Abstract
Plants are protected from microbial infection by a robust immune system. Two of the earliest responses mediated by surface-localized immune receptors include an increase in cytosolic calcium (Ca2+) and a burst of apoplastic reactive oxygen species (ROS). The Arabidopsis plasma membrane-associated cytoplasmic kinase BIK1 is an immediate convergent substrate of multiple surface-localized immune receptors that is genetically required for the PAMP-induced Ca2+ burst and directly regulates ROS production catalyzed by the NADPH oxidase RBOHD. We recently demonstrated that Arabidopsis plants maintain an optimal level of BIK1 through a process of continuous degradation regulated by the Ca2+-dependent protein kinase CPK28. cpk28 mutants accumulate more BIK1 protein and display enhanced immune signaling, while plants over-expressing CPK28 accumulate less BIK1 protein and display impaired immune signaling. Here, we show that CPK28 additionally contributes to the PAMP-induced Ca2+ burst, supporting its role as a negative regulator of BIK1.
Keywords: arabidopsis, BIK1, CPK28, calcium, PAMP-triggered immunity, phosphorylation, signal transduction
Plants perceive pathogen- and damage-associated molecular patterns (PAMPs or DAMPs) through pattern recognition receptors (PRRs) located at the cell surface. All known plant PRRs are plasma membrane-resident receptor kinases or receptor-like proteins that bind ligands outside the cell and transduce the signal inside the cell by forming larger complexes with several additional proteins.1 One of the earliest responses following PAMP/DAMP perception is an increase in cytosolic calcium (Ca2+), shortly followed by a burst of apoplastic reactive oxygen species (ROS) mediated in Arabidopsis by the NADPH oxidase RBOHD.1 Downstream immune signal transduction is largely achieved by phosphorylation relays mediated by different sub-classes of kinases, including mitogen-activated and Ca2+-dependent protein kinases (MAPKs and CDPKs), leading to transcriptional reprogramming.2 Together, these responses constitute PAMP-triggered immunity (PTI), which is thought to be sufficient to protect plants against most microbes.3
The plasma membrane-associated cytoplasmic kinase BIK1 is an immediate convergent substrate of several different PRRs, including FLS2 (which binds bacterial flagellin), EFR (which binds bacterial elongation factor Tu), PEPR1 (which binds endogenous AtPep peptides), and CERK1 (which binds fungal chitin), and is a key component of the plant immune system.4-8 Moreover, BIK1 is also a substrate of BAK1, an important co-receptor kinase that interacts with and phosphorylates several immune receptors and is required to achieve their full signaling potential.9-14 Importantly, plants lacking functional BIK1 and related proteins such as PBL1 (bik1 pbl1 mutants) are strongly impaired in PTI signaling and are more susceptible to bacterial and fungal pathogens.6,8,15,16
Recent work demonstrated that BIK1 interacts with and phosphorylates the NADPH oxidase RBOHD to enable PAMP-triggered ROS production and consequent stomatal immunity.17,18 Although BIK1 phosphorylates RBOHD in a Ca2+-independent manner,17,18 signaling through BIK1 is required for an appropriate PAMP-induced cytosolic Ca2+ burst.18,19 Fluxes in Ca2+ can be monitored in living cells using aequorin, a bioluminescent Ca2+-binding protein from jellyfish. Aequorin is a holoprotein composed of the apoenzyme apoaequorin and the luciferin coelenterazine. Thus, if supplied with exogenous coelenterazine, active aequorin can be reconstituted in plant cells transgenically expressing apoaequorin.20 We were interested to test if BIK1 and PBL1 are required for the Ca2+ burst activated through receptors other than FLS2. We therefore crossed bik1 pbl1 to a wild-type line expressing cytosolic apoaequorin under the control of the cauliflower mosaic virus 35S promoter (Col-0/pMAQ2),21 and examined the Ca2+ burst in homozygous bik1 pbl1/pMAQ2 plants after treatment with several elicitors. In agreement with what was previously shown for flg22 treatment in bik1 mutants,18 we found that the Ca2+ burst was severely impaired in bik1 pbl1 mutants after treatment with flg22, elf18, AtPep1, or chitin (Fig. 1). During the preparation of this manuscript, another study also reported that bik1 pbl1 mutants are compromised in the flg22-, elf18-, and AtPep1-triggered Ca2+ burst.19 These results are in support of BIK1 and related proteins functioning as key signaling integrators immediately downstream of multiple PRRs.
