Abstract
Patients with metastatic triple-negative breast cancer (mTNBC) typically have a poor prognosis. The purpose of this study was to prospectively evaluate the efficacy and toxicity of biweekly combination of vinorelbine and oxaliplatin (NVBOX) in second- or third-line setting for mTNBC. Eligible patients were female with 18–70 y old, and had mTNBC that had progressed after 1or 2 prior chemotherapy regimens in the metastatic setting. NVBOX was given biweekly every 4 week for a maximum of 6 cycles. The primary endpoint was progression-free survival (PFS). Forty-4 patients were recruited. All patients had been exposed to anthracyclines and/or taxanes; 56.8% of patients were cis/carbo-platin pretreated. Among the 38 evaluable patients, overall response rate was 31.6% and 7 lasted ≥ 6 months. The median PFS and overall survival (OS) were 4.3 (95% CI, 3.6–5.0) months and 12.6 (95% CI, 8.1–17.0) months, respectively. PFS and OS was significantly shorter in patients with interval from diagnosis to recurrence ≤ 1 y and time to progression (TTP) of 1–2 previous regimens before recruitment ≤ 3 months. For 34 patients who were treated in second line setting, prior platinum was a factor significantly compromising the PFS of NVBOX. Grade 3/4 hematologic toxicities included neutropenia (70.5%), thrombocytopenia (27.3%) and anemia (15.9%). The most frequent grade 3/4 non-hematologic toxicities were constipation/abdominal distension (20.5%) and nausea/vomiting (13.6%). We conclude that biweekly NVBOX regimen is effective with a good safety profile in the second- or third-line mTNBC, which warrants further investigation in a phase III study. This trial was registered with www.clinicaltrials.gov (no. NCT01528826).
Keywords: chemotherapy, metastatic breast cancer, oxaliplatin, triple-negative, vinorelbine
Abbreviations
- TNBC
triple-negative breast cancer
- mTNBC
metastatic triple-negative breast cancer
- ER
estrogen receptor
- PgR
progesterone receptor
- HER2
human epidermal growth factor receptor 2
- ORR
overall response rate
- MBC
metastatic breast cancer
- ECOG
Eastern Cooperative Oncology Group
- TTP
time to progression
- SD
stable disease
- CBR
rate of clinical benefit
- CR
complete response
- PR
partial response
- CI
confidence interval
- HR
hazard ratio
- AE
adverse events
- IHC
immunohistochemistry
- FISH
fluorescence in situ hybridization
- ANC
absolute neutrophil count
- ULN
upper limit of normal
- IV
intravenously
Introduction
Triple-negative breast cancer (TNBC), approximately 12–20% of all breast cancer, has an aggressive clinical course, and was associated with distant recurrence peaking at the first 3 y1,2 and early visceral metastasis.3 The treatment of metastatic TNBC (mTNBC) is especially challenging as the tumors lack recognized therapeutic molecular biology targets, such as estrogen receptor (ER), progesterone receptor (PgR) and gene amplification of human epidermal growth factor receptor (HER2).4,5 The median distant disease-free interval for TNBC subtype was 18 month while the median survival for mTNBC ranged from 6∼13.3 months.6,7
Cytotoxic chemotherapy remains the mainstay of treatment for mTNBC as currently there are no specific targeted or biologic agents available. Whether BRCA-associated or sporadic TNBC, the molecular characteristics are consistent with aberrant DNA repair and genome-wide instability,8,9 supporting the use of DNA-damaging agents such as platinum, which have been or are tested in several trials.10-19 Standard of care included antracyclines, taxanes, and cis/carbo-platin-containing regimen as first-line therapy with overall response rate (ORR) approximated 30% and progression-free survival (PFS) of 3 months as single agent treatment had resulted in less optimal outcome in patients with mTNBC. Moreover, patients with pretreated mTNBC experienced a particular dismal progressive course. Eighty-seven percent patients went on to receive second-line therapy with a median duration of 9 weeks, and 55% received third-line therapy with a median duration only 4 weeks.7 There is no standard of care for pretreated mTNBC. Thus, evaluation of newer combination as second-or third-line regimen for patients with mTNBC is urgently needed. Oxaliplatin, active in cisplatin- and carboplatin- resistant cell lines, may be worth exploring for cis/carbo-platin pretreated patients.20 An ORR of 21% had been reported for oxaliplatin as single agent and between 7.5 to 35% as combination regimen in patients with pretreated metastatic breast cancer (MBC).21 Oxaliplatin was usually combined with fluorouracil, gemcitabine or vinorelbine, considering its additive and/or synergistic activity with these cyctotoxic agents.20,22,23 Vinorelbine, a vinca alkaloid derivative, interferes with tubulin assembly during mitosis and is active as single agent or in combination in MBC, with response rates of 36–50%.24-26
The safety and efficacy of oxaliplatin and vinorelbine combination chemotherapy have been tested in breast cancer,27 lung cancer,28-30 and malignant pleural mesothelioma.31 Recently, a phase I/II study selected 4 different dose levels of vinorelbine and oxaliplatin (NVBOX) for phase I study in MBC and no dose-limiting toxicities occurred even at the highest level (vinorelbine 30 mg/m2 and oxaliplatin 90 mg/m2 every 2 weeks). Then this dosage and schedule of NVBOX as first-line treatment was recommended to the phase II study including 44 MBC patients and was found well tolerated and highly active with ORR of 59%, PFS of 9.2 months and overall survival (OS) of 18.6 months.32 In another phase II study 27 with 3-week schedule, the patients received an equivalent of 8.7 mg/m2/week of vinorelbine and 43.3 mg/m2/week of oxaliplatin, whereas in the biweekly schedule study, the dose of vinorelbine was almost double (15 mg/m2/week). Considering these data and dose-effect relationship, our phase II open-label, non-randomized, single center study (NCT 01528826) was conducted to evaluate the efficacy and tolerability of biweekly NVBOX, as second- or third-line therapy in patients with mTNBC. Preliminary results had been reported at the 36th San Antonio Breast Cancer Conference (P3–13–06).
