Abstract
Programmed cell death (PCD) is an integral cellular program by which targeted cells culminate to demise under certain developmental and pathological conditions. It is essential for controlling cell number, removing unwanted diseased or damaged cells and maintaining the cellular homeostasis. The details of PCD process has been very well elucidated and characterized in animals but similar understanding of the process in plants has not been achieved rather the field is still in its infancy that sees some sporadic reports every now and then. The plants have 2 energy generating sub-cellular organelles- mitochondria and chloroplasts unlike animals that just have mitochondria. The presence of chloroplast as an additional energy transducing and ROS generating compartment in a plant cell inclines to advocate the involvement of chloroplasts in PCD execution process. As chloroplasts are supposed to be progenies of unicellular photosynthetic organisms that evolved as a result of endosymbiosis, the possibility of retaining some of the components involved in bacterial PCD by chloroplasts cannot be ruled out. Despite several excellent reviews on PCD in plants, there is a void on an update of information at a place on the regulation of PCD by chloroplast. This review has been written to provide an update on the information supporting the involvement of chloroplast in PCD process and the possible future course of the field.
Keywords: ACD, Cid, chloroplast, holin/antiholin, Lrg, PCD, photosynthesis, ROS
Introduction
Death is final destination in the life of an organism. It represents a stage where organism reaches a permanently ametabolic state. Being an integral component of the life cycle, cell death is observed during different developmental stages of an organism as well as an outcome of its interaction with the unfavorable biotic/abiotic factors and certain pathological conditions, both in animals1and plants.2 Under stress conditions, the targeted sacrifice of unwanted or diseased cells is very important for the maintenance of total cell number and homeostasis. This process of targeted elimination/removal of selective cells by physiologically controlled death is collectively referred to as Programmed Cell Death (PCD).
Since the inception of the idea of PCD, most of the studies to decipher the process have been made on animal system and the process has been elucidated in great details and almost all the possible events involved in the process have been sketched out. Compared to studies made on animal system, the overall understanding of the plant PCD is still rudimentary and requires further extensive studies to figure out the regulatory proceedings and molecules involved in the process. Plants being mostly sessile in nature and having a cellular architecture different from animals are generally more responsive to perturbations in the environment and possibly institute different mechanisms to survive in changed and challenged environment. Absence of circulatory system, presence of cell wall and big vacuole that occupy almost 2/3rd cells volume restrict plant to follow molecular regulatory mechanisms other than animals. Based on the information available, Lam's group have proposed a working model of programmed cell death in plants.3 According to this model reactive oxygen and nitrogen species (ROS and RNS) play a crucial role in the plant PCD execution process. As chloroplast and mitochondria are the major source of ROS generation in the plants, their pivotal role in the PCD process is undisputed. In terms of total ROS generation in a cell, particularly under stress conditions, the contribution of Chloroplast is quite significant as compared to that of the mitochondria.4 Furthermore, compared to the involvement of chloroplasts, participation of the mitochondria in plant PCD process has been worked out in a relatively greater detail.5-8 Due to relative lack of comprehensive information, the step-wise execution events involved in the chloroplast/mitochondria mediated PCD process in plant is still a gray box. This review makes an attempt to put together the information available on the contribution of chloroplasts to the plant PCD and tries to bring about a coherent picture of the modulation of plant PCD by chloroplast and puts forward a possible future direction for the field.
Chloroplast Mediated PCD- Some Supportive Evidences
First indication about the involvement of chloroplast in PCD process came out from elegant studies made in epidermal peels of pea leaves by Samuilov et al. (2002 and 2003). These studies reported that light stimulated cyanide (CN−) induced destruction of the guard cells of the peels that contained both chloroplasts and mitochondria but the epidermal cells which were devoid of chloroplasts remained unaffected.9,10 Another land-mark contribution in our understanding of the chloroplast involvement in PCD emerged from research made in Greenberg's group showing that PCD triggered by biotic interaction relied on chlorophyll catabolic protein called accelerated cell death 2 (ACD2) which is generally localized in chloroplast. ACD 2 has been shown to protect cell from PCD through a dynamic localization to the mitochondria.7,11 More recently working on cell suspension of Arabidopsis sp. the McCabe group has shown that chloroplast and ROS are involved in heat induced apoptosis like PCD (AL-PCD).12 In another report, involvement of chloroplast in herpin induced hypersensitive response was studied using confocal fluorescence microscopy (CFM) and optical coherence tomography (OCT). Observations made through OCT suggests a decrease in the chloroplast back scattered signal under stress that was consistent with the change in the refractive index of the thylakoid membranes at an early time point after the infiltration of the inducer, indicating that a decrease in photosynthesis, emphasizing changes in chloroplast structure as one of the earliest hallmarks of plant hypersensitive cell death (Boccara et al., 2007). Recent studies that utilized conditional fluorescent (flu) mutant of Arabidopsis indicate that depending on the intensity of light exposure and light pre-treatment condition, singlet oxygen (1O2) produced in the chloroplasts is involved in EXECUTER dependent signaling pathway that manifested itself during acclimation and stress response. These reports advocate chloroplasts as a source and primary target of PCD signaling events.13-15 Thus data emerging from recent studies though provide the evidence of participation of chloroplasts in influencing the rate of PCD in some types of cells, the sequence of molecular events associated with chloroplasts are yet to be elucidated.
