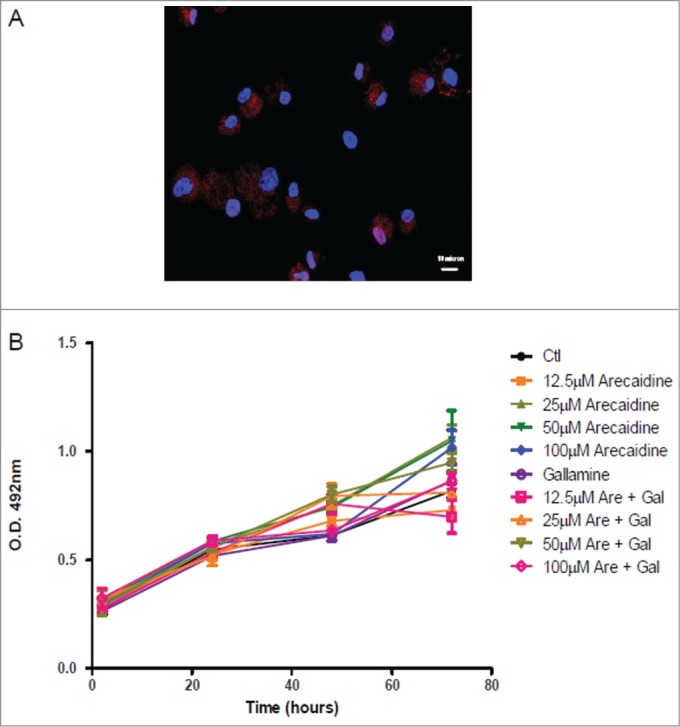
Immunofluorescence and cell viability of human dermal fibroblasts. (A) Human dermal fibroblasts expressing the M2 muscarinic receptor. Blue, DAPI; Red, M2 receptor. (B) Human dermal fibroblast cell viability/proliferation in presence of different concentrations of Arecaidine. Gallamine (10−6M) was used as M2 antagonist to counteract the Arecaidine effects. Ctl, control, Are, Arecaidine, Gal, Gallamine, O.D., optical density.
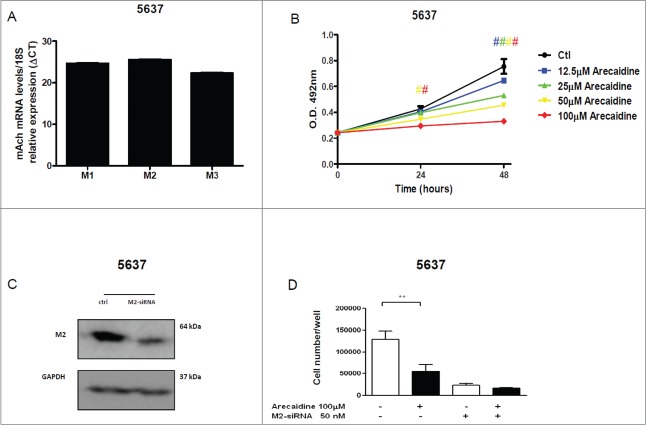
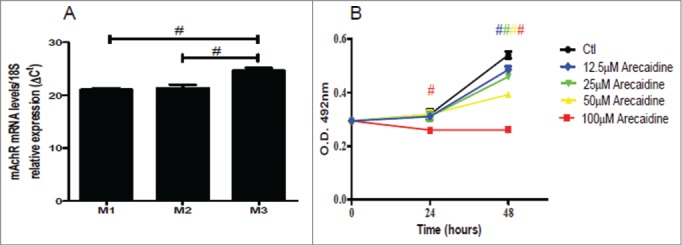
Finally, we asked whether the observed effects were T24-restricted, by testing a different cell line the 5637, a grade 2 TCC of the bladder. Given that the 5637 cell line showed a similar expression of M1, M2 and M3 receptors detected by RTPCR33 (Fig. 6A), results showed that the stimulation with Arecaidine was able to induce a statistically significant decrease even in 5637 cell proliferation in a dose dependent manner at both 24 and 48 hours (P < 0.001 at 24 hours after treatment with 50 and 100 μM of Arecaidine and at all concentrations after 48 hours, Fig. 6B). More importantly, the MTS assay performed after the M2 receptor silencing and treatment with Arecaidine, showed that 5637 cell proliferation was not inhibited, as similarly observed in T24 (Fig. 6C and D, P > 0.05).
Figure 6.
The M2 agonist Arecaidine inhibits in vitro cell proliferation of 5637 cell line. (A) M1, M2 and M3 mRNA expression levels in 5637 cell line. (B) MTS assay of 5637 cell proliferation in absence (control) or in presence of Arecaidine (12.5, 25, 50, 100 μM) for 24 and 48 hrs where cell survival was significantly decreased in a dose-dependent manner after both 24 and 48 hrs of treatment with Arecaidine. (C) Representative images of Western blot of M2 receptor expression in 5637 cells in absence and in presence of M2-siRNA pool (after 48 hrs of transfection). GAPDH was used to normalize the bands. (D) MTS analysis (reported as N. of cells) performed in 5637 cell line after 48 hrs of siRNA transfection and additional 48 hrs of Arecaidine (100 μM) treatment. The values are the mean ± SEM of 2 independent experiments performed in triplicate. #P < 0.001. Ctl, control. O.D., optical density.