Results
Patients
Between Dec 2011 and Nov 2012, 44 patients with invasive ductal carcinoma were recruited. Patient characteristics are listed in Table 1.The median age was 47 y (range: 28–70). Thirty-9 (88.6%) patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 1. Seventeen (38.6%) patients developed recurrence within one year from initial diagnosis of breast cancer. Seventeen (38.6%) patients had ≥ 3 metastatic organ sites and visceral involvement was noted in 34 (77.3%) patients. All patients had been exposed to anthracyclines and/or taxanes-containing regimens, among whom 25 (56.8%) were pretreated with cis/carbo-platin for MBC. Thirty-4 (77.3%) patients had 1 previous regimen for metastatic disease and 38.6% with time to progression (TTP) of 1–2 previous regimens before recruitment ≤ 3 months.
Table 1.
Patient characteristics at baseline
| Characteristics | No. (%) |
|---|---|
| Patients enrolled | 44 |
| Age, years | |
| Median (Range) | 47 (28–70) |
| ≤ 40 | 12 (27.3) |
| > 40 | 32 (72.7) |
| ECOG performance status | |
| 0 | 5 (11.4) |
| 1 | 39 (88.6) |
| Menopausal status | |
| Pre- or perimenopause | 20 (45.5) |
| Postmenopause | 24 (54.5) |
| Interval from diagnosis of breast cancer to recurrence | |
| ≤ 1 year | 17 (38.6) |
| > 1 year | 27 (61.4) |
| Metastatic sites | |
| Lymph nodes | 23 (52.3) |
| Liver | 13 (29.5) |
| Lung | 26 (59.1) |
| Bone | 14 (31.8) |
| Pleural effusion | 2 (4.5) |
| Local recurrence | 16 (36.4) |
| Contralateral breast | 2 (4.5) |
| Brain | 3 (6.8) |
| Type of metastasis | |
| Non-visceral | 10 (22.7) |
| Visceral | 34 (77.3) |
| No. of metastatic organ sites | |
| 1 | 9 (20.5) |
| 2 | 18 (40.9) |
| ≥ 3 | 17 (38.6) |
| Previous regimens for MBC | |
| 1 | 34 (77.3) |
| 2 | 10 (22.7) |
| Cis/carbo-platin pretreated for MBC | |
| Yes | 25 (56.8) |
| No | 19 (43.2) |
| TTP of 1–2 previous regimens before recruitment ≤ 3 months | |
| Yes | 17 (38.6) |
| No | 27 (61.4) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group; No., number; MBC, metastatic breast cancer; TTP, time to progression.
Efficacy
The median number of treatment cycles was 3 (range: 1–6 cycles). The outcomes of the patients treated with NVBOX as salvage chemotherapy in mTNBC was presented in Table 2. The ORR was 31.6% (1 complete response, 11 partial responses) and 10 achieved stable diseases (SD, 7 lasting more than 6 months) in 38 evaluable patients (2 patients without measurable disease, 4 withdrew the consent before first evaluation). The rate of clinical benefit (CBR, complete response [CR] + partial response [PR] + SD ≥ 6 months) was 50.0% (19 of 38 patients). After a median follow-up of 12.8 months, the median PFS and OS of the intent-to-treat population were 4.3 (95% confidence interval [CI], 3.6–5.0) months and 12.6 (95% CI, 8.1–17.0) months, respectively. No significant differences between the second-line and third-line were found in all terms of efficacy.
Table 2.
Summary of efficacy
| Efficacy | Total population (N = 44) No. (%) | Second-line (N = 34) No. (%) | Third-line (N = 10)* No. (%) |
|---|---|---|---|
| Progression-free survival, months | |||
| Median (95% CI) | 4.3 (3.6 to 5.0) | 4.1 (2.1 to 6.2) | 4.4 (4.0 to 4.8) |
| Overall survival, months | |||
| Median (95% CI) | 12.6 (8.1 to 17.0) | 10.0 (4.6 to 15.5) | 14.1 (12.8 to 15.5) |
| Best overall response | 38 evaluable patients | 29 evaluable patients | 9 evaluable patients |
| Complete response | 1 (2.6) | 1 (3.4) | 0 |
| Partial response | 11 (28.9) | 8 (27.6) | 3 (33.3) |
| Stable disease | 10 (26.3) | 6 (20.7) | 4 (44.4) |
| Stable disease for ≥6 months | 7 (18.4) | 5 (17.2) | 2 (22.2) |
| Progressive disease | 16 (42.1) | 14 (48.3) | 2 (22.2) |
| Overall response rate | 12 (31.6) | 9 (31.0) | 3 (33.3) |
| Clinical benefit rate | 19 (50.0) | 14 (48.3) | 5 (55.5) |
No significant differences between the second-line and third-line in all terms.