ROS Generation in the Chloroplasts
Perturbation in the components of the biotic and abiotic factors lead to the amplification of cellular ROS before physio-biochemical manifestations of the particular stress (that may lead to cell death depending on the intensity of exposed stress) is observed in the plants. In addition to mitochondria (a major source of ROS and mediator of PCD in animals), chloroplasts present in the plants produce ROS during photochemical reaction of photosynthesis as well as through tetrapyrrole intermediate molecules in chlorophyll biosynthetic pathway. In the light reactions of photosynthesis, reaction centers [Photosystem II (PSII) and Photosystem I (PSI)] in the thylakoid membranes of chloroplasts are major site of ROS generation. PSI is involved in the light mediated generation of super-oxide radical (O2−) which in turn disproportionated to produce hydrogen per oxide (H2O2), whereas photosystem II (PSII) is involved in the generation of singlet oxygen (1O2).16 The ROS are also generated in chloroplasts during chlorophyll biosynthesis in thylakoid membrane where most of the chlorophyll biosynthetic intermediates are loosely attached to thylakoid membranes without their association to reaction center.17 Among the enzymes involved in the chlorophyll biosynthesis, Protochlorophyllide oxidoreductase (PORC) is the first light-requiring enzyme that catalyzes the conversion of protochlorophyllide (Pchlide) to chlorophyllide (Chlide) in the presence of light. As reaction is photo-mediated, it acts as rate limiting step in chlorophyll biosynthesis. Thus, in dark incubated plants, the accumulated Pchlide exerts a feedback inhibition on the synthesis of other intermediates of chlorophyll biosynthesis. Interestingly application of 5 -aminolevulinic acid (ALA), a porphyrin generating photodynamic herbicide that induces over-accumulation of Pchlide in the dark, to green plants, bypasses this feedback inhibition resulting in accumulation of Mg-tetrapyrroles in the dark that in turn get excited into their triplet state in the presence of light. The photons absorbed by these tetrapyrroles, instead of being utilised in photochemical reactions generate 1O2 by energy transfer to molecular oxygen.17 Thus, chloroplasts present an organelle that generates ROS at multiple events.
Chloroplast Generated ROS and PCD
As chloroplast is one of the major cellular ROS producing organelle, its involvement in the PCD process is believed to be linked to ROS generation process. Chloroplasts generate ROS at 2 independent sites i.e. photochemical reactions of photosynthesis and chlorophyll biosynthetic pathway making them a major focus for the studies to figure out the molecular events involved in ROS mediated cell death. Reports indicate that chloroplastic ROS played important role in inducing cell death in a variety of conditions like abiotic and biotic interaction as well as during developmental program i.e., senescence.18,19 The chloroplast generated ROS and reduced plastoquinone from photosynthetic electron transport chain have been reported to be involved in CN− induced cell death in pea guard cells10 (Samuilov et al., 2003). A study focused on developmental PCD in transgenic tobacco plants defective in plastid ndh gene showed a link between consequent lower level of ROS generation and a delay in the senescence.20 A recent report suggests that loss of function of FZl gene that encodes a membrane GTPase involved in the determination of thylakoid and chloroplast morphology, in lesion mimic mutant of Arabidopsis is involved in ROS accumulation triggered by damage to the chloroplast membranes, is a signal sufficient to start the HR signaling cascade.21 Phenylalanine ammonia lyase (PAL) mediated salicylic acid (SA) accumulation has been suggested to be involved in Fumonisin B1 triggered light dependent generation of H2O2 that induces degradation of chloroplast proteins and HR induction in Arabidopsis.13,19
Chlorophyll break down leading to yellowing of leaves is one of the signatures of senescence that is believed to be a type of PCD.22 Similarly chlorophyll catabolism is also observed during biotic and abiotic interactions.23 Further evidence of light dependent ROS generation from chloroplasts during some HR induced cell death comes from HR induction in Arabidopsis following the interaction with Turnip crinkle virus.24 Seminal studies by Mur et al. (2010), and Yoshimura et al. (2007), further shed light on the role of chlorophyll catabolites in Pseudomonas syringae induced HR in Arabidopsis seedlings which were engineered to over-express a chloroplast protein STAYGREEN (SGR).25,26 This study reports that SGR is involved in triggering the production of reactive oxygen species that is generated as a result of the disruption of the light-harvesting complex (LHC) that further leads to disruption in the photosynthetic electron transport. Based on this study and available data from previous reports about the role of SGR, Mur et al. (2010) proposed a model for the role of chlorophyll catabolites in HR induction. As per this model, the disruption of LHC causes the removal of Chlorophyll porphyrin moiety that is first dechelated forming pheophytin and then dephytylated by pheophytinase to form chlorophyllase.27 Further, removal of central magnesium (Mg) by Mg dechelatase results into the formation of pheophorbide a (Pheide a), which is localized in the chloroplasts stroma. Pheide – a oxygenase (PaO), an enzyme of chlorophyll catabolic pathway cleaves Pheide – a to form red chlorophyll catabolite (RCC). RCC is further processed by RCC reductase (encoded by ACD2 in Arabidopsis) to form colorless Chlorophyll catabolites. These photodynamic breakdown intermediates (porphyrin and tetrapyrrole) being unlinked to reaction center, upon excitation by light give rise to singlet oxygen. Hortensteiner has comprehensively reviewed this pathway (Hortensteiner, 2006). With high turn-over of PaO or in its mutant, under stress conditions, the reduced PaO concentration result in exacerbated accumulation of Pheide from LHCs.28 As a consequence, photo activation of Pheide-a that causes photobleaching is supposed to make a significant contribution to the overall light-dependent ROS production during the HR.25
Chloroplast Based Executioners of PCD
Proteases