Supplementary experiments have indicated a statistically significant decrease in cell proliferation in a dose dependent manner after treatment with Arecaidine even in a further different cell line expressing the M2 receptors, the HT1197, a grade 4 TCC of the bladder (P < 0.001 at all concentrations after 24 and 48 hours; see Fig. S9A and B) and no inhibition of the cell growth in presence of Arecaidine after silencing of the M2 receptor (Fig. S9C).
The M2 agonist arecaidine inhibits in vitro migration of the T24 and 5637 cell lines
Finally, we investigated the effect of Arecaidine under different concentrations on the migratory activity of the T24 urothelial cancer cells by transwell migration assay. Migration as proliferation was severely affected by Arecaidine (Fig. 7A-F) in a concentration dependent manner where concentrations of 12.5, 25 and 50 μM of Arecaidine induced a significant and progressive reduction of the number of migrated cells compared to control of 10% (P < 0.05), 51% and 87.3% (P < 0.001), respectively. After 100 μM Arecaidine treatment, 98% of tumor cells were blocked in the upper side of the chamber (P < 0.001, Fig. 7F), suggesting a potent inhibitory effect of M2 agonist on cell migration. Similar effects were observed on 5637 cell line where a statistically significant and dose-dependent decreased number of migrated cells were found after treatment with arecaidine (Fig. 8A and B, P < 0.01 at 25, 50 and 100 μM). Experiments performed on HT1197 cell line showed no influence on the migration property because of its attenuated ability to migrate even in basal conditions (data not shown).
Figure 7.
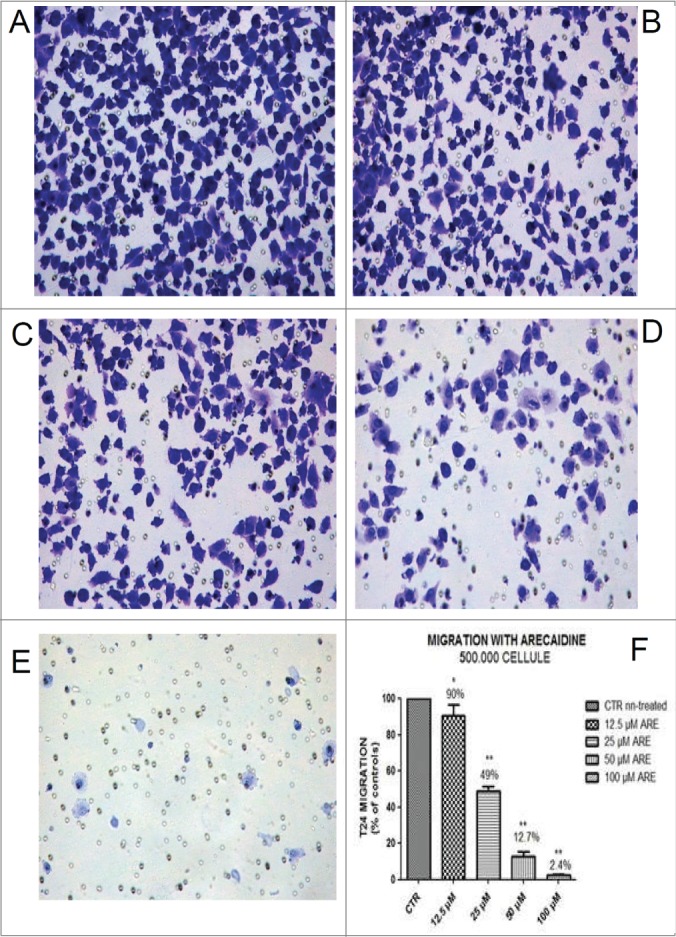
The M2 agonist Arecaidine inhibits in vitro cell migration of T24 cell line. Representative microscopic fields of T24 cell migration across an 8 μm pore size filter in absence (A) and in presence of 12.5 μM, (B) 25 μM, (C) 50 μM (D) and 100 μM (E) Arecaidine for 21 hours. Magnification 25×. (F) The graph shows that 12.5, 25 and 50 μM of Arecaidine were able to significantly decrease the number of migrated cells (10, 51 and 87%, respectively) with a better effect at 100 μM of Arecaidine. The bars represent the mean ± SD. *P < 0.05, **P < 0.001 vs. untreated cells. CTR, control, ARE, Arecaidine.
Figure 8.