Predictors of outcome
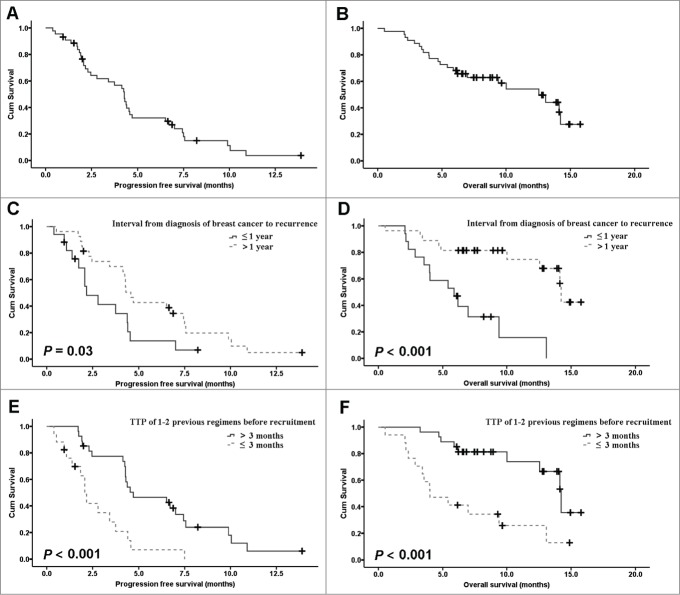
Age, menopausal status, interval from diagnosis of breast cancer to recurrence, extent of disease, prior cis/carbo-platin for MBC, and TTP of 1–2 previous regimens before recruitment ≤ 3 months, were included in the prognostic factors model. PFS and OS was significantly shorter in patients with interval from diagnosis of breast cancer to recurrence ≤ 1 y (Hazard ratio [HR] = 2.10; 95% CI, 1.05–4.21; P = 0.037 and HR = 5.45; 95% CI, 2.08–14.32; P < 0.001) and TTP of 1–2 previous regimens before recruitment ≤ 3 months (HR = 3.39; 95% CI, 1.66–6.89; P < 0.001 and HR = 4.09; 95% CI, 1.73–9.68; P < 0.001)(Fig. 1). Multivariate analysis showed that TTP of 1–2 previous regimens before recruitment ≤ 3 months was the only independent predictor for PFS, while interval from diagnosis of breast cancer to recurrence was the only independent factor to influence OS (Table 3). For 34 patients who received NVBOX as second line treatment, prior 1st-line platinum treatment was a factor significantly compromising the PFS of NVBOX (HR = 2.29; 95% CI, 1.03–5.10; P = 0.043).
Figure 1.
Kaplan-Meier estimates of progression-free survival (PFS) (A) and overall survival (OS) (B) for the ITT population. Kaplan-Meier estimates of PFS (C) and OS (D) for different interval from diagnosis of breast cancer to recurrence (≤ 1 y vs. > 1 year).Kaplan-Meier estimates of PFS (C) and OS (D) for different TTP of 1–2 previous regimens before recruitment (≤ 3 months vs. > 3 months).
Table 3.
Univariate and multivariate models for progression free survival and overall survival in patients with metastatic triple negative breast cancer
| Univariate |
Multivariate |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progression Free | Overall | Progression Free | Overall | ||||||||||
| Factor |
No. |
Median(months) |
95% CI |
P |
Median (months) |
95% CI |
P |
HR |
95% CI |
P |
HR |
95%CI |
P |
| Age | .59 | .61 | |||||||||||
| ≤ 40 | 12 | 3.8 | 2.5 to 5.0 | 13.1 | - | ||||||||
| > 40 | 32 | 4.3 | 4.1 to 4.4 | 12.6 | 7.3 to 17.8 | ||||||||
| Menopausal status | .91 | .59 | |||||||||||
| Pre- or perimenopause | 20 | 3.8 | 2.2 to 5.3 | 12.6 | 7.3 to 17.8 | ||||||||
| Postmenopause | 24 | 4.3 | 4.1 to 4.5 | 14.2 | 4.3 to 24.1 | ||||||||
| Interval* | .03 | <.001 | <.001 | ||||||||||
| ≤ 1 year | 17 | 2.2 | 0.8 to 3.5 | 5.9 | 3.2 to 8.6 | 5.45 | 2.08 to 14.32 | ||||||
| > 1 year | 27 | 4.6 | 4.1 to 5.0 | 14.2 | 14.0 to 14.5 | ||||||||
| Visceral involvement | .71 | .20 | |||||||||||
| No | 10 | 4.4 | 4.0 to 4.8 | Not reached | |||||||||
| Yes | 34 | 4.1 | 3.2 to 5.1 | 10.0 | 3.4 to 16.7 | ||||||||
| No. of metastatic sites | .62 | .18 | |||||||||||
| 1 | 9 | 4.6 | 3.3 to 5.8 | 14.1 | 12.2 to 16.1 | ||||||||
| 2 | 18 | 4.3 | 0.0 to 8.6 | 9.4 | 4.5 to 14.3 | ||||||||
| ≥ 3 | 17 | 4.2 | 1.5 to 7.0 | 12.6 | 8.1 to 17.0 | ||||||||
| Cis/carbo-platin pretreated for MBC | .11 | .14 | |||||||||||
| Yes | 25 | 2.2 | 1.6 to 2.8 | 10.0 | 3.1 to 16.9 | ||||||||
| No | 19 | 4.5 | 4.1 to 5.0 | Not reached | |||||||||
| TTP of 1–2 previous regimens before recruitment ≤ 3 month | <.001 | <.001 | <.001 | ||||||||||
| Yes | 17 | 2.1 | 1.7 to 2.5 | 4.0 | 1.5 to 6.5 | 3.39 | 1.66 to 6.89 | ||||||
| No | 27 | 4.7 | 2.0 to 7.4 | 14.2 | 12.4 to 16.1 | ||||||||
Interval from diagnosis of breast cancer to recurrence
Toxicity
Toxicity profile of the combination was acceptable and manageable. The most common AEs were presented in Table 4. The most common grade 3/4 hematologic toxicities were neutropenia (70.5%), thrombocytopenia (27.3%) and anemia (15.9%). Four (9.1%) patients experienced febrile neutropenia. The most frequent grade 3/4 non-hematologic toxicities were constipation/abdominal distension (20.5%) and nausea/vomiting (13.6%). Two patients developed grade 3 peripheral sensory neurotoxicity. Dose adjustment due to adverse events (AEs) occurred in 14 patients (31.8%). Median total dose intensity for vinorelbine and oxaliplatin was 0.90 and 0.88, respectively. There were no treatment-related deaths.