Proteases are one of the key executioners of PCD process. Caspases form a major group of cysteine containing intracellular proteases which are involved in the disassembly of target animal cells into apoptotic bodies. Caspases cleave their target substrates at aspartic acid residue. Though the plant genomes studied so far do not code for caspases per se, Caspase-like activity has been observed and reported by several workers.29,30 The role of caspase-like proteases in plant PCD was first established with identification and characterization of metacaspases, a distant relative of caspases that have arginine as a preferred P1 amino acid in their cleavage site. There are 9 metacaspases identified in arabidopsis genome itself which are grouped in 2 different sub-classes based on the sequence similarities in Caspase-like region and the overall predicted domain structure.31 Recently another subfamily of metacaspase has been identified from phytoplankton protist that shows a domain structure distinct from that of subfamily metacaspase I or II. This new subfamily of metacaspase shows a rearrangement of domain structure between N- and C-terminus. These metacaspases have been designated as type III metacaspase.32 Initial report on metacaspases type II in Picea abies (mc II Pa) embryo suggest that McIIPa are generally localized in the cytoplasm and translocate to nuclei of the target cells destined for elimination.33
Apart from metacaspases, vacuoler processing enzymes (VPEs) have been reported as another class of proteases involved in cell dismantling process (Hara-Nishimura et al., 2005). Like metacaspases, VPEs also use catalytic cysteine that is activated by catalytic Histidine for the nucleophilic attack but unlike metacaspases which cleave their substrates after Arg, VPEs cleave substrate after Asn residue.34 Immunolocalization studies reveal that VPEs are primarily localized in vacuoles and vesicles and play role in PCD and pathogen infection.35,36
The available data on chloroplastic proteases and their putative link with PCD process is very limited. Consistent with chloroplasts being of endosymbiotic origin, chloroplastic proteases identified and described till the date are found to be closely related to bacterial enzymes.37 Among 11 different families of proteases represented in chloroplasts, 6 families (4 families from class serine protease and 2 families from metalloprotease) are found to be involved in the degradation process while others work as processing peptidases.38 Despite reported presence of several proteases and peptidases in the chloroplast only a few namely,Clp, FtsH, Lon, DegP, CTP and SPP4 have been given generous attention. These proteases are involved in the regulation of various events involved in chloroplast biogenesis and maintenance including degradation of partially assembled complexes and damaged proteins.39 Huesgen first showed the direct involvement of the chloroplastic Deg proteases in PCD process.40 Deg proteins, named after a null mutant phenotype (degradation of periplasmic proteins) in E.coli, are periplasmic ATP independent serine proteases comprise of a N-terminal proteolytic domain and a C-terminal domain with 2 PDZ domains that regulate proteolytic activity of Deg proteins.40-42 In a seminal study carried out by Carrion et al. (2013), the researchers using Arabidopsis as a model system demonstrated, that during senescence process the proteins targeted for degradation are relocalized into small senescence associated vacuoles (SAVs) in chloroplast with high cystein peptidase activity. Presence of cystein protease activity in the SAVs was evaluated by pretreatment of the leaf discs with cystein protease inhibitor E-64. This treatment effectively blocked RuBisCo degradation implying the presence of cysteine proteases like activity in SAVs that could be at least partly responsible for the degradation of stromal proteins of the chloroplast during senescence.43 Among metacaspases, type I metacaspase AtMC1b is found to be localized in the chloroplasts of Arabidopsis, whose expression is induced in vascular tissues on exposure to various biotic and abiotic stresses.44 Interestingly LSD1 (Lysine specific demethylase 1) which is a known regulator of HR induced PCD is associated with AtMC1a zinc finger domain containing protein.45 Information available on chloroplastic proteases in PCD execution process is still a gray box and further studies are required to reveal mechanistic details.
PCD Signature Triggered by Chloroplastic Residents
Mitochondria have been postulated as the key executioner of apoptosis in animal model systems. The mitochondria associated events during animal cell PCD include Bcl2 mediated release of Cytochrome c (Cyt c), Smac/DIABLO, endoG, AIF, and Omi/HtrA2 from the space between outer and inner-mitochondrial membrane to the cytosol. Cytosolic Cyt c then binds to Apaf-1 to induce apoptosome formation, leading to caspase-9 and caspase-3/7 activation.46 Cytochrome c, apart from being involved in respiratory electron transport chain, after being released to cytosol play an active part in apoptosome formation.47,48
In chloroplasts, similar to the release of cytochrome c, cytochrome f that is a component of photosynthetic electron transport machinery has been shown to get released from the thylakoid membranes to the cytosol during cell death induced by palmitoleic acid in eggplant49 and during heat stress induced PCD in a unicellular green alga chlorella.50 These observations indicate the possible involvement of the components of chloroplast electron transport machinery in PCD execution process. In a recent report, Wang and co-workers (2014) have reported the release of cytochrome f from chloroplast to cytoplasm to induce caspase-3 like activity that could be inhibited by 26S proteasome inhibitor MG 132 during senescence in detached rice leaves.51 Furthermore, these authors have shown that Cyt f might interact with E3-ubiquitin ligase and RPN9b, the subunits of the ubiquitin proteasome system (UPS), and other PCD-related proteins suggesting that released Cyt f to the cytoplasm may induce caspase-like activity that acts on the downstream degradation process through ubiquitin processing system.51 In addition, cleavage of the 55 kDa large sub-unit of RuBisCO mediated by subtiline like proteases during PCD triggered by biotic (virus infection) and abiotic (oxidative and osmotic) stresses is evident but not universal.52 In an elegant classic study that was carried out by Dickman's group, a trans-kingdom approach was applied to ascertain the potential mechanistic overlap between plant and animal PCD. In this study human Bcl-2; Bcl-2DBH4; chicken Bcl-xL, and C. elegans CED-9 were introduced in tobacco plant (Nicotiana tobaccum). Sub- cellular localization of the above indicated gene products revealed their localization in the usual expected places the nucleus and mitochondria similar to animal cells, along with the chloroplast – the suspected or bonafide PCD executioner in plants. Furthermore, the study assigned a functional significance to the localization of animal PCD executioner in chloroplast based on the observation that the selected chloroplast-directed herbicides killed tobacco cells in an apoptotic-like, light-requiring manner, but the transgenic tobacco expressing anti-apoptotic genes survived and did not exhibit apoptotic characteristics further strengthening the notion that under the conditions favoring oxidative stress, chloroplast is involved in the execution event during PCD.53
