Abstract
Recently, we found NHX1, the gene encoding a Na+/H+ exchanger, participated in plant disease defense. Although NHX1 has been confirmed to be involved in plant salt tolerance, whether the NHX1 transgenic plants exhibit both salt tolerance and disease resistance has not been investigated. The T1 progenies of Nicotiana tabacum L. lines expressing SeNHX1 (from Salicornia europaea) were generated for the present study. Compared with PBI-type control plants, SeNHX1 transgenic tobaccos exhibited more biomass, longer root length, and higher K+/Na+ ratio at post germination or seedling stage under NaCl treatment, indicating enhanced salt tolerance. The vacuolar H+ efflux in SeNHX1 transgenic tobacco was increased after treatment of NaCl with different concentration. Meanwhile, the SeNHX1 transgenic tobaccos showed smaller wilted spot area, less H2O2 accumulation in leaves after infection of Phytophthora parasitica var. nicotianae. Further investigation demonstrated a larger NAD(P)(H) pool in SeNHX1 transgenic tobacco. These evidences revealed that overexpression of SeNHX1 intensified the compartmentation of Na+ into vacuole under salt stress and improved the ability of eliminating ROS after pathogen attack, which then enhanced salt tolerance and disease resistance simultaneously in tobacco. Our findings indicate NHX1 has potential value in creating crops with both improved salt tolerance and disease resistance.
Keywords: disease resistance, Na+/H+ exchanger, salt tolerance, SeNHX1, tobacco, vacuolar H+ flux
Plants have developed various biochemical and physiological processes to respond to environmental stresses because of their sessile nature. Sequestering excessive cytoplasmic Na+ into the vacuole through Na+/H+ exchanger 1 (NHX1) is an important strategy in plants to deal with salt stress for maintaining ionic homeostasis.1 Evidences in the NHX1 transgenic plants and yeasts support the enhanced tolerance to salt stress by overexpressing Na+/H+ exchangers.2-4 Recent studies suggest NHX1 affect the lumen pH5 by regulating H+ transportation, which is associated with cellular ROS metabolism.6-8 In our previous study, we found NHX1 could regulate cellular ROS level, and participate in plant disease defense.9 However, no evidence demonstrates that transformation of NHX1 could improve both salt tolerance and disease defense in plant. In this study, the T1 progenies of Nicotiana tabacum L. (Wisconsin 38) lines expressing SeNHX1 (from Salicornia europaea) were generated,4 which were used to investigate the resistance to Phytophthora parasitica var nicotianae (Ppn) and the salt tolerance.
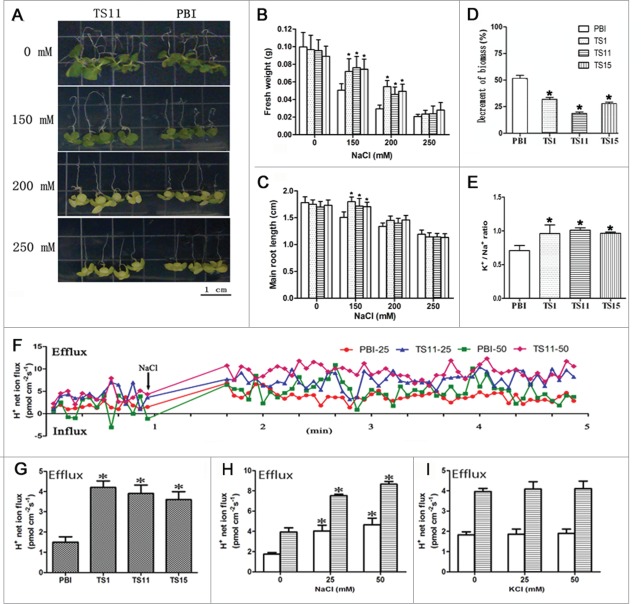
The analysis of salt tolerance was performed both at post germination and seedling stages between SeNHX1 transgenic lines and PBI-type plants (pBI121 empty vector transformed tobaccos, as control). At post germination stage, SeNHX1 transgenic tobacco exhibited longer main roots under 150 mM NaCl treatment, and higher fresh weight under 150 and 200 mM NaCl treatment (Fig. 1A, B and C). In addition, the 6-week-old seedlings of SeNHX1 transgenic lines (TS1, TS11 and TS15) showed markedly higher biomass and K+/Na+ ratios than PBI-type plants after 6 weeks of 200 mM NaCl treatment (Fig. 1D and E).