Figure 1.
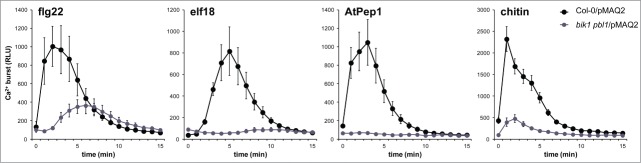
The PAMP-triggered Ca2+ burst is altered in bik1 pbl1 mutants. To normalize expression of the apoaequorin transgene, bik1 pbl1/pMAQ2 was generated by crossing. Leaf discs were harvested from individual 4-week old plants grown on soil in short-day chambers as described previously29 and were floated in 12.5 μM coelenterazine (LUX Innovate) overnight in the dark prior to treatment with 100 nM flg22 (EZBiolab), 100 nM elf18 (EZBiolab), 1 μM AtPep1 (EZBiolab), or 100 μg/mL chitin (Sigma Aldrich). The induced cytosolic Ca2+ burst was measured as relative light units (RLU) using a charge-coupled device camera (Photek Ltd.) as described previously.17 Values are means ± standard deviation (n=8). Experiments were performed at least 3 times with similar results.
Although very little is known about the molecular mechanisms underlying the PAMP/DAMP-induced Ca2+ burst, it is clear that changes in intracellular [Ca2+] are prerequisite to many downstream immune responses.22 Ca2+ signals are thought to be decoded into cellular responses by Ca2+-binding proteins such as calmodulins, calcineurin B-like proteins, and/or CDPKs.22 Several Ca2+-binding proteins including CDPKs are involved in biotic and abiotic stress responses.23 For example, western blot and in-gel kinase assays demonstrated that PAMP perception results in phosphorylation and activation of several CDPKs in Arabidopsis.24 In particular, it was recently shown that PAMP treatment results in hyper-phosphorylation and activation of CPK5 and CPK6,25 which are involved in PTI signaling.24 Notably, in addition to the critical Ca2+-independent regulation by BIK1,17,18 the NADPH oxidase RBOHD is an in vitro and in vivo phosphorylation target of CPK5 and related Ca2+-dependent protein kinases CPK4, CPK6, and CPK11.17,25 Furthermore, RBOHD itself contains 2 cytoplasmic Ca2+-binding EF-hands26 and PAMP-triggered ROS production is Ca2+-dependent,17,27,28 indicating a very tight interplay between ROS and Ca2+ signaling during PTI.
Adding to the complexity of Ca2+-dependent and Ca2+-independent mechanisms underlying immune responses, we recently demonstrated that the Ca2+-dependent protein kinase CPK28 regulates BIK1 turnover to buffer immune signaling.29 Plants over-expressing CPK28 (CPK28-OE1) accumulate lower levels of BIK1 protein compared to wild-type plants and are strongly impaired in PAMP-triggered responses and anti-bacterial immunity.29 Biochemical characterization demonstrated that CPK28 kinase activity is Ca2+-dependent.30 However, the effect on BIK1 turnover occurs in both the presence and absence of pathogen elicitors,29 suggesting that CPK28 is active even in the absence of PAMP/DAMP-induced Ca2+ fluxes. To test if CPK28 activity is altered by PAMP treatment, we extracted total protein from wild-type and functionally complemented cpk28-1/35S:CPK28-YFP plants treated with water or flg22 and conducted in-gel kinase assays using radioactive phosphate (Fig. 2A). We additionally transfected protoplasts harvested from cpk28-1 plants with 35S:CPK28-YFP and similarly conducted kinase assays treated with or without flg22 (Fig. 2B). In both seedlings and protoplasts, CPK28 showed constitutive (auto-) phosphorylating activity in untreated and treated samples. This is in contrast to CPK5 and CPK6, which become strongly phosphorylated after PAMP treatment and thus activated.25 While more accurate methods are required to detail and quantify site-specific phosphorylation changes following PAMP treatment, these results suggest that basal cellular levels of Ca2+ are already sufficient for CPK28 activation in vivo. Thus, CPK28 activity may not be altered in response to PAMP perception, or additional mechanisms may be involved beyond PAMP-induced Ca2+-binding.