After the establishment of the chloroplastic protein Accelerated Cell Death 2 (ACD2) in modulating PCD triggered by Pseudomonas syringae infiltration and protoporphyrin IX (PPIX) treatment,7 further mechanistic details of ACD2 modulated PCD was reported by the same group using a complementation assay based on ACD2 and acd2 sector plants. This study have shown that ACD2 localizes dynamically within chloroplast and mitochondria during infection to protect cells from mobile pro-death substrate molecules, some of which may originate in chloroplasts, but do display major effect on mitochondria suggesting a possibility of the role of inter organelle cross-talk in the PCD execution event in plants.11 A report based on studies made in a conditional flu mutant of Arabidopsis ascribed another important function to chloroplasts, namely an environmental sensor that helps perceive and respond to a broad range of stresses.54 Furthermore, based on the analysis of Executer 1 and Executer 2 proteins (the nuclear encoded and chloroplast localized proteins involved in singlet oxygen related gene expression) independently as well as inactivated Executer1 and Executer 2 in flu background, authors suggest that Retrograde control of 1O2-responsive genes required the concerted action of both Executer proteins within the plastid compartment. The information discussed above suggest that the chloroplast s act as sensor for various environmental signals and relay the signal to the nucleus to coordinate the execution of PCD which may involve several chloroplast borne components.
Chloroplast Mediated PCD: A Bacterial PCD Connection
Chloroplasts and mitochondria have been proposed to have evolved from an endosymbiotic acquisition of bacteria into the eukaryotic cell.55 It is believed that mechanistically PCD is an evolutionary conserved process, therefore it is presumed that the major executioners of the PCD i.e. chloroplast and mitochondria would have retained some of the regulatory molecules from their ancient bacterial progenitors.56 This line of thinking is further strengthened by the presence of holins and antiholin proteins in bacteriophages that helps in the lysis of host cells to release newly formed viral particles57 and CidA and LrgA proteins of Staphylococcus aureus that is involved in cell death, lysis and biofilm development.58 A recent report shows that CidA and LrgA show structural, biochemical and functional similarities with Holin group of proteins.59 Interestingly, putative orthorlogs of bacterial Cid and Lrg proteins have been identified in the chloroplast envelope membrane proteome.60 Characterization of AtLrgB found in Arabidopsis has been shown to function as an anti-apoptotic factor that acts against the cell death signals similar to bacterial Lrg protein.61 Wang and Bayles proposed an excellent model of conservation of cell death among bacteria and chloroplast.56 According to this theory, chloroplasts and bacteria share common executioners of the cell death. The plant CidAB and LrgAB have been proposed to function as holins and antiholins of bacteria. Plant CidAB may function as cell death effector, and the bax inhibitor – has been hypothesized to interact with Cid AB and block its activity.56 The effectors Cid and Lrg both function to regulate cell death but target different molecules to modulate death process. In bacteria they stimulate peptidoglycan hydrolases while in plants protease similar to Caspase cascade are stimulated to initiate the cell death.56
Based on the available information discussed above and detailed in Figure 1, it can be safely assumed that the chloroplasts act as one of the prominent executioner of the PCD process in plants. It is clearly involved in the amplification of cellular ROS and the cellular redox changes effected by it could act as a signaling event that depending on the physiological state of the cell could lead to PCD.14,62 In the execution event, certain proteins that are involved in chlorophyll catabolic process like ACD2, target mitochondria to execute the process.11 In another sequence of events, singlet oxygen generated in the chloroplasts activates 2 chloroplastic executer proteins namely 1 and 2 that in turn relay the signal to nucleus for respective gene expression.14 In a parallel chloroplastic event, chloroplast envelope proteins Cid and Lrg execute the cell death process through their own interaction as well as through the interaction with bax inhibitor. Simultaneously certain proteases like Deg, MCP1b, and other proteins with caspase like function are activated that act on the dismantling core complexes of electron transport system. Chloroplasts mediated cell death execution process is evolutionary conserved at certain degree.
Figure 1.
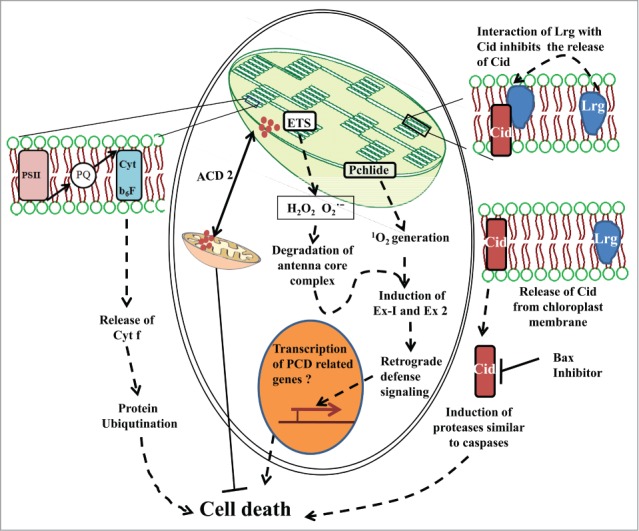
Chloroplast associated events modulating PCD: reactive oxygen species (ROS) generated through photosynthetic electron transport and chlorophyll biosynthetic pathway are involved in degradation of antenna core complex, simultaneously ROS activate chloroplastic executer 1 and 2 proteins that in- turn relay signal to the nucleus for the activation of transcription for cell death related genes. In a separate event, Cytochrome f released from electron transport chain induces caspase 3 like activity leading to cell death involving ubiquitin processing system. In another chloroplastic event cid and lrg protein located on chloroplast envelop membrane execute cell death process involving caspase like proteases. Bax inhibitor (BI) has been shown to interact with cid thus preventing downstream proteolytic activities and PCD process. Similarly binding of lrg to Cid prevents release of Cid showing its antiapoptotic function.