Table 4.
Adverse events
| Description of Toxicity† | Any No. (%) | Grade 3 No. (%) | Grade 4 No. (%) |
|---|---|---|---|
| Hematologic | |||
| Neutropenia | 38 (86.4) | 14 (31.8) | 17 (38.6) |
| Thrombocytopenia | 23 (52.3) | 11 (25.0) | 1 (2.3) |
| Anemia | 30 (68.2) | 7 (15.9) | 0 |
| Febrile neutropenia | 4 (9.1) | 4 (9.1) | 0 |
| Nonhematologic | |||
| Sensory neuropathy | 21 (47.7) | 2 (4.5) | 0 |
| Constipation/Abdominal distension | 20 (45.5) | 9 (20.5) | 0 |
| Nausea/Vomiting | 20 (45.5) | 6 (13.6) | 0 |
| Fatigue | 15 (34.1) | 1 (2.3) | 0 |
| Anorexia | 14 (31.8) | 0 | 0 |
| Increased ALT/AST* | 2 (4.5) | 0 | 0 |
| Arthralgia | 2 (4.5) | 0 | 0 |
| Allergic reaction | 1 (2.3) | 1 (2.3) | 0 |
Grade used was the worst recorded per patient.
Abbreviation: ALT = alanine transaminase; AST = aspartate transaminase.
Discussion
Our report is the first prospective phase II study evaluating the safety and activity of a third-generation platinum (other than cis/carbo-platin) based regimen as second or third-line treatment for mTNBC. The CBR was 50.0%, with 31.6% of patients achieving responses, with median PFS of 4.3 months, and median OS of 12.6 months. While direct inter-trial comparisons of response rates and PFS may not be possible due to large patient heterogeneity, our results with biweekly NVBOX are nevertheless promising, particularly in view of the high proportion (56.8%) of cis/carbo-platin pretreatment seen in the study. Given the limited effective treatment options available for pretreated patients with mTNBC, these results are encouraging, with the testing of oxaliplatin impact in larger size phase III trial and earlier disease stages the next step to consider.
Platinum single agent in this population was of poor efficacy. In a randomized phase II BALI-1 trial,33 the control group, cisplatin had only modest activity (ORR 6%) as second-line treatment in sporadic mTNBC patients. Similarly, in another phase II study (TBCRC009), single agent cisplatin 75 mg/m2 or carboplatin AUC = 6 every 21 d in second-line setting was tested with the ORR 20%.14 For patients with more extensive, rapidly progressive, or symptomatic disease, many oncologists prefer combination therapies because, even if the advantage in terms of survival has not been demonstrated to date, they seem to offer better results in terms of response rate, PFS and CBR that could be translated to better control of symptoms. However, even with platinum-based combination chemotherapy GC (gemcitabine plus carboplatin), the PFS and OS for the second- or third-line mTNBC population (n = 222) were only 2.9 and 9.1 months, respectively.11
In this study, interval from diagnosis of breast cancer to recurrence ≤ 1 y and TTP of 1–2 previous regimens before recruitment ≤ 3 months were identified as significant unfavorable factors for PFS and OS of previously treated mTNBC patients. Multivariate analysis also revealed that they were independent predictor for OS and PFS, respectively. Our study is the first to show the role of TTP of 1–2 previous regimens before recruitment ≤ 3 months in predicting the significantly poor prognosis (median PFS only 2.1 months and OS 4.0 months). A short TTP of the second- and/or first-line therapy (≤ 3 months) might suggest the tumor be primarily or secondarily resistant to the cytotoxic agents, indicating primarily resistance to NVBOX that resulted in shorter survival. To these patients, chemotherapy may not be able to improve survival for this group of patients. Best supportive care (BSC) might be a better option. A randomized study including patients with TTP of previous therapies ≤ 3 months to compare the salvage treatment and BSC is worth to be encouraged.
Prior first-line treatment with cis/carbo-platin–containing regimen was a factor significantly compromising the PFS of NVBOX. Oxaliplatin has a target mechanism of action and mechanisms of resistance different from cis/carbo-platin, thus shows little or no cross-resistance with cis/carbo-platin-resistant human breast, ovarian, cervix squamous cell carcinoma, non-small-cell lung cancer, germ cell cancer and mouse leukemia cell lines.23 However, the non-cross resistance may not be absolute, as 1,2-diaminocyclohexane (DACH) -Pt complexes are not effective in all platinum-resistant cell lines.34,35 A methodology of biomarkers to predict the efficacy of oxaliplatin in cis/carbo-platin pretreated patients remains to be explored.