Figure 2.
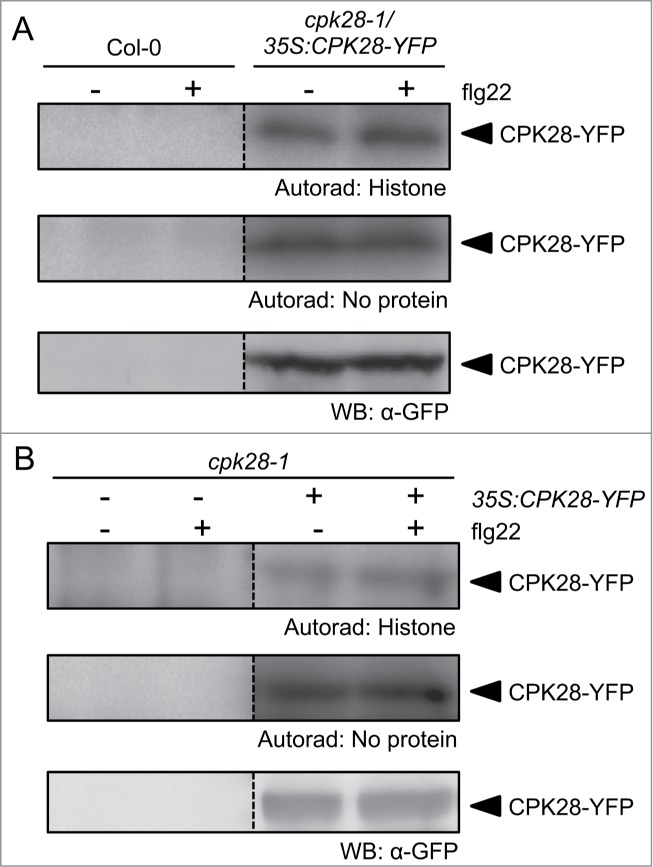
CPK28 is active before and after PAMP treatment. (A) Wild-type Col-0 or functionally complemented cpk28-1/35S:CPK28-YFP seedlings were grown in liquid MS media for 2 weeks prior to treatment with water or 100 nM flg22 for 10 minutes as described previously.29 (B) Protoplasts were prepared from 5-week-old soil-grown cpk28-1 plants and transfected with 35S:CPK28-YFP as described previously.25 Protoplasts were treated 14 hours after transfection with water or 100 nM flg22 for 10 minutes. Total protein extracts were separated by SDS-PAGE and subject to histone in-gel kinase assays (autoradiography), as described previously,25 or western blot (WB: α-GFP), as described previously.30 Results are presented from the same blots; some lanes were removed for clarity as indicated by dashed lines. These assays were repeated at least 3 times with similar results.
Our previous work demonstrated that CPK28 negatively regulates BIK1 and is therefore genetically upstream of the PAMP-triggered Ca2+ burst. This led us to hypothesize that the PAMP-induced Ca2+ burst would be impaired in plants overexpressing CPK28. To test this, we crossed CPK28-OE1 with Col-0/pMAQ2 and found that the cytosolic Ca2+ burst was indeed strongly reduced in homozygous CPK28-OE1/pMAQ2 plants after treatment with flg22, elf18, AtPep1, or chitin (Fig. 3), similar to what we observed in bik1 pbl1 plants (Fig. 1). These results suggest that CPK28 resides in an activated state prior to the PAMP-induced Ca2+ burst and further support its role as a negative regulator of BIK1.
Figure 3.