Future Perspectives
Studies looking at the regulation of PCD in plants and particularly those evaluating the role of chloroplasts have not been that extensive and exhaustive to draw a clear cut picture of the sequence of events in plant PCD and the executioners involved and their particular role. Studies at best can be described as preliminary and exploratory which can indicate the events and complexity but not be able to provide the in depth comprehensive understanding of the involvement of chloroplast in the overall process. More studies are required to figure out the details of the pathways involved in plant PCD and particularly decipher the role of chloroplast. To increase the overall understanding of the PCD process in plants, future studies may be geared to gather more detailed information on:
Upstream of execution pathway, i.e., identification of stress signal receptors and the specific target molecules of ROS/RNS generated that is involved in further execution events.
Analyzing PCD degradome in chloroplast and mitochondria to identify targets of the proteases associated with/involved in PCD.
Emerging recent information suggest a possibility that PCD regulation process involve co—ordinated action of chloroplasts and mitochondria. Therefore analyses of cross talk between chloroplast, mitochondria and nucleus could be made a priority as it may provide a wealth of information to better understand the PCD in plants.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work has been executed with financial support from DBT, Govt. India as a part of Ramlingaswami fellowship program to BST.
References
- 1. Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell 2011; 147:742-58; PMID:22078876; http://dx.doi.org/ 10.1016/j.cell.2011.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gadjev I, Stone JM, Gechev TS. Programmed cell death in plants: new insights into redox regulation and the role of hydrogen peroxide. Int Rev Cell Mol Biol 2008; 270:87-144; PMID:19081535; http://dx.doi.org/ 10.1016/S1937-6448(08)01403-2 [DOI] [PubMed] [Google Scholar]
- 3. Watanabe N, Lam E. Programmed cell death in plants: apoptotic but not quite. In: Yin X-MaD Z., ed. Apoptosis: A Guide for Basic and Clinical Research: Humana Press, 2009: 301-24. [Google Scholar]
- 4. Zurbriggen MD, Carrillo N, Hajirezaei MR. ROS signaling in the hypersensitive response: when, where and what for? Plant Signaling Behav 2010; 5:393-6; PMID:20383072; http://dx.doi.org/ 10.4161/psb.5.4.10793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao C, Xing D, Li L, Zhang L. Implication of reactive oxygen species and mitochondrial dysfunction in the early stages of plant programmed cell death induced by ultraviolet-C overexposure. Planta 2008; 227:755-67; PMID:17972096; http://dx.doi.org/ 10.1007/s00425-007-0654-4 [DOI] [PubMed] [Google Scholar]
- 6. Lord CE, Gunawardena AH. Programmed cell death in C. elegans, mammals and plants. Eur J Cell Biol 2012; 91:603-13; PMID:22512890; http://dx.doi.org/ 10.1016/j.ejcb.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 7. Yao N, Greenberg JT. Arabidopsis Accelerated Cell Death2 modulates programmed cell death. Plant Cell 2006; 18:397-411; PMID:16387834; http://dx.doi.org/ 10.1105/tpc.105.036251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang L, Xing D. Methyl jasmonate induces production of reactive oxygen species and alterations in mitochondrial dynamics that precede photosynthetic dysfunction and subsequent cell death. Plant Cell Physiol 2008; 49:1092-111; PMID:18535010; http://dx.doi.org/ 10.1093/pcp/pcn086 [DOI] [PubMed] [Google Scholar]
- 9. Samuilov VD, Lagunova EM, Dzyubinskaya EV, Izyumov DS, Kiselevsky DB, Makarova YV. Involvement of chloroplasts in the programmed death of plant cells. Biochem Biokhimiia 2002; 67:627-34; PMID:12126469; http://dx.doi.org/ 10.1023/A:1016138003183 [DOI] [PubMed] [Google Scholar]
- 10. Samuilov VD, Lagunova EM, Kiselevsky DB, Dzyubinskaya EV, Makarova YV, Gusev MV. Participation of chloroplasts in plant apoptosis. Biosci Rep 2003; 23:103-17; PMID:14570380; http://dx.doi.org/ 10.1023/A:1025576307912 [DOI] [PubMed] [Google Scholar]