The most frequently reported grade 3/4 AEs of NVBOX were neutropenia, thrombocytopenia and constipation/abdominal distension. Neutropenia was widespread and often grade 3/4 (70.4%), similar to triweekly NVBOX (78.6%),27 as expected with vinorelbine considering the population's pre-treatment profile. Four patient (9.1%) experienced febrile neutropenia, the incidence of which was a bit higher than other combinations such as 5-fluorouracil/oxaliplatin (0–2%) or vinorelbine/cisplatin (0%), but much lower than the 5-fluorouracil/vinorelbine combination (up to 33%) in later-line treatment setting.36-39 It should be noted that in the previous phase I/II study of biweekly NVBOX, not only the grade 3/4 neutropenia (45.5%), but also the grade 3/4 constipation/abdominal distension (2.3%) were much less frequent than those in our study. The less incidence of grade 3/4 neutropenia in the previous study might be due to the recruitment of previous untreated MBC patients, while the higher frequency of constipation/abdominal distension observed in our study might be due to the fact that all patients were taxane-pretreated and 47.7% were cisplatin pretreated. A possible role of ethnic differences (Asian vs. Spanish) in genetic backgrounds may also contribute to the difference. Most of the constipation/abdominal distension were considered autonomic nerve damage related, but was manageable by appropriate medications and dose interruption or reduction in this study. Taking into account the prior exposure of taxane and/or cisplatin, NVBOX regimen was considered reasonably well tolerated.
Although cytotoxic chemotherapy remains the mainstay of treatment for mTNBC, chemotherapy combined with targeted agents such as PARP inhibitors,11 EGFR inhibitors,12,13 antiangiogenic agents40 and Chk1 inhibitor 41 were recently investigated in the later-line setting, some of which 11,13,40 showed gains in response rate and PFS and were worthy of being further explored.
In conclusion, our study demonstrated that biweekly NVBOX regimen is effective and well-tolerated as second- or third-line treatment for patients with mTNBC. Future trials of NVBOX that focus on its interaction and role with target agents for mTNBC in the later-line setting are urgently required.
Patients and Methods
Patients
Women age between 18–70 y with histologically confirmed MBC documented as ER negative (IHC <10 %), PgR negative (IHC <10 %), and HER2 negative, were eligible. HER2 status was assessed by immunohistochemistry (IHC) and/or fluorescence in situ hybridization (FISH). HER2 negative was defined as no staining by IHC, and HER2 gene amplification by FISH was performed for those cases of 1+ and 2+ by IHC and confirmed absence of gene amplification. All patients must have progressed after 1or 2 prior chemotherapy regimens for metastatic disease. Pretreatment with vinorelbine and/or oxaliplatin was not permitted. Patients must have at least one measurable disease according to RECIST 1.1 criteria, a life expectancy of no less than 3 months, ECOG performance status ≤ 1 and adequate hematologic, renal, and hepatic function, as indicated by hemoglobin ≥ 9 g /dl, absolute neutrophil count (ANC) ≥ 1.5×109 /L, platelet count ≥ 75×109 /L, total serum bilirubin ≤ 1.5 × upper limit of normal (ULN), AST/ALT ≤ 2.5×ULN (≤ 5× ULN in case of liver metastases) and serum creatinine ≤ 1.0 × ULN (calculated creatinine clearance ≥ 50 mL/min). Patients who had received radiotherapy, chemotherapy, endocrine therapy, target therapy or any other investigated drugs within 4 weeks before the recruitment or has not recovered from the treatment-related toxicity(such as the severity of peripheral neuropathy> grade 1) were excluded. Patients with symptomatic central nervous system metastases or who were pregnant were ineligible.
The study was approved by the Fudan University Cancer Hospital Ethic Committee for Clinical Investigation (approval number: 1111104–12).The study was carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients prior to enrollment.
Treatment
Patients were treated with vinorelbine 30 mg/m2 intravenously (IV) and oxaliplatin 90 mg/m2 IV on day 1 every 2 weeks until disease progression or unacceptable toxicity or up to 6 cycles. A period of 4 weeks (NVBOX twice) was considered as one treatment cycle. Administration of prophylactic G-CSF was not permitted in the study. Dose modifications were made if Grade 4 neutropenia lasted longer than 3 days, Grade 3–4 thrombocytopenia, febrile neutropenia and/or Grade 3 non-hematological toxicity (except alopecia and inadequately treated nausea/vomiting). In this case, treatment was interrupted for up to 2 weeks until resolution to Grade ≤ 2, and doses of vinorelbine and oxaliplatin were reduced permanently by 20% in subsequent cycles. If toxicity did not resolve after 2 weeks or dose modifications occurred more than twice, the patient was withdrawn from the study. In addition, patients who experienced any grade 4 non-hematological toxicity were withdrawn from the study. The primary endpoint was PFS. Secondary endpoints included OS, ORR, and safety.
Assessment
Pretreatment assessment included a detailed medical history, physical examination, routine laboratory tests and performance status. Laboratory evaluation included a routine blood count, biochemistry including electrolytes, renal and liver function tests, and urinalysis. AEs and concomitant medications were recorded at the end of each cycle throughout the study period until 30 d after the last dose of a study treatment was administered. Toxicity was evaluated and graded according to National Cancer Institute Common Terminology Criteria for AEs, version 4.0.
Radiographic scans (CT scan or MRI) for efficacy evaluation were conducted at baseline and every 2 treatment cycles thereafter per RECIST 1.1 guidelines. The best overall response was reported. For patients without progress at the end of treatment, radiographic assessment was performed every 2 months within the first 6 months and every 3 months thereafter until disease progress. Survival status was assessed every 3 months after disease progress.