Figure 1.
Analysis of vacuole net H+ fluxes and NaCl tolerance of SeNHX1 transgenic tobaccos. A to C, Phenotype changes (A), and fresh weights (B), and root lengths (C) of 6-day-old seedlings after 0 (MS), 150, 200 or 250 mM NaCl treatment for 12 d. Data are means ± SE (n = 3 plates, 4 seedlings in each plate). D-E, Effect of 200 mM NaCl on biomass (D) and K+/Na+ ratio (E) of PBI-type and 3 transgenic lines (TS1, TS11 and TS15) subjected to 200 mM NaCl for 6 weeks. Data are means ± SE (n = 6 seedlings). F, Time-course dynamic curves of vacuolar net H+ fluxes under different concentrations of NaCl shock. PBI-25/50 represents vacuoles of PBI-type plants under 25 mM or 50 mM NaCl shock, respectively. TS11-25/50 represents vacuoles of TS11 plants under 25 mM or 50 mM NaCl shock, respectively. G to I, Mean net H+ fluxes in vacuoles for 12 min test under normal condition (G), and supplied with 0, 25 and 50 mM NaCl (H) or KCl (I). The value obtained from NMT indicates net ion flux, the positive values of ion flux in figures represent cation efflux or anion influx and vice versa. Data are means ± SE (n = 6 vacuoles from 6 independent transgenic lines). PBI means tobacco plants transformed with pBI121 empty vector. TS1, TS11, and TS15 represent T1 progenies of SeNHX1 transgenic tobacco lines. Asterisks on bars indicate significant differences from PBI-type plants (difference from untreated plants in H and I) under the same treatment at P ≤ 0.05.
The NMT10-11 (Non-Invasive Micro-Test electrophysiological Technology) was further used to detect trans-tonoplast net H+ fluxes in SeNHX1 transgenic plants. The results showed the average net H+ efflux (H+ transportation out of vacuole) was 1.5 pmol m−2 s−1 in PBI-type plants, while it was two-fold higher in SeNHX1 transgenic tobacco lines (4.2, 3.9 and 3.7 pmol m−2 s−1 in TS1, TS11 and TS15, respectively) without NaCl treatment (Fig. 1F and G). In addition, both of TS11 line and PBI-type tobacco plants exhibited enhanced net H+ efflux in vacuoles along with increasing concentrations of NaCl instead of KCl (Fig. 1H and I). These results indicated SeNHX1 functions as a Na+/H+ exchanger in enhancement of H+ efflux and Na+ compartment in vacuole.
Besides, we found that transformation of SeNHX1 improved the resistance of tobacco to Ppn. The PBI-type plants exhibited larger wilted spots than SeNHX1 transgenic tobaccos at 60 hpi (hour post inoculation) after Ppn inoculation (Fig. 2A and B). The disease classification9 indicated more severe infection occurred in PBI-type plants compared with transgenic lines (Fig. 2C). The quantitative analysis of H2O2 contents showed PBI-type tobaccos accumulated more H2O2 than SeNHX1 transgenic plants at 60 hpi (Fig. 2D). In addition, SeNHX1 transgenic tobaccos exhibited higher contents of NAD(P)(H) components and a larger NAD(P)(H) pool (Fig. 2E and F), contributing to the enhancement of ROS elimination ability.12
Figure 2.
