Abstract
Gibberellins (GAs) are diterpenoid hormones that regulate growth and development in flowering plants. The moss Physcomitrella patens has part of the GA biosynthetic pathway from geranylgeranyl diphosphate to ent-kaurenoic acid via ent-kaurene, but it does not produce GA. Disruption of the ent-kaurene synthase gene in P. patens suppressed caulonemal differentiation. Application of ent-kaurene or ent-kaurenoic acid restored differentiation, suggesting that derivative(s) of ent-kaurenoic acid, but not GAs, are endogenous regulator(s) of caulonemal cell differentiation. The protonemal growth of P. patens shows an avoidance response under unilateral blue light. Physiological studies using gene mutants involved in ent-kaurene biosynthesis confirmed that diterpenoid(s) regulate the blue-light response. Here, we discuss the implications of these findings, and provide data for the ent-kaurene oxidase gene-disrupted mutant.
Keywords: cytochrome P450 monooxygenase, CYP701B1, ent-kaurenoic acid, blue light, Physcomitrella patens, ent-kaurene, gibberellin, gene disruption, protonema
Abbreviations
- GA
gibberellin
- KO
ent-kaurene oxidase
- KAO
ent-kaurenoic acid oxidase
- CPS
ent-copalyl diphosphate synthase
- KS
ent-kaurene synthase
- P450
cytochrome P450 monooxygenase
- nptII
neomycin phosphotransferase II
The diterpenoid plant hormone gibberellin (GA) regulates growth and development of vascular seed plants. In higher plants, this phytohormone has been extensively studied at the chemical, physiological, and biochemical levels. In contrast, little is known about endogenous GAs and their physiological roles in nonvascular plants. Here, we studied the roles of GA-like compounds in the moss Physcomitrella patens. The genome database of P. patens was released in 2008.1 Homology searches of the database revealed that putative GA biosynthetic genes were present in the P. patens genome. Based on these results, we characterized a bifunctional ent-kaurene synthase (CPS/KS) and CYP701B1 ent-kaurene oxidase (KO), which catalyze reactions from geranylgeranyl diphosphate to ent-kaurene via ent-copalyl diphosphate and from ent-kaurene to ent-kaurenoic acid, respectively (Fig. 1A).2,3 Previous studies on a Ppcps/ks-disrupted (ent-kaurene-deficient) mutant of P. patens showed that its caulonemal differentiation was suppressed under red light. The caulonemal differentiation was restored by application of either ent-kaurene or ent-kaurenoic acid, but not GA4.4 The germination and growth of positively photoblastic seeds of vascular plants such as Arabidopsis and lettuce are regulated by the red-light photoreceptor, phytochrome, and GAs.5,6 The growth and development of photoblastic seed plants are positively regulated by GA biosynthesis and signaling under red light. These results suggested that caulonemal differentiation may be regulated by both red light and ent-kaurene-derived diterpenoids, but not by GAs in P. patens (Fig. 1B).
Figure 1.