- 11. Pattanayak GK, Venkataramani S, Hortensteiner S, Kunz L, Christ B, Moulin M, Smith AG, Okamoto Y, Tamiaki H, Sugishima M, et al. Accelerated cell death 2 suppresses mitochondrial oxidative bursts and modulates cell death in Arabidopsis. Plant J: Cell Mol Biol 2012; 69:589-600; PMID:21988537; http://dx.doi.org/ 10.1111/j.1365-313X.2011.04814.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doyle SM, Diamond M, McCabe PF. Chloroplast and reactive oxygen species involvement in apoptotic-like programmed cell death in Arabidopsis suspension cultures. J Exp Bot 2010; 61:473-82; PMID:19933317; http://dx.doi.org/ 10.1093/jxb/erp320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gutierrez J, Gonzalez-Perez S, Garcia-Garcia F, Daly CT, Lorenzo O, Revuelta JL, McCabe PF, Arellano JB. Programmed cell death activated by Rose Bengal in Arabidopsis thaliana cell suspension cultures requires functional chloroplasts. J Exp Bot 2014; 65:3081-95; PMID:24723397; http://dx.doi.org/ 10.1093/jxb/eru151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim C, Meskauskiene R, Zhang S, Lee KP, Lakshmanan Ashok M, Blajecka K, Herrfurth C, Feussner I, Apel K. Chloroplasts of Arabidopsis are the source and a primary target of a plant-specific programmed cell death signaling pathway. Plant Cell 2012; 24:3026-39; PMID:22797473; http://dx.doi.org/ 10.1105/tpc.112.100479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang S, Apel K, Kim C. Singlet oxygen-mediated and EXECUTER-dependent signalling and acclimation of Arabidopsis thaliana exposed to light stress. Philosophical Trans Royal Soc London Series B, Biol Sci 2014; 369:20130227; PMID:24591714; http://dx.doi.org/ 10.1098/rstb.2013.0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 2006; 141:391-6; PMID:16760493; http://dx.doi.org/ 10.1104/pp.106.082040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tripathy BC, Oelmuller R. Reactive oxygen species generation and signaling in plants. Plant Signaling Behav 2012; 7:1621-33; PMID:23072988; http://dx.doi.org/ 10.4161/psb.22455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Breusegem F, Dat JF. Reactive oxygen species in plant cell death. Plant Physiol 2006; 141:384-90; PMID:16760492; http://dx.doi.org/ 10.1104/pp.106.078295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xing F, Li Z, Sun A, Xing D. Reactive oxygen species promote chloroplast dysfunction and salicylic acid accumulation in fumonisin B1-induced cell death. FEBS Lett 2013; 587:2164-72; PMID:23711368; http://dx.doi.org/ 10.1016/j.febslet.2013.05.034 [DOI] [PubMed] [Google Scholar]
- 20. Zapata JM, Guera A, Esteban-Carrasco A, Martin M, Sabater B. Chloroplasts regulate leaf senescence: delayed senescence in transgenic ndhF-defective tobacco. Cell Death Differ 2005; 12:1277-84; PMID:15905880; http://dx.doi.org/ 10.1038/sj.cdd.4401657 [DOI] [PubMed] [Google Scholar]
- 21. Landoni M, De Francesco A, Bellatti S, Delledonne M, Ferrarini A, Venturini L, Pilu R, Bononi M, Tonelli C. A mutation in the FZL gene of Arabidopsis causing alteration in chloroplast morphology results in a lesion mimic phenotype. J Exp Bot 2013; 64:4313-28; PMID:23963675; http://dx.doi.org/ 10.1093/jxb/ert237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vergeiner C, Banala S, Krautler B. Chlorophyll breakdown in senescent banana leaves: catabolism reprogrammed for biosynthesis of persistent blue fluorescent tetrapyrroles. Chemistry 2013; 19:12294-305; PMID:23946204; http://dx.doi.org/ 10.1002/chem.201301907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sakuraba Y, Kim D, Kim YS, Hortensteiner S, Paek NC. Arabidopsis STAYGREEN-LIKE (SGRL) promotes abiotic stress-induced leaf yellowing during vegetative growth. FEBS Lett 2014; 588:3830-7; PMID:25261252; http://dx.doi.org/ 10.1016/j.febslet.2014.09.018 [DOI] [PubMed] [Google Scholar]
- 24. Chandra-Shekara AC, Gupte M, Navarre D, Raina S, Raina R, Klessig D, Kachroo P. Light-dependent hypersensitive response and resistance signaling against turnip crinkle virus in Arabidopsis. Plant J: Cell Mol Biology 2006; 45:320-34; PMID:16412080; http://dx.doi.org/ 10.1111/j.1365-313X.2005.02618.x [DOI] [PubMed] [Google Scholar]
- 25. Mur LA, Aubry S, Mondhe M, Kingston-Smith A, Gallagher J, Timms-Taravella E, James C, Papp I, Hörtensteiner S, Thomas H, et al. Accumulation of chlorophyll catabolites photosensitizes the hypersensitive response elicited by Pseudomonas syringae in Arabidopsis. New Phytol 2010; 188:161-74; PMID:20704660; http://dx.doi.org/ 10.1111/j.1469-8137.2010.03377.x [DOI] [PubMed] [Google Scholar]
- 26. Yoshimura H, Okamoto S, Tsumuraya Y, Ohmori M. Group 3 sigma factor gene, sigJ, a key regulator of desiccation tolerance, regulates the synthesis of extracellular polysaccharide in cyanobacterium Anabaena sp. strain PCC 7120. DNA Res: Int J Rapid Pub Rep Genes Genomes 2007; 14:13-24; PMID:17376888; http://dx.doi.org/ 10.1093/dnares/dsm003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schelbert S, Aubry S, Burla B, Agne B, Kessler F, Krupinska K, Hörtensteiner S. Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell 2009; 21:767-85; PMID:19304936; http://dx.doi.org/ 10.1105/tpc.108.064089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Christ B, Egert A, Sussenbacher I, Krautler B, Bartels D, Peters S, Hörtensteiner S. Water deficit induces chlorophyll degradation via the ‘PAO/phyllobilin’ pathway in leaves of homoio- (Craterostigma pumilum) and poikilochlorophyllous (Xerophyta viscosa) resurrection plants. Plant, Cell Environ 2014; 37:2521-31; PMID:24697723; http://dx.doi.org/ 10.1111/pce.12308 [DOI] [PubMed] [Google Scholar]