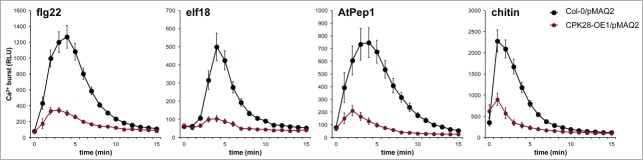
The PAMP-triggered Ca2+ burst is altered by CPK28 over-expression. The PAMP-triggered Ca2+ burst in Col-0/pMAQ2 compared to CPK28-OE1/ Experiments were conducted as described in Figure 1 and were performed at least 3 times with similar results. Values are means ± standard deviation (n = 8).
In accordance with CPK28 regulating BIK1 turnover, loss-of-function cpk28-1 mutants accumulate higher levels of BIK1 protein compared to wild-type plants and display strongly enhanced PAMP-triggered responses.29 To test the effect of enhanced accumulation of BIK1 on the PAMP-triggered Ca2+ burst, we generated a homozygous cpk28-1/pMAQ2 line through crossing. We found that treatment with flg22, elf18, AtPep1, or chitin resulted in an enhanced cytosolic Ca2+ burst in cpk28-1/pMAQ2 compared to the wild-type (Fig. 4). This supports our previous findings that increased BIK1 accumulation results in increased PAMP-triggered signaling including ROS production.29
Figure 4.
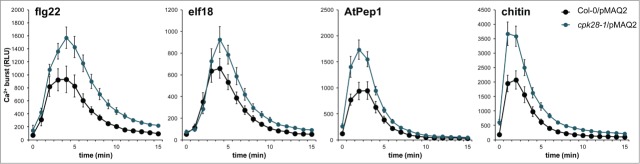
The PAMP-triggered Ca2+ burst is altered in cpk28-1. Experiments were conducted as described in Figure 1 and were performed at least 3 times with similar results. Values are means ± standard deviation (n=8).
Secondary messengers such as Ca2+ and ROS are rapidly and transiently induced following PAMP perception and contribute to plant immune signaling. Here we show that CPK28 is genetically upstream of the PAMP-induced Ca2+ burst, supporting our previous findings that CPK28 is a negative regulator of BIK1. Several immune receptors rely on the signaling integrator BIK1 to relay microbial perception at the cell surface to intracellular responses.5,6,8 While BIK1 is positively regulated by phosphorylation mediated by PRRs,5-8 our previous work suggests that BIK1 is also negatively regulated by phosphorylation mediated by CPK28.29 This raises the intriguing possibility that residue-specific or quantitative differences contribute to the 2 states. We propose that CPK28-mediated phosphorylation of BIK1 influences its ubiquitination by a currently unknown E3 ligase, resulting in its degradation. Identification of ubiquitination and CPK28-mediated phosphorylation sites on BIK1 will allow us to directly test this hypothesis. Moreover, future work to unravel the molecular mechanisms underlying the PAMP-induced Ca2+ burst and subsequent signaling events will enhance our understanding of this important branch of the plant immune system.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Yasuhiro Kadota for helpful comments on the manuscript and all members of the Zipfel laboratory for discussion. We thank Gerald Berkowitz and Jian-Min Zhou for generously sharing Col-0/pMAQ2 and bik1 pbl1 seeds, respectively. The John Innes Center horticultural staff is acknowledged for growing plants and maintaining growth facilities.
Funding
This research was funded by grants from The Gatsby Foundation and The European Research Council (ERC) to CZ and the Deutsche Forschungsgemeinschaft (DFG) to TR. JM was the recipient of a Long-Term Fellowship from the European Molecular Biology Organization (EMBO) and is currently supported by an Anniversary Future Leader Fellowship from the UK Biotechnology and Biological Sciences Research Council (BBSRC).