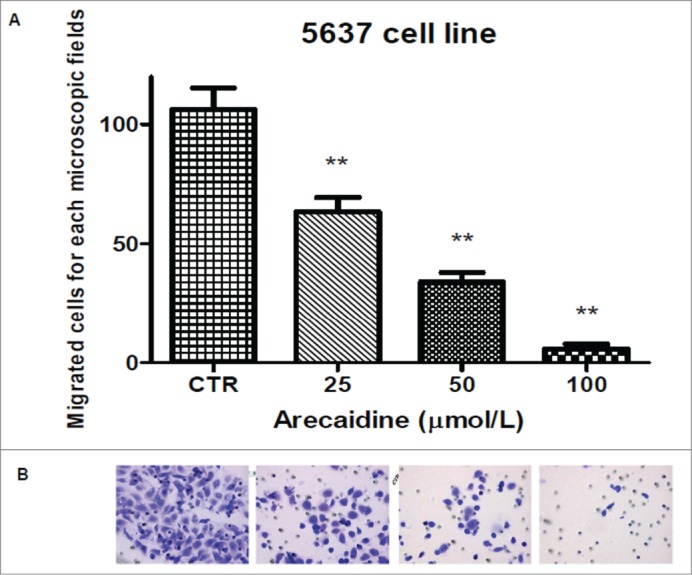
The M2 agonist Arecaidine inhibits in vitro cell migration of 5637 cell line. (A) Graph showing that 25, 50 and 100 μM of Arecaidine were able to significantly decrease the number of migrated cells. (B). Representative microscopic fields of 5637 cell migration across an 8 μm pore size filter in absence and in presence of 25, 50 and 100 μM Arecaidine for 21 hours. Magnification 25×. The bars represent the mean ± SD. **P < 0.01 vs. untreated cells. CTR, control.
Discussion
Recently, the potential involvement of acetylcholine and muscarinic receptors in the modulation of tumor cell proliferation has been highlighted.29,30 In particular, several reports have shown that M3 muscarinic receptor activation enhances tumor cell proliferation, migration and angiogenesis.19,24,31 However, this question is far by being settled. Very recently, we have demonstrated that selective activation of M2 receptors induces cell growth arrest and apoptosis of glioma tumor cells in vitro,27,28 suggesting that M2 receptors might play a counteracting role in tumor cell proliferation.
In the present study we examined the expression of mAChR subtypes (M1, M2, M3) muscarinic receptor subtypes in TCC, showing that the expression of M2 receptor is the highest in the high grade samples (Fig. S10), however no statistical significant difference was found between low-grade and normal samples, probably due to the number of cases still low. On the contrary, M1 and M3 are significantly upregulated only in low-grade samples.
The results on the M2 expression and its protein distribution in tumor samples lead us to hypothesize that this specific type of muscarinic receptor might play a potential permissive role in bladder cancer cell biology. In fact, in non-neuronal tissues such as bladder, the acetylcholine could be released in an autocrine or paracrine fashion, modulating the functions of the neighbouring cells.32 In addition, in pathological conditions including cancer, an altered sensitivity of tumor cells to muscarinic receptors has been described.33,34 Both these aforementioned explanations need further experiments to elucidate the biological function of M2 muscarinic receptors in bladder cancer.
Notably, our study also shows that the M2 change is not limited to the cancer site, but the transition from the normal to the tumoral area through the hyperplasic region is apparently accompanied by a progressive increase in M2 expression. This observation is not far from a study showing a similar behaviour of a different receptor such as Endothelial growth factor receptor (EGFR), whose differential tissue expression is reported in urothelial carcinoma, correlating with clinic-pathological parameters.35
Muscarinic receptors can be detected in urothelial cancer cell lines. To date, the evidence for the expression of these receptors is quite conflicting. In fact, some authors reported that T24, one of the most studied urothelial cancer cell lines, expresses only the M2 and M5 receptors, at variance with other cell lines positive for M1, M2, and M3.33 Our data show that the M3 receptor subtype is abundantly expressed in T24 cell line, and that both M1 and M2 and M2 receptors are similarly represented. Therefore, this apparent contrast with previous publications33 could be overcome, if we consider that we have used real time PCR, a more sensitive technique than PCR.
By pharmacological competition with selective M2 antagonists (gallamine or methoctramine) and silencing of M2 receptors by siRNAs, we have previously confirmed that Arecaidine was able to activate M2 receptor subtypes in normal and pathological cell types (i.e. Schwann cells, oligodendrocytes and glioblastoma cells).28,36-38
In the light of the above findings and given that the expression of M2 receptors is associated with the urothelial tumor grade in vivo, we aimed to investigate the effects of the M2 agonist Arecaidine on the T24 cell line.
Arecaidine is able to inhibit cell proliferation in T24 cells in concentration- and time-dependent manner as in glioblastoma cells,28 where the highest concentration (100μM) already affects cell proliferation after 24 hours, whereas the lower concentrations are effective only over a prolonged time of treatment (48 hours), probably due to the different dynamic of the gradual activation of the M2 receptor. The involvement of the M2 receptors in mediating the inhibition of cell proliferation in T24 cell line was confirmed by the silencing of the M2 receptor by siRNA. In fact, after siRNA transfection the M2 receptor expression was completely abolished in T24 cells, and the T24 cell proliferation appeared unmodified after Arecaidine treatment.