Statistical methods
The primary objective of this study was to evaluate the NVBOX regimen as second- or third-line treatment for mTNBC. The primary endpoint was PFS. The sample size was based on testing the hypothesis that NVBOX is superior to historical data from the report of Kassam et al.7 With a 2-sided test of survival time differences between NVBOX doublet and historical data (12 months enrollment duration, 12 months follow up duration after enrollment), a sample of 38 evaluable patients would allow detecting an increase of 2.0 months (from 2.0 to 3.4 months) in the PFS on therapy, with 90% power and 5% significance level. A total of 42 patients were to be enrolled assuming < 10% patient discontinuation rate due to noncompliance or toxicity.
PFS was defined as the interval between treatment start and documented disease progression, or death as a result of any cause in patients with no evidence of disease progression. OS was defined as the interval between the initiation of treatment and death. CBR was defined as the percentage of patients who had a complete response, a partial response, or stable disease for at least 6 months. Safety issues including incidence and severity of AEs were also investigated.
All statistical analyses were carried out using SPSS 19.0 (SPSS, Inc..). PFS and OS were estimated and 95% confidence intervals were calculated by means of the Kaplan–Meier method. All P values and confidence intervals reported are 2-sided, and all analyses are of data for the ITT population unless otherwise noted. Univariate survival curves were generated by the Kaplan-Meier method and differences in survival among the variables were assessed by the log-rank test. Prognostic variables identified by univariate analysis, with P < 0.1, were analyzed in the multivariate Cox model. All tests were 2-sided and P values <0 .05 were considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the all the patients and investigators. Presented partly at the San Antonio Breast Cancer Conference (P3–13–06), December 10–14, 2013, San Antonio, TX.
References
- 1. Schneider BP, Winer EP, Foulkes WD, Garber J, Perou CM, Richardson A, Sledge GW, Carey LA. Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res 2008; 14:8010-18; PMID:19088017; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-1208 [DOI] [PubMed] [Google Scholar]
- 2. Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology 2008; 52:108-18; PMID:18171422; http://dx.doi.org/ 10.1111/j.1365-2559.2007.02889.x [DOI] [PubMed] [Google Scholar]
- 3. Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat 2009; 115:423-28; PMID:18543098; http://dx.doi.org/ 10.1007/s10549-008-0086-2 [DOI] [PubMed] [Google Scholar]
- 4. Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. . Molecular portraits of human breast tumours. Nature 2000; 406:747-52; PMID:10963602; http://dx.doi.org/ 10.1038/35021093 [DOI] [PubMed] [Google Scholar]
- 5. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010; 363:1938-48; PMID:21067385; http://dx.doi.org/ 10.1056/NEJMra1001389 [DOI] [PubMed] [Google Scholar]
- 6. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer:clinical features and patterns of recurrence. Clin Cancer Res 2007; 13:4429-34; PMID:17671126; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-3045 [DOI] [PubMed] [Google Scholar]
- 7. Kassam F, Enright K, Dent R, Dranitsaris G, Myers J, Flynn C, Fralick M, Kumar R, Clemons M. Survival outcomes for patients with metastatic triple-negative breast cancer:implications for clinical practice and trial design. Clin Breast Cancer 2009; 9:29-33; PMID:19299237; http://dx.doi.org/ 10.3816/CBC.2009.n.005 [DOI] [PubMed] [Google Scholar]
- 8. Andre F, Job B, Dessen P, Tordai A, Michiels S, Liedtke C, Richon C, Yan K, Wang B, Vassal G, et al. . Molecular characterization of breast cancer with high-resolution oligonucleotide comparative genomic hybridization array. Clin Cancer Res 2009; 15:441-51; PMID:19147748; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-1791 [DOI] [PubMed] [Google Scholar]
- 9. Bergamaschi A, Kim YH, Wang P, Sorlie T, Hernandez-Boussard T, Lonning PE, Tibshirani R, Borresen-Dale AL, Pollack JR. Distinct patterns of DNA copy number alteration are associated with different clinicopathological features and gene-expression subtypes of breast cancer. Genes Chromosomes Cancer 2006; 45:1033-40; PMID:16897746; http://dx.doi.org/ 10.1002/gcc.20366 [DOI] [PubMed] [Google Scholar]
- 10. O’Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, Koo IC, Sherman BM, Bradley C. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med 2011; 364:205-14; http://dx.doi.org/ 10.1056/NEJMoa1011418 [DOI] [PubMed] [Google Scholar]