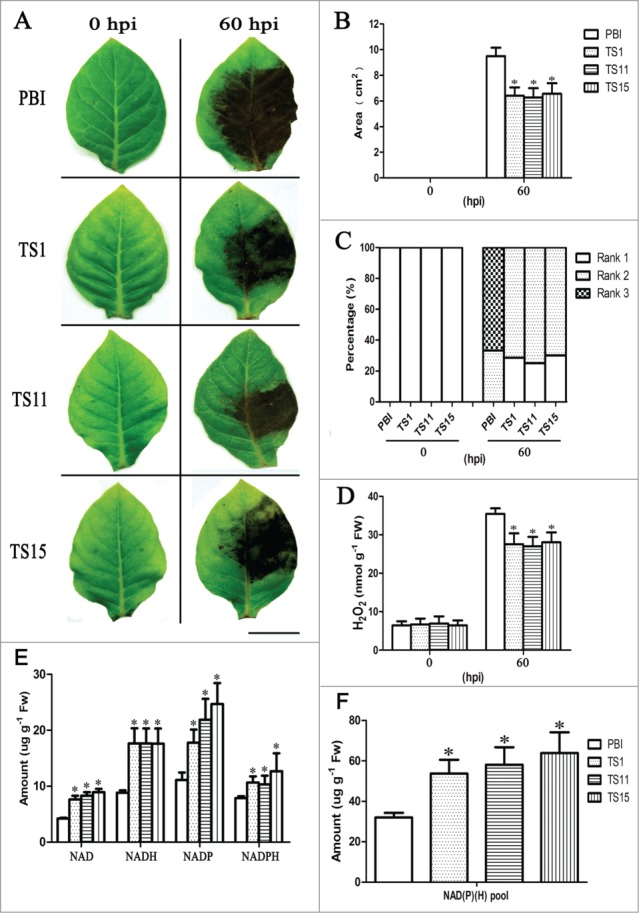
Comparison of NAD(P)(H) pool and the disease development between PBI-type and SeNHX1 transgenic tobaccos. (A) Symptoms on tobacco leaves of PBI-type and 3 SeNHX1 transgenic lines (TS1, TS11, and TS15) at 0 and 60 hpi. Left side of leaf with wounding-only treatment was served as control (Scale bar = 2 cm). (B) Area of wilt spots after Ppn inoculation. (C) Evaluation on disease courses. The rank of disease symptom in leaves was classified into 3 levels from Rank 1 to 3 according to area of wilt spots.9 The higher rank means more serious infection. (D) H2O2 contents in leaf tissue at 0 and 60 hpi. Data are means ± SE (n = 30 leaves). (E) The content of NAD(P)(H) components. (F) NAD(P)(H) pool as NAD(P) + NAD(P)H. Data are means ± SE (n = 6 seedlings). PBI means tobacco plants transformed with pBI121 empty vector. TS1, TS11, and TS15 represent T1 progenies of SeNHX1 transgenic tobacco lines, respectively. Asterisks on bars indicate significant differences from PBI-type plants under the same treatment at P ≤ 0.05.
Several molecules including transcriptional factors, kinases and hormones are involved in crosstalk between abiotic and biotic stress responses. In this study, we reported that SeNHX1, a gene encoding Na+/H+ exchanger 1, participated in both salt stress responses and Ppn resistance in tobacco. Our previous study revealed that involvement in Ppn resistance was not only the characteristic of SeNHX1, but also of AtNHX1 from Arabidopsis thaliana and NbNHX1 from Nicotiana benthamiana.9 Therefore, the regulation of vacuolar Na+ and H+ flux by NHX1 can not only compartment Na+ in vacuole in response to salt stress, but also affect lumen pH and the cellular ROS level, thus participate in plant disease defense.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Research Programs from the Chinese Ministry of Agriculture (Grant No. 2013ZX08009-003-002)and the National Natural Science Foundation of China (Grant No. 31200201).