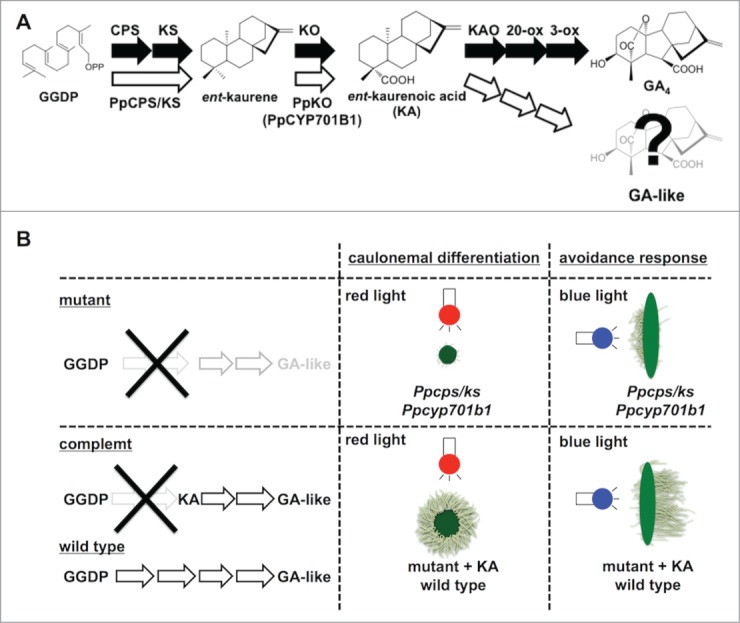
The biosynthetic pathway of diterpenoid regulator(s) and physiological responses under light sources in P. patens. (A), In P. patens, novel diterpenoid (GA-like) regulator(s) synthesized from ent-kaurenoic acid. PpCPS/KS and PpKO (CYP701B1) are involved in ent-kaurene and ent-kaurenoic acid biosynthesis, respectively. Black arrow shows the GA biosynthetic pathway in flowering plant. White arrow shows the GA-like biosynthetic pathway in Physcomitrella patens. (B), GA-like regulator(s) from ent-kaurenoic acid regulates the differentiation to caulonemal cells under red light and the avoidance response of protonemal cells to blue light. Mutants show the suppression of caulonemal differentiation under red light and that of avoidance response under unilateral blue light. These phenotypes are restored by application of ent-kaurenoic acid.
Recently, we observed unique responses of P. patens irradiated by blue light (Fig. 1B).7 The wild-type protonema grew in the opposite direction to the light source under unilateral blue light, whereas the Ppcps/ks disruption mutant did not show this response. This avoidance response of the Ppcps/ks disruption mutant under blue light was rescued by application of ent-kaurenoic acid. In seed plants, blue light reduces the endogenous GA level and decreases shoot length.8 In P. patens, diterpenoid regulator(s) derived from ent-kaurenoic acid may regulate its unique phototropism under blue light. To investigate the regulation of the diterpenoid biosynthetic genes, we analyzed the expression patterns of PpCPS/KS and PpKO in P. patens under red and blue light. PpCPS/KS gene expression was induced by blue light while that of PpKO was not regulated by either blue or red light. PpKO is a member of the cytochrome P450 monooxygenase (P450) superfamily, and the recombinant PpKO protein catalyzes the oxidation of ent-kaurene to ent-kaurenoic acid in vitro.3 The responsiveness of PpKO gene expression to light stimuli was significantly different from that of PpCPS/KS. Since the level of PpKO expression was lower than that of PpCPS/KS and it did not respond to either blue or red light, we focused on the biosynthetic role of PpKO during protonemal growth and generated knockout mutant lines for PpKO by gene targeting according to the procedure reported previously.9 There is a single copy of the KO gene, PpKO (CYP701B1), in the P. patens genome database.3 To generate the Δcyp701b1 (Ppko-disruption) mutant, the exons of CYP701B1 were amplified and then inserted into the pTN182 vector containing the G418-resistance cassette (nptII, encoding neomycin phosphotransferase II) (Fig. 2A).10-12 The transformants were selected on G418-containing medium. We obtained 2 mutant lines of CYP701B1 (Ppcyp701b1). The insertion of the resistance cassette into the correct region in each line was confirmed by PCR (Fig. 2B and C). These two Ppcyp701b1 mutants did not show any obvious differences in colony size and gametophore growth, compared with wild type. Under red-light irradiation, the caulonemal differentiation of Ppcyp701b1 was suppressed as the same response as Ppcps/ks disruption mutant (Fig. 3). Protonemal colonies of Ppcyp701b1 were inoculated into BCD-ATG medium containing 1 μM ent-kaurene or ent-kaurenoic acid under red-light. The suppression of caulonemal differentiation was recovered by the application of only ent-kaurenoic acid but not by the ent-kaurene (Fig. 3). Moreover, phenotypic analysis of Ppcyp701b1 mutants revealed that neither Ppcyp701b1 nor Ppcps/ks showed the avoidance response of protonemal growth to unilateral blue-light irradiation (Fig. 4B and C).7 Protonemal colonies of Ppcyp701b1 were inoculated into BCD-ATG medium containing 1 μM ent-kaurene or ent-kaurenoic acid and were incubated under blue-light conditions for 7 d As shown in Fig. 4D, protonemal growth was unchanged after ent-kaurene application. In contrast, application of ent-kaurenoic acid rescued the blue-light avoidance phenotype, and the protonemal growth occurred on the opposite side to the light source, just like in wild type (Fig. 4A and E). These results indicated that ent-kaurenoic acid is synthesized by PpKO (CYP701B1) in P. patens, and that PpKO is involved in the synthesis of the diterpene-derived regulator(s) involved in the blue-light avoidance response.