References
- 1. Macho AP, Zipfel C. Plant PRRs and the activation of innate immune signaling. Mol Cell 2014; 54:263-72; PMID:24766890; http://dx.doi.org/ 10.1016/j.molcel.2014.03.028 [DOI] [PubMed] [Google Scholar]
- 2. Tena G, Boudsocq M, Sheen J. Protein kinase signaling networks in plant innate immunity. Curr Opin Plant Biol 2011; 14:519-29; PMID:21704551; http://dx.doi.org/ 10.1016/j.pbi.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 2009; 60:379-406; PMID:19400727; http://dx.doi.org/ 10.1146/annurev.arplant.57.032905.105346 [DOI] [PubMed] [Google Scholar]
- 4. Lin W, Lu D, Gao X, Jiang S, Ma X, Wang Z, Mengiste T, He P, Shan L. Inverse modulation of plant immune and brassinosteroid signaling pathways by the receptor-like cytoplasmic kinase BIK1. Proc Natl Acad Sci U S A 2013; 110:12114-9; PMID:23818580; http://dx.doi.org/ 10.1073/pnas.1302154110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu Z, Wu Y, Yang F, Zhang Y, Chen S, Xie Q, Tian X, Zhou JM. BIK1 interacts with PEPRs to mediate ethylene-induced immunity. Proc Natl Acad Sci U S A 2013; 110:6205-10; PMID:23431184; http://dx.doi.org/ 10.1073/pnas.1215543110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu D, Wu S, Gao X, Zhang Y, Shan L, He P. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc Natl Acad Sci U S A 2010; 107:496-501; PMID:20018686; http://dx.doi.org/ 10.1073/pnas.0909705107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu J, Wei X, Yan L, Liu D, Ma Y, Guo Y, Peng C, Zhou H, Yang C, Lou Z, et al. . Identification and functional analysis of phosphorylation residues of the Arabidopsis BOTRYTIS-INDUCED KINASE1. Protein Cell 2013; 4:771-81; PMID:24104392; http://dx.doi.org/ 10.1007/s13238-013-3053-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang J, Li W, Xiang T, Liu Z, Laluk K, Ding X, Zou Y, Gao M, Zhang X, Chen S, et al. . Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 2010; 7:290-301; PMID:20413097; http://dx.doi.org/ 10.1016/j.chom.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 9. Sun Y, Li L, Macho AP, Han Z, Hu Z, Zipfel C, Zhou JM, Chai J. Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 2013; 342:624-8; PMID:24114786; http://dx.doi.org/ 10.1126/science.1243825 [DOI] [PubMed] [Google Scholar]
- 10. Schwessinger B, Roux M, Kadota Y, Ntoukakis V, Sklenar J, Jones A, Zipfel C. Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet 2011; 7:e1002046; PMID:21593986; http://dx.doi.org/ 10.1371/journal.pgen.1002046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schulze B, Mentzel T, Jehle AK, Mueller K, Beeler S, Boller T, Felix G, Chinchilla D. Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J Biol Chem 2010; 285:9444-51; PMID:20103591; http://dx.doi.org/ 10.1074/jbc.M109.096842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tor M, de Vries S, Zipfel C. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 2011; 23:2440-55; PMID:21693696; http://dx.doi.org/ 10.1105/tpc.111.084301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci U S A 2007; 104:12217-22; PMID:17626179; http://dx.doi.org/ 10.1073/pnas.0705306104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nurnberger T, Jones JD, Felix G, Boller T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 2007; 448:497-500; PMID:17625569; http://dx.doi.org/ 10.1038/nature05999 [DOI] [PubMed] [Google Scholar]
- 15. Veronese P, Nakagami H, Bluhm B, Abuqamar S, Chen X, Salmeron J, Dietrich RA, Hirt H, Mengiste T. The membrane-anchored BOTRYTIS-INDUCED KINASE1 plays distinct roles in Arabidopsis resistance to necrotrophic and biotrophic pathogens. Plant Cell 2006; 18:257-73; PMID:16339855; http://dx.doi.org/ 10.1105/tpc.105.035576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng F, Yang F, Rong W, Wu X, Zhang J, Chen S, He C, Zhou JM. A Xanthomonas uridine 5'-monophosphate transferase inhibits plant immune kinases. Nature 2012; 485:114-8; PMID:22504181; http://dx.doi.org/ 10.1038/nature10962 [DOI] [PubMed] [Google Scholar]