References
- 1. Zhu JK. Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 2003; 6:441-5; PMID:12972044; http://dx.doi.org/ 10.1016/S1369-5266(03)00085-2; http://www.sciencedirect.com/science/article/pii/S1369526603000852 [DOI] [PubMed] [Google Scholar]
- 2. Apse MP, Aharon GS, Snedden WA, Blumwald E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 1999; 285:1256-8; PMID:10455050; http://dx.doi.org/ 10.1126/science.285.5431.1256; http://www.sciencemag.org/content/285/5431/1256.long [DOI] [PubMed] [Google Scholar]
- 3. Yu JN, Huang H, Wang ZN, Zhang JS, Chen SY. An Na+/H+ antiporter gene from wheat plays an important role in stress tolerance. J Biosciences 2007; 32:1153-61; PMID:17954976; http://dx.doi.org/ 10.1007/s12038-007-0117-x; http://link.springer.com/article/10.1007%2Fs12038-007-0117-x [DOI] [PubMed] [Google Scholar]
- 4. Zhou SF, Chen XY, Zhang XG, Li YX. Improved salt tolerance in tobacco plants by co-transformation of a betaine synthesis gene BADH and a vacuolar Na+/H+ antiporter gene SeNHX1. Biotechnol Lett 2008; 30:369-76; PMID:17968511; http://dx.doi.org/ 10.1007/s10529-007-9548-6; http://link.springer.com/article/10.1007%2Fs10529-007-9548-6 [DOI] [PubMed] [Google Scholar]
- 5. Yamaguchi T, Fukada-Tanaka S, Inagaki Y, Saito N, Yonekura-Sakakibara K, Tanaka Y, Kusumi T, Iida S. Genes encoding the vacuolar Na+/H+ exchanger and flower coloration. Plant Cell Physiol 2001; 42:451-1; PMID:11382810; http://dx.doi.org/ 10.1093/pcp/pce080; http://pcp.oxfordjournals.org/content/42/5/451.long [DOI] [PubMed] [Google Scholar]
- 6. Lamb FS, Moreland JG, Miller FJ. Electrophysiology of reactive oxygen production in signaling endosomes. Antioxid Redox Sign 2009; 11:1335-47; PMID:19207039; http://dx.doi.org/ 10.1089/ars.2008.2448; http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2872256/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valentine JS, Curtis AB. A convenient preparation of solutions of superoxide anion and the reaction of superoxide anion with a copper (II) complex. J Am Chem Soc 1975; 97:224-6; PMID:166105; http://dx.doi.org/ 10.1021/ja00834a058; http://pubs.acs.org/doi/abs/10.1021/ja00834a058">http://pubs.acs.org/doi/abs/10.1021/ja00834a058 [DOI] [PubMed] [Google Scholar]
- 8. Mumbengegwi DR, Li Q, Li C, Bear CE, Engelhardt JF. Evidence for a superoxide permeability pathway in endosomal membranes. Mol Cell Biol 2008; 28:3700-12; PMID:18378695; http://dx.doi.org/ 10.1128/MCB.02038-07; http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2423302/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen XY, Bao H, Guo J, Jia WT, Tai F, Nie LL, Jiang P, Feng JJ, Lv SL, Li YX. Na+/H+ exchanger 1 participates in tobacco disease defence against Phytophthora parasitica var. nicotianae by affecting vacuolar pH and priming the antioxidative system. J Exp Bot 2014; 65:6107-22; PMID:25170102; http://dx.doi.org/ 10.1093/jxb/eru351; http://jxb.oxfordjournals.org/content/early/2014/08/27/jxb.eru351.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen XY, Nie LL, Bao HXG, Jiang P, Lv SL, Li YX. Modified non-invasive micro-test electrophysiological technology for vacuolar H+ flux detection. Anal Biochem 2011; 418:295-7; PMID:21839717; http://dx.doi.org/ 10.1016/j.ab.2011.07.018; http://www.sciencedirect.com/science/article/pii/S000326971100488X [DOI] [PubMed] [Google Scholar]
- 11. Sun J, Chen SL, Dai SX, Wang RG, Li NY, Shen X, Zhou XY, Lu CF, Zheng XJ, Hu ZM, et al. . NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol 2009; 149:1141-53; PMID:19028881; http://dx.doi.org/ 10.1104/pp.108.129494; http://www.plantphysiol.org/content/149/2/1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayashi M, Takahashi H, Tamura K, Huang J, Yu LH, Kawai-Yamada M, Tezuka T, Uchimiya H. Enhanced dihydroflavonol-4-reductase activity and NAD homeostasis leading to cell death tolerance in transgenic rice. Proc Natl Acad Sci USA 2005; 102:7020-5; PMID:15863611; http://dx.doi.org/ 10.1073/pnas.0502556102; http://www.pnas.org/content/102/19/7020.long [DOI] [PMC free article] [PubMed] [Google Scholar]