Figure 2.
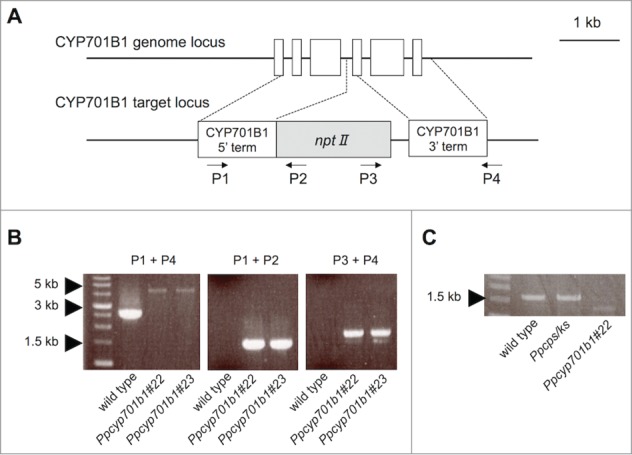
Generation of CYP701B1 mutant. (A), Structure of genomic locus of CYP701B1 and disruption constructs. White boxes represent exons; the line between the boxes represents an intron. Arrows show the primers used for PCR in (B). (B), Insertion of nptII in the correct region of CYP701B1. (C), The open reading frame of CYP701B1 was amplified from cDNA by PCR.
Figure 3.

Photomorphogenesis of protonema in the wild type and mutant of P. patens under red-light irradiation. (A), Ppcyp701b1 mutant. (B), Ppcps/ks mutant. (C), Wild type. (D), Ppcyp701b1 mutant with application of 1 μM ent-kaurene. (E), Ppcps/ks mutant with application of 1 μM ent-kaurene. (F), Ppcyp701b1 mutant with application of 1 μM ent-kaurenoic acid. (G), Ppcps/ks mutant with application of 1 μM ent-kaurenoic acid. Bars = 1 mm.
Figure 4.

Photomorphogenesis of protonema in the wild type and mutant of P. patens under unilateral blue-light irradiation. (A), Wild type. (B), Ppcps/ks mutant. (C), Ppcyp701b1 mutant. (D), Ppcyp701b1 mutant with application of 1 μM ent-kaurene. (E), Ppcyp701b1 mutant with application of 1 μM ent-kaurenoic acid. Bars = 5 mm.
Our research group has shown that diterpenoid compounds synthesized from ent-kaurenoic acid are involved in protonemal differentiation and the avoidance response to blue light (Fig 1). There is a significant difference between vascular and nonvascular plants, because no CYP88A P450 homolog was found in P. patens. CYP88A catalyzes the oxidation reaction of ent-kaurenoic acid to form GA12. We hypothesize that the moss P. patens may convert ent-kaurenoic acid to a novel diterpenoid, but not GA12, by a moss-specific member of the P450 family. Our current aim is to identify ent-kaurenoic acid oxidase and the metabolites converted from ent-kaurenoic acid in P. patens.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Mitsuyasu Hasebe, National Institute for Basic Biology (NIBB) for sharing the vectors for transformation. We thank Professors H. Nozaki and K. Hayashi (Okayama University of Science, Okayama, Japan) for providing the wild type and ent-kaurene-deficient mutant of P. patens.