Moreover, the use of M1 and M3 antagonists did not counteract the decrease in cell proliferation induced by Arecaidine. Interestingly, in presence of M3 antagonist we observed that the Arecaidine effect was enhanced, suggesting either that high doses of Arecaidine bind a lesser extend M3 receptors and that on the other the block of the M3 receptor enhances the Arecaidine mediated effect. As suggested for the M2 upregulation in cancer bioptic specimens, we could hypothesize that also in T24 an enhanced sensitivity to M2 receptor occurs, likely due to a dysregulation of the muscarinic receptor signaling.
Cancer cell migration is an issue of particular interest because of the genesis of urothelial bladder carcinoma, often described as a multifocal tumor in the urothelial tract of a single patient. Several molecular evidences suggest that these lesions are descendants of the intraepithelial (or intraluminal) spread of a single transformed cell.39,40 It is interesting that Arecaidine seems also to influence T24 cell migration as well as proliferation.
The hypothesis of our study is also validated as the involvement of M2 receptors in proliferation and migration can be further extended to 2 TCC lines with different grade beyond T24 such as HT1197 and 5637 (grade 4 and 2, respectively), reflecting also in some way the variability in response to the Arecaidine treatment and considering their different genetic background.
Although further experiments are required to elucidate the mechanism downstream M2 receptor activation, the decreased migration at lower concentrations may suggest an impact on cell adhesion molecules such as Focal adhesion kinase (FAK), whose specific activation is required for dynamic process as migration. All together these data support the evidence that the M2 receptor is specifically involved in the regulation of tumor cell proliferation and migration.
Our observations, although restricted to cancer cell lines, may contribute to a more detailed biological and molecular knowledge of the role of muscarinic receptors in urothelial bladder cancer cells.
Conclusions
In conclusion, for the first time we show that M2 is upregulated in high grade urothelial bladder cancer specimens and that its agonistic stimulation affect both in vitro proliferation and migration of urothelial cancer cell line. We believe that our data could help to clarify the role of the M2 muscarinic receptors in urothelial bladder cancer.
Methods
Cell lines and tumor samples
Human bladder cancer 5637, T24 and HT1197 cell lines (grade 2, 3, 4, respectively) were purchased from Cell Lines Service (CLS–Eppelheim, Germany) and ICLC (ICLC, Genova, Italy), respectively.40 Cells were maintained in RPMI, DMEM:Ham's F12 mixture (1:1) and DMEM, respectively, and supplemented with 2 mM L-glutamine, and 10% fetal calf serum (FCS, Sigma-Aldrich).
Bladder cancer tissue was obtained from a total of 16 patients underwent to surgical treatment (8 with non-muscle invasive low and 8 with high grade of TCC). Four urothelial samples, obtained from patients underwent bladder biopsy for diseases other than bladder tumor (all selected cases suffering from ureteral lithiasis) were used as controls. All patients gave informed consent to tissue donation. The biopsy withdrawal has been approved by the Ethical Committee Protocol N. CE/7779. The research has been conducted in accordance with the Declaration of Helsinki. Only sections adjacent to tissue areas containing a minimal rate of 60% tumor cells were further processed for immunohistochemistry and qPCR analysis. Histological grading was performed according to WHO 2004 tumor classification.
Isolation and expansion of human fibroblasts
Isolation of human primary dermal fibroblasts culture was performed as previously described.41,42
Drugs, cell survival and proliferation
The M2 agonist Arecaidine was purchased from Sigma-Aldrich. The muscarinic antagonists used were: gallamine, (final concentration 10−6M, Sigma, Cat. N. G-8134) as M2 antagonist, pirenzepine (final concentration 10−6M, Tocris, Cat. N. 1071) as M1 antagonist and 4-DAMP (final concentration 10−8M, Tocris, Cat. N. 0482) as M3 antagonist.28 Cell growth of T24 cell lines (2000 cells/well) and human dermal fibroblasts41,42 was quantified using a colorimetric method (CellTiter 96 AQueus One Solution Cell Proliferation Assay, Promega) according to the manufacturer's instructions.42,43
M2 receptor silencing
The human M2 muscarinic receptor (CHRM2) (ID1129) expression was inhibited in T24, 5637 and HT1197 cells using 4 different siRNAs. The Chromo GAPDH siRNA (20 nM/well) were also used as positive control of transfection. The sequences of the 4 CHRM2 siRNAs (Riboxx Life Sciences) were as follows: 1.(siRNA1129-1) sense, 5′- AUUUACUACUAAAUCCUCCCCC-3’, antisense 5′-GGGGGAGGAUUUAGUAGUAAAU-3′;
2. (siRNA1129-2) sense 5′- AUGUAGCCCAUUUCUUCCCCC-3’, antisense 5′-GGGGGAAGAAAUGGGCUACUA; 3.(siRNA1129-3) sense 5′-UCCUUUGAGUUUCAGGCUGCCCCC-3′, antisense 5′- GGGGGCAGCCUGAAACUCAAAGGA-3′; 4.(siRNA1129-4) sense 5′-AGUUACACCUUGACCUAACCCCC-3’, antisense 5′-GGGGGUUAGGUCAAGGUGUAACU-3’. Cells were plated in 24 multiwells (2,5 × 103 cells/well) for MTS assay and in 60 mm dishes (40 × 104 cell/dish) for western blot analysis and cultured in complete medium. The siRNAs were pre-mixed with RiboxxFect according to manufacturer's instructions and then added to wells 24 h after plating. The efficiency of the transfection was evaluated transfecting, in separate wells, Chromo-GAPDH siRNA. The ability of the siRNA pool to affected CHRM2 expression was tested using pool siRNA at the final concentration of 50 nM/each and then evaluating M2 receptor expression by western blot analysis 48 h after transfection.