- 11. O’Shaughnessy J, Schwartzberg LS, Danso MA, Rugo HS, Miller K, Yardley R, Al E. A randomized phase III study of iniparib (BSI-201) in combination with gemcitabine/carboplatin (G/C) in metastatic triple-negative breast cancer (TNBC). J Clin Oncol 2011; 29:1007; http://dx.doi.org/ 10.1200/JCO.2009.27.8960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carey LA, Rugo HS, Marcom PK, Mayer EL, Esteva FJ, Ma CX, Liu MC, Storniolo AM, Rimawi MF, Forero-Torres A, et al. . TBCRC 001:randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol 2012; 30:2615-23; PMID:22665533; http://dx.doi.org/ 10.1200/JCO.2010.34.5579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baselga J, Gomez P, Greil R, Braga S, Climent MA, Wardley AM, Kaufman B, Stemmer SM, Pego A, Chan A, et al. . Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol 2013; 31:2586-92; PMID:23733761; http://dx.doi.org/ 10.1200/JCO.2012.46.2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Isakoff SJ, Goss PE, Mayer EL, Traina TA, Carey LA, Krag K, Rugo HS. TBCRC009:A multicenter phase II study of cisplatin or carboplatin for metastatic triple-negative breast cancer and evaluation of p63/p73 as a biomarker of response. J Clin Oncol 2011; 29:1025; http://dx.doi.org/ 10.1200/JCO.2010.31.6679 [DOI] [Google Scholar]
- 15. ClinicalTrials.gov:Triple negative breast cancer trial (TNT). [Google Scholar]
- 16. Staudacher L, Cottu PH, Dieras V, Vincent-Salomon A, Guilhaume MN, Escalup L, Dorval T, Beuzeboc P, Mignot L, Pierga JY. Platinum-based chemotherapy in metastatic triple-negative breast cancer:the Institut Curie experience. Ann Oncol 2011; 22:848-56; PMID:20924076; http://dx.doi.org/ 10.1093/annonc/mdq461 [DOI] [PubMed] [Google Scholar]
- 17. Fan Y, Xu BH, Yuan P, Ma F, Wang JY, Ding XY, Zhang P, Li Q, Cai RG. Docetaxel-cisplatin might be superior to docetaxel-capecitabine in the first-line treatment of metastatic triple-negative breast cancer. Ann Oncol 2013; 24:1219-25; PMID:23223332; http://dx.doi.org/ 10.1093/annonc/mds603 [DOI] [PubMed] [Google Scholar]
- 18. Bhattacharyya GS, Basu S, Agarwal V, Aaa A. Single institute phase II study of weekly cisplatinum and metronomic dosing of Endoxan and methotrexate in second line metastatic breast cancer triplenegative. Eur J Cancer 2009; 7:41; http://dx.doi.org/ 10.1016/S1359-6349(09)72076-2 [DOI] [Google Scholar]
- 19. ClinicalTrials.gov:Gemcitabine Plus Cisplatin Versus Gemcitabine Plus Paclitaxel in Triple Negative Breast Cancer (TNBC); [Google Scholar]
- 20. Raymond E, Faivre S, Woynarowski JM, Chaney SG. Oxaliplatin:mechanism of action and antineoplastic activity. Semin Oncol 1998; 25:4-12; PMID:9609103 [PubMed] [Google Scholar]
- 21. Delpeuch A, Leveque D, Rob L, Bergerat JP. Off-label use of oxaliplatin in patients with metastatic breast cancer. Anticancer Res 2011; 31:1765-67; PMID:21617237 [PubMed] [Google Scholar]
- 22. Rixe O, Ortuzar W, Alvarez M, Parker R, Reed E, Paull K, Fojo T. Oxaliplatin, tetraplatin, cisplatin, and carboplatin:spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute's Anticancer Drug Screen panel. Biochem Pharmacol 1996; 52:1855-65; PMID:8951344; http://dx.doi.org/ 10.1016/S0006-2952(97)81490-6 [DOI] [PubMed] [Google Scholar]
- 23. Raymond E, Chaney SG, Taamma A, Cvitkovic E. Oxaliplatin:a review of preclinical and clinical studies. Ann Oncol 1998; 9:1053-71; PMID:9834817; http://dx.doi.org/ 10.1023/A:1008213732429 [DOI] [PubMed] [Google Scholar]
- 24. Martin M, Ruiz A, Munoz M, Balil A, Garcia-Mata J, Calvo L, Carrasco E, Mahillo E, Casado A, Garcia-Saenz JA, et al. . Gemcitabine plus vinorelbine versus vinorelbine monotherapy in patients with metastatic breast cancer previously treated with anthracyclines and taxanes:final results of the phase III Spanish Breast Cancer Research Group (GEICAM) trial. Lancet Oncol 2007; 8:219-25; PMID:17329192; http://dx.doi.org/ 10.1016/S1470-2045(07)70041-4 [DOI] [PubMed] [Google Scholar]
- 25. Fumoleau P, Delgado FM, Delozier T, Monnier A, Gil DM, Kerbrat P, Garcia-Giralt E, Keiling R, Namer M, Closon MT, et al. . Phase II trial of weekly intravenous vinorelbine in first-line advanced breast cancer chemotherapy. J Clin Oncol 1993; 11:1245-52; PMID:8315421 [DOI] [PubMed] [Google Scholar]
- 26. Garcia-Conde J, Lluch A, Martin M, Casado A, Gervasio H, De Oliveira C, De Pablo JL, Gorostiaga J, Giron GC, Cervantes A, et al. . Phase II trial of weekly IV vinorelbine in first-line advanced breast cancer chemotherapy. Ann Oncol 1994; 5:854-57; PMID:7848890 [DOI] [PubMed] [Google Scholar]
- 27. Petit T, Benider A, Yovine A, Bougnoux P, Spaeth D, Maindrault-Goebel F, Serin D, Tigaud JD, Eymard JC, Simon H, et al. . Phase II study of an oxaliplatin/vinorelbine combination in patients with anthracycline- and taxane-pre-treated metastatic breast cancer. Anticancer Drugs 2006; 17:337-43; PMID:16520663; http://dx.doi.org/ 10.1097/00001813-200603000-00013 [DOI] [PubMed] [Google Scholar]