- 17. Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, Ntoukakis V, Jones JD, Shirasu K, Menke F, Jones A, et al. . Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol Cell 2014; 54:43-55; PMID:24630626; http://dx.doi.org/ 10.1016/j.molcel.2014.02.021 [DOI] [PubMed] [Google Scholar]
- 18. Li L, Li M, Yu L, Zhou Z, Liang X, Liu Z, Cai G, Gao L, Zhang X, Wang Y, et al. . The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 2014; 15:329-38; PMID:24629339; http://dx.doi.org/ 10.1016/j.chom.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 19. Ranf S, Eschen-Lippold L, Frohlich K, Westphal L, Scheel D, Lee J. Microbe-associated molecular pattern-induced calcium signaling requires the receptor-like cytoplasmic kinases, PBL1 and BIK1. BMC Plant Biol 2014; 14:374; PMID:25522736; http://dx.doi.org/ 10.1186/s12870-014-0374-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knight MR, Campbell AK, Smith SM, Trewavas AJ. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 1991; 352:524-6; PMID:1865907; http://dx.doi.org/ 10.1038/352524a0 [DOI] [PubMed] [Google Scholar]
- 21. Knight H, Trewavas AJ, Knight MR. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 1996; 8:489-503; PMID:8721751; http://dx.doi.org/ 10.1105/tpc.8.3.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seybold H, Trempel F, Ranf S, Scheel D, Romeis T, Lee J. Calcium signalling in plant immune response: from pattern recognition receptors to calcium decoding mechanisms. New Phytol 2014; 204:782-90; PMID:25539002; http://dx.doi.org/ 10.1111/nph.13031 [DOI] [PubMed] [Google Scholar]
- 23. Schulz P, Herde M, Romeis T. Calcium-dependent protein kinases: hubs in plant stress signaling and development. Plant Physiol 2014; 163:523-30; PMID:24014579; http://dx.doi.org/ 10.1104/pp.113.222539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, He P, Bush J, Cheng SH, Sheen J. Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 2010; 464:418-22; PMID:20164835; http://dx.doi.org/ 10.1038/nature08794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dubiella U, Seybold H, Durian G, Komander E, Lassig R, Witte CP, Schulze WX, Romeis T. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc Natl Acad Sci U S A 2013; 110:8744-9; PMID:23650383; http://dx.doi.org/ 10.1073/pnas.1221294110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, Hishinuma H, Senzaki E, Yamagoe S, Nagata K, et al. . Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem 2008; 283:8885-92; PMID:18218618; http://dx.doi.org/ 10.1074/jbc.M708106200 [DOI] [PubMed] [Google Scholar]
- 27. Segonzac C, Feike D, Gimenez-Ibanez S, Hann DR, Zipfel C, Rathjen JP. Hierarchy and roles of pathogen-associated molecular pattern-induced responses in Nicotiana benthamiana. Plant Physiol 2011; 156:687-99; PMID:21478366; http://dx.doi.org/ 10.1104/pp.110.171249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ranf S, Eschen-Lippold L, Pecher P, Lee J, Scheel D. Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage-associated molecular patterns. Plant J 2011; 68:100-13; PMID:21668535; http://dx.doi.org/ 10.1111/j.1365-313X.2011.04671.x [DOI] [PubMed] [Google Scholar]
- 29. Monaghan J, Matschi S, Shorinola O, Rovenich H, Matei A, Segonzac C, Gro-Malinovsky F, Rathjen J, MacLean D, Romeis T, et al. . The calcium dependent protein kinase CPK28 buffers plant immunity and regulates BIK1 turnover. Cell Host Microbe 2014; 16:605-15; PMID:25525792; http://dx.doi.org/ 10.1016/j.chom.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 30. Matschi S, Werner S, Schulze WX, Legen J, Hilger HH, Romeis T. Function of calcium-dependent protein kinase CPK28 of Arabidopsis thaliana in plant stem elongation and vascular development. Plant J 2013; 73:883-96; PMID:23252373; http://dx.doi.org/ 10.1111/tpj.12090 [DOI] [PubMed] [Google Scholar]