Western blot
Western Blot Analysis on T24, 5637 and HT1197 for M2 receptor was performed as previously described.28 The primary antibody against human anti-M2 mAChR was purchased from Abcam (Cat. N. ab2805) (dilution 1:500 v/v). U251 glioblastoma cell line was used as positive control for M2 receptor expression.28
Cell migration assay
Transwell filter chambers were used for the assay (8 μm pores, BD Biosciences). Semi-confluent T24, HT1997 or 5637 cells were resuspended in serum free medium, seeded at 5×104/well in the upper Boyden chamber and incubated at 37°C for 21 hours. The lower chamber contained DMEM-F12, DMEM or RPMI with 10% FCS. Afterwards, the inner side of the insert was wiped with a wet swab, in order to remove the cells and the outer side of the insert gently rinsed with PBS and stained with 0.25% crystal violet (Sigma) for 15 min, rinsed again, and then allowed to dry. Migrated cells were counted (8 fields per chamber) under a light microscope. Arecaidine was added at different concentrations in both the upper and the lower part of each chamber. Experiments were repeated 3 times in duplicate.
Immunohistochemistry
Sections from each of the FFPE normal and cancer samples were examined by immunohistochemistry using the Vectastain ABC kit (Vector Laboratories) after de-paraffination in xylene/ethanol. Antigen retrieval was performed in Tris-EDTA pH 9.0 prior to incubation with the anti-human M2 receptor at 4°C overnight (5 μg/ml, Abcam Cat. N. ab2805). The sections were counterstained with Mayer's hematoxylin. Ten representative fields/section were selected and the positive cells were counted under a light microscope.
Immunofluorescence
Human primary dermal fibroblast cultures were fixed with 2% paraformaldehyde and permeabilized with 0.1% Triton X-100 solution (Sigma-Aldrich). Cells were then incubated overnight at 4°C with anti-human M2 receptor purified antibody (Abcam) followed by incubation with secondary antibody fluorescein-conjugated (AlexaFluor 488 or 594, Invitrogen). Cells were counterstained with DAPI (diamidino-2-phenylindole) before analyzed by a fluorescence microscope LEICA DM 4000B (Leica Microsystems).
Quantitative PCR (qPCR)
Total RNA was extracted from FFPE sections and TCC cell lines with the “RNeasy FFPE Kit” (Qiagen, Venlo, Netherlands) and quantified by spectrophotometer. RNA (1 μg) was reverse transcribed with the “Quantitek Reverse Transcription Kit” (Qiagen, Netherlands). Primers for qPCR were designed by using the GenBank nucleotide database and the online software available from Genscript (www.Genscript.com). Primer sequences used are: 1) 18 s; Forward primer: 5′-gcaattattccccatgaacg-3′; Reverse primer: 5′-gggacttaatcaacgcaagc-3′; 2) M2; Forward primer: 5′-gatggcctggagcacaaca-3′; Reverse primer: 5′-gctgcttagtcatcttcacaac-3′; 3) M1; Forward primer: 5′-acgctctactggcgcatcta-3′, Reverse primer: 5′-gccttcgtcctcttcctctt-3′; 4) M3; Forward primer: 5′-cgctccaaccaggaggaagta-3′, Reverse primer: 5′-ggagttgaggatggtgctgt-3′. qPCR amplification was performed by using the Biorad CHROMO4 System (Bio-rad Laboratories, USA) and the Power SYBR green Master Mix (Applied Biosystems, USA). PCR conditions included: 2 min at 50°C, and 10 min at 95°C, followed by 45 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s. qPCR data were analyzed by using the Bio-Rad Instrument Gene Expression Analysis Software. Quantification was performed using a comparative CT method (CT = threshold cycle value).28
Statistical analysis
Statistical analysis was performed using unpaired 2-tail t-test. For multiple comparisons, the One-way analysis of variance (ANOVA) test and Bonferroni post-hoc test were used. Data are expressed as mean ± SD unless specified and plotted using GraphPad Prism 5 software (San Diego, USA). The results were considered statistically significant at P < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We acknowledge Fondazione Roma. We also thank Prof Giuseppe Ragona and Dr Rosa Puca for their support and help.