- 29. Bonneau L, Ge Y, Drury GE, Gallois P. What happened to plant caspases? J Exp Bot 2008; 59:491-9; PMID:18272922; http://dx.doi.org/ 10.1093/jxb/erm352 [DOI] [PubMed] [Google Scholar]
- 30. Reape TJ, McCabe PF. Apoptotic-like regulation of programmed cell death in plants. Apoptosis: Int J Programmed Cell Death 2010; 15:249-56; PMID:20094801; http://dx.doi.org/ 10.1007/s10495-009-0447-2 [DOI] [PubMed] [Google Scholar]
- 31. Watanabe N, Lam E. Recent advance in the study of caspase-like proteases and Bax inhibitor-1 in plants: their possible roles as regulator of programmed cell death. Mol Plant Pathol 2004; 5:65-70; PMID:20565583; http://dx.doi.org/ 10.1111/j.1364-3703.2004.00206.x [DOI] [PubMed] [Google Scholar]
- 32. Choi CJ, Berges JA. New types of metacaspases in phytoplankton reveal diverse origins of cell death proteases. Cell Death Dis 2013; 4:e490; PMID:23412383; http://dx.doi.org/ 10.1038/cddis.2013.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bozhkov PV, Suarez MF, Filonova LH, Daniel G, Zamyatnin AA, Jr, Rodriguez-Nieto S, Zhivotovsky B, Smertenko A. Cysteine protease mcII-Pa executes programmed cell death during plant embryogenesis. Proc Nat Acad Sci U S A 2005; 102:14463-8; PMID:16183741; http://dx.doi.org/ 10.1073/pnas.0506948102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van der Hoorn RA. Plant proteases: from phenotypes to molecular mechanisms. Annu Rev Plant Biol 2008; 59:191-223; PMID:18257708; http://dx.doi.org/ 10.1146/annurev.arplant.59.032607.092835 [DOI] [PubMed] [Google Scholar]
- 35. Kuroyanagi M, Yamada K, Hatsugai N, Kondo M, Nishimura M, Hara-Nishimura I. Vacuolar processing enzyme is essential for mycotoxin-induced cell death in Arabidopsis thaliana. J Biol Chem 2005; 280:32914-20; PMID:16043487; http://dx.doi.org/ 10.1074/jbc.M504476200 [DOI] [PubMed] [Google Scholar]
- 36. Rojo E, Martin R, Carter C, Zouhar J, Pan S, Plotnikova J, Jin H, Paneque M, Sánchez-Serrano JJ, Baker B, et al. VPEgamma exhibits a caspase-like activity that contributes to defense against pathogens. Curr Biol: CB 2004; 14:1897-906; PMID:15530390; http://dx.doi.org/ 10.1016/j.cub.2004.09.056 [DOI] [PubMed] [Google Scholar]
- 37. Estelle M. Proteases and cellular regulation in plants. Curr Opin Plant Biol 2001; 4:254-60; PMID:11312137; http://dx.doi.org/ 10.1016/S1369-5266(00)00169-2 [DOI] [PubMed] [Google Scholar]
- 38. Garcia-Lorenzo M, Sjodin A, Jansson S, Funk C. Protease gene families in Populus and Arabidopsis. BMC Plant Biol 2006; 6:30; PMID:17181860; http://dx.doi.org/ 10.1186/1471-2229-6-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adam Z, Rudella A, van Wijk KJ. Recent advances in the study of Clp, FtsH and other proteases located in chloroplasts. Curr Opin Plant Biol 2006; 9:234-40; PMID:16603408; http://dx.doi.org/ 10.1016/j.pbi.2006.03.010 [DOI] [PubMed] [Google Scholar]
- 40. Huesgen PF, Scholz P, Adamska I. The serine protease HhoA from Synechocystis sp. strain PCC 6803: substrate specificity and formation of a hexameric complex are regulated by the PDZ domain. J Bacteriol 2007; 189:6611-8; PMID:17616590; http://dx.doi.org/ 10.1128/JB.00883-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clausen T, Southan C, Ehrmann M. The HtrA family of proteases: implications for protein composition and cell fate. Mol Cell 2002; 10:443-55; PMID:12408815; http://dx.doi.org/ 10.1016/S1097-2765(02)00658-5 [DOI] [PubMed] [Google Scholar]
- 42. Skorko-Glonek J, Wawrzynow A, Krzewski K, Kurpierz K, Lipinska B. Site-directed mutagenesis of the HtrA (DegP) serine protease, whose proteolytic activity is indispensable for Escherichia coli survival at elevated temperatures. Gene 1995; 163:47-52; PMID:7557477; http://dx.doi.org/ 10.1016/0378-1119(95)00406-V [DOI] [PubMed] [Google Scholar]
- 43. Carrion CA, Costa ML, Martinez DE, Mohr C, Humbeck K, Guiamet JJ. In vivo inhibition of cysteine proteases provides evidence for the involvement of ‘senescence-associated vacuoles’ in chloroplast protein degradation during dark-induced senescence of tobacco leaves. J Exp Bot 2013; 64:4967-80; PMID:24106291; http://dx.doi.org/ 10.1093/jxb/ert285 [DOI] [PubMed] [Google Scholar]
- 44. Castillo-Olamendi L, Bravo-Garcia A, Moran J., Rocha-Sosa M., Porta H. AtMCP1b, a chloroplast-localised metacaspase, is induced in vascular tissue after wounding or pathogen infection. Funct Plant Biol 2007; 34:1061-71; http://dx.doi.org/ 10.1071/FP07153 [DOI] [PubMed] [Google Scholar]
