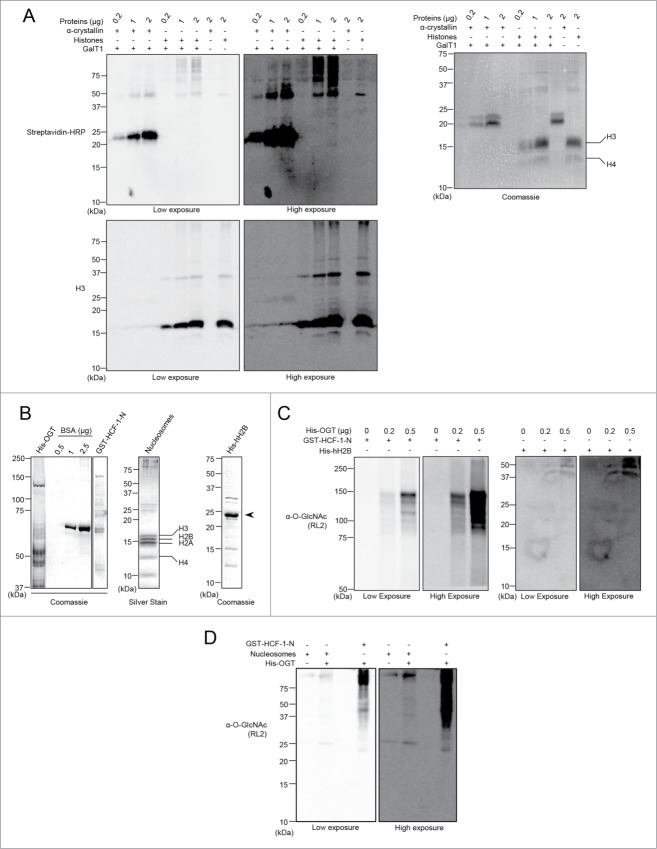
Figure 7.
(See previous page). Histones are not modified by Click-iT biotin-alkyl chemistry or by in vitro OGT-mediated O-GlcNAcylation. (A) The poorly O-GlcNAcylated α-crystallin is detected with the Click-iT biotin-alkyl chemistry but not histones. HeLa cells were harvested and acid extraction followed by acetone precipitation was performed to purify histones. The GalNAz labeling reaction was carried out for a minimum of 16 hours and the biotin-alkyl reaction was performed in the presence or absence of the GalT1 enzyme. (Left panel) O-GlcNAcylation was analyzed by blotting with streptavidin-HRP. (Right panel) Coomassie brilliant blue staining shows the indicated amounts of used purified proteins. (B–D) Histones are not O-GlcNAcylated in vitro by OGT. (B) Purified proteins analyzed by Coomassie brilliant blue or silver staining as indicated. (Left panel) Relative quantification of purified recombinant His-OGT and GST-HCF-1-N to known amounts of BSA. (Middle and Right panels) Integrity of Flag-H2A-purified mammalian nucleosomes and recombinant His-hH2B. (C) In vitro O-GlcNAcylation reaction of the purified recombinant His-hH2B. GST-HCF-1-N and recombinant His-hH2B were incubated with increasing amounts of recombinant His-OGT for 4 hours. O-GlcNAcylation was detected by western blotting using the anti-O-GlcNAc antibody (RL2). (D) In vitro O-GlcNAcylation reaction of the purified mammalian nucleosomes by OGT. GST-HCF-1-N and nucleosomes were incubated in the absence or presence of His-OGT for 11 hours. The O-GlcNAcylation was detected as in (C). kDa: Molecular weight marker in kilodalton.