Funding
This manuscript was financially supported by University of Rome “Sapienza,” Department of Medical-Surgical Sciences and Biotechnologies, Latina, Italy.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Authors’ Contribution
LP, AC, CS, DP have performed the experiments; ALP, AC have performed surgery and provided bioptic samples; VP has performed immunohistochemistry; MDB has performed M2 silencing experiments; EDF and AMT have been involved in drafting and revising the manuscript; AC has designed the study and written the manuscript.
References
- 1. van Rhijn BW, Burger M, Lotan Y, Solsona E, Stief CG, Sylvester RJ, Witjes JA, Zlotta AR. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol 2009; 56:430-42; PMID:19576682; http://dx.doi.org/ 10.1016/j.eururo.2009.06.028 [DOI] [PubMed] [Google Scholar]
- 2. Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol 2009; 27:289-93; PMID:19219610; http://dx.doi.org/ 10.1007/s00345-009-0383-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan KS, Volkmer JP, Weissman I. Cancer stem cells in bladder cancer: a revisited and evolving concept. Curr Opin Urol 2010; 20:393-97; PMID:20657288; http://dx.doi.org/ 10.1097/MOU.0b013e32833cc9df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int 2007; 72:1057-64; PMID:17667988; http://dx.doi.org/ 10.1038/sj.ki.5002439 [DOI] [PubMed] [Google Scholar]
- 5. Datta SN, Roosen A, Pullen A, Popat R, Rosenbaum TP, Elneil S, Dasgupta P, Fowler CJ, Apostolidis A. Immunohistochemical expression of muscarinic receptors in the urothelium and suburothelium of neurogenic and idiopathic overactive human bladders and changes with botulinum neurotoxin administration. The J Urol 2010; 184:2578-85; http://dx.doi.org/ 10.1016/j.juro.2010.07.034 [DOI] [PubMed] [Google Scholar]
- 6. Ochodnický P, Humphreys S, Eccles R, Poljakovic M, Wiklund P, Michel MC. Expression profiling of G-protein-coupled receptors in human urothelium and related cell lines. BJU Int 2012; 110:E293-E300; PMID:22551294; http://dx.doi.org/ 10.1111/j.1464-410X.2012.011145.x [DOI] [PubMed] [Google Scholar]
- 7. Nile CJ, Gillespie JI. Interactions between cholinergic and prostaglandin signaling elements in the urothelium: role of muscarinic type 2 receptors. Urol 2011; 79:e17-e23. [DOI] [PubMed] [Google Scholar]
- 8. Braverman AS, Lebed B, Linder M, Ruggieri MR. M2 mediated contractions of human bladder from organ donors is associated with an increase in urothelial muscarinic receptors. Neurourol Urodyn 2007; 26:63-70; PMID:17123299; http://dx.doi.org/ 10.1002/nau.20378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giglio D, Tobin G. Muscarinic receptor subtypes in the lower urinary tract. Pharmacology 2009; 83:259-69; PMID:19295256; http://dx.doi.org/ 10.1159/000209255 [DOI] [PubMed] [Google Scholar]
- 10. Bschleipfer T, Schukowski K, Weidner W, Grando SA, Schwantes U, Kummer W, Lips KS. Expression and distribution of cholinergic receptors in the human urothelium. Life Sci 2007; 80:2303-7; PMID:17335853; http://dx.doi.org/ 10.1016/j.lfs.2007.01.053 [DOI] [PubMed] [Google Scholar]
- 11. Arrighi N, Bodei S, Zani D, Michel MC, Simeone C, Cosciani Cunico S, Spano P, Sigala S. Different muscarinic receptor subtypes modulate proliferation of primary human detrusor smooth muscle cells via AktPI3K and map kinases. Pharmacol Res 2013; 74:1-6; PMID:23628881; http://dx.doi.org/ 10.1016/j.phrs.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 12. Kullmann FA, Artim D, Beckel J, Barrick S, de Groat WC, Birder LA. Heterogeneity of muscarinic receptor-mediated Ca2+ responses in cultured urothelial cells from rat. Am J Physiol Ren Physiol 2008; 294:971-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kullmann FA, Artim DE, Birder LA, de Groat WC. Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J Neurosci 2008; 28:1977-87; PMID:18287514; http://dx.doi.org/ 10.1523/JNEUROSCI.4694-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mansfield KJ, Liu L, Moore KH, Vaux KJ, Millard RJ, Burcher E. Molecular characterization of M2 and M3 muscarinic receptor expression in bladder from women with refractory idiopathic detrusor overactivity. BJU Int 2007; 99:1433-8; PMID:17428242; http://dx.doi.org/ 10.1111/j.1464-410X.2007.06866.x [DOI] [PubMed] [Google Scholar]