- 28. Monnet I, Soulie P, de Cremoux H, Saltiel-Voisin S, Bekradda M, Saltiel JC, Brain E, Dupont-Andre G, Cvitkovic E. Phase I/II study of escalating doses of vinorelbine in combination with oxaliplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol 2001; 19:458-63; PMID:11208839 [DOI] [PubMed] [Google Scholar]
- 29. Monnet I, de CH, Soulie P, Saltiel-Voisin S, Bekradda M, Saltiel JC, Brain E, Rixe O, Yataghene Y, Misset JL, et al. . Oxaliplatin plus vinorelbine in advanced non-small-cell lung cancer:final results of a multicenter phase II study. Ann Oncol 2002; 13:103-07; PMID:11863089; http://dx.doi.org/ 10.1093/annonc/mdf006 [DOI] [PubMed] [Google Scholar]
- 30. Mir O, Alexandre J, Ropert S, Montheil V, Martin I, Durand JP, Goldwasser F. Vinorelbine and oxaliplatin in stage IV nonsmall cell lung cancer patients unfit for cisplatin:a single-center experience. Anticancer Drugs 2009; 20:105-08; PMID:19209026; http://dx.doi.org/ 10.1097/CAD.0b013e32831cdb51 [DOI] [PubMed] [Google Scholar]
- 31. Fennell DA, C SJ, Shamash J, Sheaff MT, Evans MT, Goonewardene TI, Nystrom ML, Gower NH, Rudd RM. Phase II trial of vinorelbine and oxaliplatin as first-line therapy in malignant pleural mesothelioma. Lung Cancer-J Iaslc 2005; 47:277-81; http://dx.doi.org/ 10.1016/j.lungcan.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 32. Guerrero A, Servitja S, Rodriguez-Lescure A, Calvo L, Del BS, Quintanar MT, Juarez JI, Gayo J, Llombart A, Tusquets I. Phase I/II study of biweekly vinorelbine and oxaliplatin as first-line treatment in patients with metastatic breast cancer. Anticancer Drugs 2011; 22:283-89; PMID:21150776; http://dx.doi.org/ 10.1097/CAD.0b013e3283425c55 [DOI] [PubMed] [Google Scholar]
- 33. Baselga J, Gomez P, Greil R, Braga S, Climent MA, Wardley AM, Kaufman B, Stemmer SM, Pego A, Chan A, et al. . Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol 2013; 31:2586-92; PMID:23733761; http://dx.doi.org/ 10.1200/JCO.2012.46.2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hills CA, Kelland LR, Abel G, Siracky J, Wilson AP, Harrap KR. Biological properties of ten human ovarian carcinoma cell lines:calibration in vitro against four platinum complexes. Br J Cancer 1989; 59:527-34; PMID:2653399; http://dx.doi.org/ 10.1038/bjc.1989.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perez RP, O’Dwyer PJ, Handel LM, Ozols RF, Hamilton TC. Comparative cytotoxicity of CI-973, cisplatin, carboplatin and tetraplatin in human ovarian carcinoma cell lines. Int J Cancer 1991; 48:265-69; PMID:2019469; http://dx.doi.org/ 10.1002/ijc.2910480219 [DOI] [PubMed] [Google Scholar]
- 36. Zelek L, Cottu P, Tubiana-Hulin M, Vannetzel JM, Chollet P, Misset JL, Chouaki N, Marty M, Gamelin E, Culine S, et al. . Phase II study of oxaliplatin and fluorouracil in taxane- and anthracycline-pretreated breast cancer patients. J Clin Oncol 2002; 20:2551-58; PMID:12011135; http://dx.doi.org/ 10.1200/JCO.2002.06.164 [DOI] [PubMed] [Google Scholar]
- 37. Vassilomanolakis M, Koumakis G, Demiri M, Missitzis J, Barbounis V, Efremidis AP. Vinorelbine and cisplatin for metastatic breast cancer:a salvage regimen in patients progressing after docetaxel and anthracycline treatment. Cancer Invest 2003; 21:497-504; PMID:14533438; http://dx.doi.org/ 10.1081/CNV-120022358 [DOI] [PubMed] [Google Scholar]
- 38. Girre V, Dalenc F, Laurence V, Jouve M, Dieras V, Pierga J, Et A. Vinorelbine-5-fluorouracil combination as second line chemotherapy in metastatic breast cancer (MBC) after anthracyclinetaxanes combination (AT). Ann Oncol 2000; 11(Suppl 4):24. [Google Scholar]
- 39. Delaloge S, Tubiana-Hulin M, Wardley A, Mastro LD, Santoro A, Zambelli A, Bonetti A, Boni C, Habib F, Brienza S. A multistep randomized phase II/III trial comparing oxaliplatin (OXA)+5 fluorouracil (FU) to vinorelbine (VIN)+FU (FUN) after taxane (T)/anthracycline (A) failure in advanced/metastatic breast cancer (MBC) patients (pts):Final results. Proc Am Soc Clin Oncol 2004; 22:660. [Google Scholar]
- 40. Brufsky A, Valero V, Tiangco B, Dakhil S, Brize A, Rugo HS, Rivera R, Duenne A, Bousfoul N, Yardley DA. Second-line bevacizumab-containing therapy in patients with triple-negative breast cancer:subgroup analysis of the RIBBON-2 trial. Breast Cancer Res Treat 2012; 133:1067-75; PMID:22415477; http://dx.doi.org/ 10.1007/s10549-012-2008-6 [DOI] [PubMed] [Google Scholar]
- 41. Ma CX, Ellis MJ, Petroni GR, Guo Z, Cai SR, Ryan CE, Craig LA, Naughton MJ, Pluard TJ, Brenin CM, et al. . A phase II study of UCN-01 in combination with irinotecan in patients with metastatic triple negative breast cancer. Breast Cancer Res Treat 2013; 137:483-92; PMID:23242585; http://dx.doi.org/ 10.1007/s10549-012-2378-9 [DOI] [PMC free article] [PubMed] [Google Scholar]