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research B (24380060 to M. Nakajima), and for Scientific Research for Young Scientists (243051 to S. Miyazaki) from the Japan Society for the Promotion of Science (JSPS).
References
- 1. Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, Shapiro H, Nishiyama T, Perroud PF, Lindquist EA, Kamisugi Y, et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 2008; 319:64-9; PMID:18079367 [DOI] [PubMed] [Google Scholar]
- 2. Hayashi K, Kawaide H, Notomi M, Sakigi Y, Matsuo A, Nozaki H. Identification and functional analysis of bifunctional ent -kaurene synthase from the moss Physcomitrella patens. FEBS Lett 2006; 580:6175-81; PMID:17064690 [DOI] [PubMed] [Google Scholar]
- 3. Miyazaki S, Katsumata T, Natsume M, Kawaide H. The CYP701B1 of Physcomitrella patens is an ent -kaurene oxidase that resists inhibition by uniconazole-P. FEBS Lett 2011; 585:1879-83; PMID:21545802; http://dx.doi.org/ 10.1016/j.febslet.2011.04.057 [DOI] [PubMed] [Google Scholar]
- 4. Hayashi K, Horie K, Hiwatashi Y, Kawaide H, Yamaguchi S, Hanada A, Nakashima T, Nakajima M, Mander LN, Yamane H, et al. Endogenous diterpenes derived from ent -kaurene, a common gibberellin precursor, regulate protonema differentiation of the moss Physcomitrella patens. Plant Physiol 2010; 153:1085-97; PMID:20488896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Toyomasu T, Kawaide H, Mitsuhashi W, Inoue Y, Kamiya Y. Phytochrome regulates gibberellin biosynthesis during germination of photoblastic lettuce seeds. Plant Physiol 1998; 118:1517-23; PMID:9847128; http://dx.doi.org/ 10.1104/pp.118.4.1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamaguchi S, Smith MW, Brown RG, Kamiya Y, Sun T. Phytochrome regulation and differential expression of gibberellin 3β-hydroxylase genes in germinating Arabidopsis seeds. Plant cell 1998; 10:2115-26; PMID:9836749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miyazaki S, Toyoshima H, Natsume M, Nakajima M, Kawaide H. Blue-light irradiation up-regulates the ent -kaurene synthase gene and affects the avoidance response of protonemal growth in Physcomitrella patens. Planta 2014; 240:117-24; PMID:24715198; http://dx.doi.org/ 10.1007/s00425-014 [DOI] [PubMed] [Google Scholar]
- 8. Zhao X, Yu X, Foo E, Symons GM, Lopez J, Bendehakkalu KT, Xiang J, Weller JL, Liu X, Reid JB, et al. A study of gibberellin homeostasis and cryptochrome-mediated blue light inhibition of hypocotyl elongation. Plant Physiol 2007; 145:106-18; PMID:17644628; http://dx.doi.org/ 10.1104/pp.107.099838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nishiyama T, Hiwatashi Y, Sakakibara I, Kato M, Hasebe M. Tagged mutagenesis and gene-trap in the moss, Physcomitrella patens by shuttle mutagenesis. DNA Res 2000; 7:9-17; PMID:10718194 [DOI] [PubMed] [Google Scholar]
- 10. Beck E, Ludwig G, Auerswald EA, Reiss B, Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. gene 1982; 19:327-36; PMID:6295884; http://dx.doi.org/ 10.1016/0378-1119(82)90023-3 [DOI] [PubMed] [Google Scholar]
- 11. Odell JT, Nagy F, Chua NH. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 1985; 313:810-2; PMID:3974711; http://dx.doi.org/ 10.1038/313810a0 [DOI] [PubMed] [Google Scholar]
- 12. Guerineau F, Brooks L, Meadows J, Lucy A, Robinson C, Mullineaux P. Sulfonamide resistance gene for plant transformation. Plant Mol Biol 1990; 15:127-36; PMID:2103427; http://dx.doi.org/ 10.1007/BF00017730 [DOI] [PubMed] [Google Scholar]