- 15. Mukerji G, Yiangou Y, Agarwal SK, Anand P. Transient receptor potential vanilloid receptor subtype 1 in painful bladder syndrome and its correlation with pain. J Urol 2006; 176:797-801; PMID:16813950; http://dx.doi.org/ 10.1016/j.juro.2006.03.074 [DOI] [PubMed] [Google Scholar]
- 16. Hanna-Mitchell AT, Beckel JM, Barbadora S, Kanai AJ, de Groat WC, Birder LA. Non neuronal acetylcholine and urinary bladder urothelium. Life Sci 2007; 80:2298-302; PMID:17363007; http://dx.doi.org/ 10.1016/j.lfs.2007.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Braverman AS. Alterations in muscarinic receptor subtype function in the bladder. Curr Bladder Dysf Rep 2009; 4:47-52; http://dx.doi.org/ 10.1007/s11884-009-0007-1 [DOI] [Google Scholar]
- 18. Rayford W, Noble MJ, Austenfeld MA, Weigel J, Mebust WK, Shah GV. Muscarinic cholinergic receptors promote growth of human prostate cancer cells. The prostate 1997; 30:160-66; PMID:9122040; http://dx.doi.org/ 10.1002/(SICI)1097-0045(19970215)30:3<160::AID-PROS3>3.0.CO;2-Q [DOI] [PubMed] [Google Scholar]
- 19. Frucht H, Jensen RT, Dexter D, Yang WL, Xiao Y. Human colon cancer cell proliferation mediated by the M3 muscarinic cholinergic receptor. Clinical Cancer Res 1999; 5:2532-39. [PubMed] [Google Scholar]
- 20. Oppitz M, Möbus V, Brock S, Drews U. Muscarinic receptors in cell lines from ovarian carcinoma: negative correlation with survival of patients. Gynecologic Oncol 2002; 85:159-64; http://dx.doi.org/ 10.1006/gyno.2002.6597 [DOI] [PubMed] [Google Scholar]
- 21. Song P, Sekhon HS, Lu A, Arredondo J, Sauer D, Gravett C. M3 muscarinic receptor antagonists inhibit small cell lung carcinoma growth and mitogen-activated protein kinase phosphorylation induced by acetylcholine secretion. Cancer Res 2007; 67:3936-42; PMID:17440109; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-2484 [DOI] [PubMed] [Google Scholar]
- 22. Lammerding-Köppel M, Noda S, Blum A, Schaumburg-Lever G, Rassner G, Drews U. Immunohistochemical localization of muscarinic acetylcholine receptors in primary and metastatic malignant melanomas. J Cutan Pathol 1997; 24:137-44; PMID:9085148; http://dx.doi.org/ 10.1111/j.1600-0560.1997.tb01567.x [DOI] [PubMed] [Google Scholar]
- 23. Fiszman GL, Sales ME. Antibodies against muscarinic receptors in breast cancer: Agonizing tumor growth. Curr Immunol 2008; 4:1-7. [Google Scholar]
- 24. Boss A, Oppitz M, Drews U. Muscarinic cholinergic receptors in the human melanoma cell line SK-Mel 28: modulation of chemotaxis. Clin Exp Dermatol 2005; 30:557-64; PMID:16045692; http://dx.doi.org/ 10.1111/j.1365-2230.2005.01865.x [DOI] [PubMed] [Google Scholar]
- 25. Rimmaudo LE, de la Torre E, Sacerdote de Lustig E, Sales ME. Muscarinic receptors are involved in LMM3 tumor cells proliferation and angiogenesis. Bioch. Bioph. Res. Com 2005; 334:1359-136. [DOI] [PubMed] [Google Scholar]
- 26. Song P, Sekhon HS, Proskocil B, Blusztajn JK, Mark GP, Spindel ER. Synthesis of acetylcholine by lung cancer. Life Sci 2003; 19:2159-68; http://dx.doi.org/ 10.1016/S0024-3205(03)00078-X [DOI] [PubMed] [Google Scholar]
- 27. Ferretti M, Fabbiano C, Di Bari M, Ponti D, Calogero A, Tata AM. M2 muscarinic receptors inhibit cell proliferation in human glioblastoma cell lines. Life Sci 2012; 91:1134-37; PMID:22575825; http://dx.doi.org/ 10.1016/j.lfs.2012.04.033 [DOI] [PubMed] [Google Scholar]
- 28. Ferretti M, Fabbiano C, Di Bari M, Conte C, Castigli E, Sciaccaluga M, Ponti D, Ruggieri P, Raco A, Ricordy R, Calogero A, Tata AM. M2 receptor activation inhibits cell cycle progression and survival in human glioblastoma cells. J Cell Mol Med 2013; 17:552-66; PMID:23490231; http://dx.doi.org/ 10.1111/jcmm.12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greig NH, Reale M, Tata AM. New pharmacological approaches to the cholinergic system: an overview on muscarinic receptor ligands and cholinesterase inhibitors. Recent Pat CNS Drug Discov 2013; 8:123-41; PMID:23597304; http://dx.doi.org/ 10.2174/1574889811308020003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nguyen VT, Chernyavsky AI, Arredondo J, Bercovich D, Orr-Urtreger A, Vetter DE. Synergistic control of keratinocyte adhesion through muscarinic and nicotinic acetylcholine receptor subtypes. Exp Cell Res 2004; 294:534-49; PMID:15023540; http://dx.doi.org/ 10.1016/j.yexcr.2003.12.010 [DOI] [PubMed] [Google Scholar]