- 45. Dietrich RA, Richberg MH, Schmidt R, Dean C, Dangl JL. A novel zinc finger protein is encoded by the Arabidopsis LSD1 gene and functions as a negative regulator of plant cell death. Cell 1997; 88:685-94; PMID:9054508; http://dx.doi.org/ 10.1016/S0092-8674(00)81911-X [DOI] [PubMed] [Google Scholar]
- 46. Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet 2009; 43:95-118; PMID:19659442; http://dx.doi.org/ 10.1146/annurev-genet-102108-134850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol 2000; 10:369-77; PMID:10932094; http://dx.doi.org/ 10.1016/S0962-8924(00)01803-1 [DOI] [PubMed] [Google Scholar]
- 48. Goodsell DS. The molecular perspective: cytochrome C and apoptosis. The Oncologist 2004; 9:226-7; PMID:15047927; http://dx.doi.org/ 10.1634/theoncologist.9-2-226 [DOI] [PubMed] [Google Scholar]
- 49. Peters JS, Chin C. Evidence for cytochrome f involvement in eggplant cell death induced by palmitoleic acid. Cell Death Differ 2005; 12:405-7; PMID:15706353; http://dx.doi.org/ 10.1038/sj.cdd.4401551 [DOI] [PubMed] [Google Scholar]
- 50. Zuppini A, Gerotto C, Moscatiello R, Bergantino E, Baldan B. Chlorella saccharophila cytochrome f and its involvement in the heat shock response. J Exp Bot 2009; 60:4189-200; PMID:19773387; http://dx.doi.org/ 10.1093/jxb/erp264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang H, Zhu X, Li H, Cui J, Liu C, Chen X, Zhang W. Induction of caspase-3-like activity in rice following release of cytochrome-f from the chloroplast and subsequent interaction with the ubiquitin-proteasome system. Sci Rep 2014; 4:5989; PMID:25103621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vartapetian AB, Tuzhikov AI, Chichkova NV, Taliansky M, Wolpert TJ. A plant alternative to animal caspases: subtilisin-like proteases. Cell Death Differ 2011; 18:1289-97; PMID:21546909; http://dx.doi.org/ 10.1038/cdd.2011.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen S, Dickman MB. Bcl-2 family members localize to tobacco chloroplasts and inhibit programmed cell death induced by chloroplast-targeted herbicides. J Exp Bot 2004; 55:2617-23; PMID:15475374; http://dx.doi.org/ 10.1093/jxb/erh275 [DOI] [PubMed] [Google Scholar]
- 54. Lee KP, Kim C, Landgraf F, Apel K. EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc Nat Acad Sci U S A 2007; 104:10270-5; PMID:17540731; http://dx.doi.org/ 10.1073/pnas.0702061104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dyall SD, Brown MT, Johnson PJ. Ancient invasions: from endosymbionts to organelles. Science 2004; 304:253-7; PMID:15073369; http://dx.doi.org/ 10.1126/science.1094884 [DOI] [PubMed] [Google Scholar]
- 56. Wang J, Bayles KW. Programmed cell death in plants: lessons from bacteria? Trends Plant Sci 2013; 18:133-9; PMID:23083702; http://dx.doi.org/ 10.1016/j.tplants.2012.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang IN, Smith DL, Young R. Holins: the protein clocks of bacteriophage infections. Annu Rev Microbiol 2000; 54:799-825; PMID:11018145; http://dx.doi.org/ 10.1146/annurev.micro.54.1.799 [DOI] [PubMed] [Google Scholar]
- 58. Bayles KW. The biological role of death and lysis in biofilm development. Nat Rev Microbiol 2007; 5:721-6; PMID:17694072; http://dx.doi.org/ 10.1038/nrmicro1743 [DOI] [PubMed] [Google Scholar]
- 59. Ranjit DK, Endres JL, Bayles KW. Staphylococcus aureus CidA and LrgA proteins exhibit holin-like properties. J Bacteriol 2011; 193:2468-76; PMID:21421752; http://dx.doi.org/ 10.1128/JB.01545-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang Y, Jin H, Chen Y, Lin W, Wang C, Chen Z, Han N, Bian H, Zhu M, Wang J. A chloroplast envelope membrane protein containing a putative LrgB domain related to the control of bacterial death and lysis is required for chloroplast development in Arabidopsis thaliana. New Phytol 2012; 193:81-95; PMID:21916894; http://dx.doi.org/ 10.1111/j.1469-8137.2011.03867.x [DOI] [PubMed] [Google Scholar]
- 61. Yamaguchi M, Takechi K, Myouga F, Imura S, Sato H, Takio S, Shinozaki K, Takano H. Loss of the plastid envelope protein AtLrgB causes spontaneous chlorotic cell death in Arabidopsis thaliana. Plant Cell Physiol 2012; 53:125-34; PMID:22180599; http://dx.doi.org/ 10.1093/pcp/pcr180 [DOI] [PubMed] [Google Scholar]
- 62. Gechev T, Gadjev I, Van Breusegem F, Inze D, Dukiandjiev S, Toneva V, Minkov I. Hydrogen peroxide protects tobacco from oxidative stress by inducing a set of antioxidant enzymes. Cell Mol Life Sci: CMLS 2002; 59:708-14; PMID:12022476; http://dx.doi.org/ 10.1007/s00018-002-8459-x [DOI] [PMC free article] [PubMed] [Google Scholar]