- 31. Bordey A, Sontheimer H, Trouslard J. Muscarinic activation of BK channels induces membrane oscillations in glioma cells and leads to inhibition of cell migration. J Membr Biol 2000; 176:31-40; PMID:10882426; http://dx.doi.org/ 10.1007/s002320001073 [DOI] [PubMed] [Google Scholar]
- 32. Shah N, Khurana S, Cheng K, Raufman JP. Muscarinic receptors and ligands in cancer. Am J Physiol Cell Physiol 2009; 296:C221-C232; PMID:19036940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tully BT, Li M, Sun Y, Berkowitz J, Chai TC. Defects in muscarinic receptor cell signalling in bladder urothelial cancer cell lines. Urology 2009; 74:467-73; PMID:19573899; http://dx.doi.org/ 10.1016/j.urology.2009.02.043 [DOI] [PubMed] [Google Scholar]
- 34. Ikeda Y, Kanai A. Urotheliogenic modulation of intrinsic activity in spinal cord-transected rat bladders: role of mucosal muscarinic receptors. Am J Physiol Renal Physiol 2008; 295:F454-F461.x; PMID:18550643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ehsani L, Osunkoya AO. Human epidermal growth factor receptor 2 expression in urothelial carcinoma of the renal pelvis: correlation with clinicopathologic parameters. Int J Clin Exp Pathol 2014; 7(5):2544-50; PMID:24966967 [PMC free article] [PubMed] [Google Scholar]
- 36. Barlow RB. and Weston-Smith P. The relative potencies of some agonists at M2 muscarinic receptors in guinea-pig ileum, atria and bronchi. Br J Pharmacol 1985; 85:437-40; PMID:3896364; http://dx.doi.org/ 10.1111/j.1476-5381.1985.tb08879.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Loreti S, Ricordy R, De Stefano ME, Augusti-Tocco G, Tata AM. Acetylcholine inhibits cell cycle progression in rat Schwann cells by activation of the M2 receptor subtype. Neuron Glia Biol 2007; 3:269-79; PMID:18634559; http://dx.doi.org/ 10.1017/S1740925X08000045 [DOI] [PubMed] [Google Scholar]
- 38. De Angelis F, Bernardo A, Magnaghi V, Minghetti L, Tata AM. Muscarinic receptor subtypes as potential targets to modulate oligodendrocyte progenitor survival, proliferation and differentiation. Dev. Neurobiol 2012; 72:713-28; PMID:21913336; http://dx.doi.org/ 10.1002/dneu.20976 [DOI] [PubMed] [Google Scholar]
- 39. x Habuchi T. Origin of multifocal carcinomas of the bladder and upper urinary tract: molecular analysis and clinical implications. Int J Urol 2005; 12:709-16; PMID:16174043 [DOI] [PubMed] [Google Scholar]
- 40. Ueno K, Hirata H, Majid S, Yamamura S, Shahryari V, Tabatabai ZL, Hinoda Y, Dahiya R. Tumor suppressor microRNA-493 decreases cell motility and migration ability in human bladder cancer cells by down-regulating RhoC and FZD4. Mol Cancer Ther 2012; 11:244-53; PMID:22057916; http://dx.doi.org/ 10.1158/1535-7163.MCT-11-0592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Coccia A, Bastianelli D, Mosca L, Monticolo R, Panuccio I, Carbone A, Calogero A, Lendaro E. Extra Virgin Olive Oil Phenols Suppress Migration and Invasion of T24 Human Bladder Cancer Cells Through Modulation of Matrix Metalloproteinase-2. Nutr Cancer 2014. Jun 11:1-9; http://dx.doi.org/10.108001635581.2014.922204 [DOI] [PubMed] [Google Scholar]
- 42. De Falco E, Scafetta G, Napoletano C, Puca R, Vingolo EM, Ragona G, Iorio O, Frati G. A standardized laboratory and surgical method for in vitro culture isolation and expansion of primary human Tenon's fibroblasts. Cell Tissue Banking 2013; 14:277-87; PMID:22820760; http://dx.doi.org/ 10.1007/s10561-012-9325-1 [DOI] [PubMed] [Google Scholar]
- 43. Scafetta G, Tricoli E, Siciliano C, Napoletano C, Puca R, Vingolo EM, Cavallaro G, Polistena A, Frati G, De Falco E. Suitability of Human Tenon's Fibroblasts as Feeder Cells for Culturing Human Limbal Epithelial Stem Cells. Stem Cell Rev 2013, 9:847-57; PMID:23832306; http://dx.doi.org/ 10.1007/s12015-013-9451-6 [DOI] [PubMed] [Google Scholar]
- 44. Menna C, De Falco E, Pacini L, Scafetta G, Ruggieri P, Puca R, Petrozza V, Ciccone AM, Rendina EA, Calogero A, Ibrahim M. Axitinib affects cell viability and migration of a primary foetal lung adenocarcinoma culture. Cancer Invest 2014; 32:13-21; PMID:24380379; http://dx.doi.org/ 10.3109/07357907.2013.861472 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.