Abstract
Abnormal glucose metabolism mediated by pyruvate kinase M2 (PKM2) fuels cancer overgrowth and propagation. However, its expression and oncogenic roles in in oral squamous cell carcinoma (OSCC) remains incompletely known. Here, we aimed to investigate the expression of PKM2, its prognostic values and oncogenic functions using 7,12-dimethyl-1,2-bezan-tracene (DMBA)-induced hamster buccal pouch SCC model, primary OSCC specimens as well as in vitro cellular assays. We found that in DMBA-induced OSCC model, negative PKM2 expression was commonly observed in normal epithelial, while more PKM2 abundance was detected in hyperplasia, dysplasia and SCC. Overexpression of PKM2 in a major fraction of OSCC significantly associated with tumor size (P = 0.027), cervical node metastasis (P = 0.004) and clinical stages (P = 0.000). Patients with increased PKM2 had remarkably reduced overall and disease-free survival. Multivariate survival analysis further revealed that PKM served as a critical independent prognostic factor for patients' overall survival. Furthermore, impaired cell proliferation and migration, and reduced apoptosis were detected upon PKM2 knockdown in HN4 and HN12 cells. Taken together, our findings reveal that PKM2 is critically involved in OSCC initiation and progression probably by promoting cell proliferation and migration as well as reducing apoptosis. Its overexpression correlates with aggressive clinicopathological features and poor patients' outcome.
Keywords: aerobic glycolysis, oral squamous cell carcinoma, PKM2, pyruvate kinase M2, Warburg effect
Background
Oral squamous cell carcinoma (OSCC) cancer is one of the common cancers worldwide with well-established etiologic factors including smoking abuse and alcohol consumption, human papillomavirus (HPV) infection, et al.1 Despite the great advancement in the interdisciplinary therapy against this malignancy over the past years, however, the 5-year survival rate has not been improved too much. Locoregional recurrence and cervical metastasis are usually identified as the most prevalent factors dictating patients' prognosis.2 Multiple genetic and epigenetic factors have been identified as key players underlying OSCC pathogenesis, however, no universally-accepted biomarkers have been established for the diagnostic and prognostic management of these patients, thus suggesting our limited understanding regarding oral tumorigenesis and unresolved therapeutic challenge.3 Therefore, the pipeline of novel biomarkers discovery and validation is paramount for early detection, therapeutics development and prognostic prediction, and ultimately leading to improved treatment outcome.
Abnormal energy metabolism including glucose consumption is one of the core hallmarks of solid cancer.4 Most cancer cells exhibit significantly increased glucose uptake and lactate production irrespective of oxygen availability, which is known as the Warburg effect or aerobic glycolysis. It confers growth advantage to cancer cells through offering glycolytic intermediates.5 A line of evidence indicates that several oncogenic hyperactivation drives the metabolic switch by activating the pivotal glycolytic enzyme including pyruvate kinase (PK) in cancers. Under anaerobic conditions, PK is a rate-limiting enzyme that catalyzes the final step of glycolysis, converting the phosphoenolpyruvate to pyruvate. Particularly, PKM2, one of the alternative splicing isoforms encoded by PKM, has increasingly recognized as one of the predominant mediators behind cancer aerobic glycolysis.6,7 This findings have been supported by the facts that abnormal overexpression of PKM2 has been frequently observed in multiple human cancers and its upregulation correlates with cancer aggressive behaviors and clinicopathological features, therapeutic resistance and unfavorable treatment outcomes.8-10 Further evidence linking PKM2 to tumorigenesis also comes from previous findings that PKM2 depletion via either RNA interference or pharmacologic agents impaired cancer cell proliferation, induced cell apoptosis, enhanced therapeutic sensitivity and decreased tumorigenicity both in vitro and in vivo.11-13 Collectively, these findings have provided proof-of-principle that PKM2 is the bona fide oncogene mediating cancer metabolic reprogramming as well as an attractive therapeutic target.
Previous studies have offered several essential clues regarding PKM2 expression and biological roles in head neck cancer.14-16 However, the expression pattern of PKM2 and its clinicopathological significance in OSCC initiation and progression have not been well established thus far. In the present study, we aimed to analyze the PKM2 expression both in an chemical-induced OSCC animal model and primary human specimens and then investigate the relationship between its abundance and clinicopathological features and patients' prognosis. In addition, the oncological roles of PKM2 in OSCC cells were also determined in vitro.
Results
The expression of PKM2 in DMBA-induced OSCC animal model
We have provided evidence that PKM2 is aberrantly overexpressed in a major fraction of tongue squamous cell carcinoma (the most prevalent site for OSCC), and associates with cancer aggressiveness and patients' prognosis.16 To further unravel the oncogenic roles of PKM2 during oral carcinogenesis, we developed the hamster buccal pouch carcinogenesis model, which is one of the best characterized animal models for OSCC, and sought to determine the expression pattern of PKM2 during different stages of OSCC carcinogenesis. Upon animal euthanization, gross examinations of hamster buccal pouch revealed no apparent changes in mineral oil-treated control animals, while thickened mucosa with rough surface was observed in 4-week and 10-week DMBA-treated animals. Of note, exophytic tumor-like lesions were frequently identified in 16-week DMBA-treated animals. The following histopathological analyses were further confirmed the multiple stages induced by DMBA including epithelial hyperplasia, dysplasia, carcinoma in situ as well as squamous cell carcinoma (Fig. 1A,C,E,G). Overall, similar to previous studies, our animal model largely recapitulated the typical multiple stages of OSCC, reminiscence of human OSCC initiation and progression.17 Moreover, as shown in Figure 1B, D, F, H, immunohistochemical staining of PKM2 in animal tissue samples from diverse stages of OSCC indicted negative in most normal epithelial and positive with diverse degrees in epithelial hyperplasia, dysplasia and SCC. Furthermore, the immunohistochemistry data displayed in Table 1 revealed that PKM2 overexpression was observed in the majority of SCC samples, while much less in samples with hyperplasia and dysplasia. Together, our data indicate that PKM2 is involved in OSCC development and may functions as a key oncogene underlying oral tumorigenesis.
Figure 1.
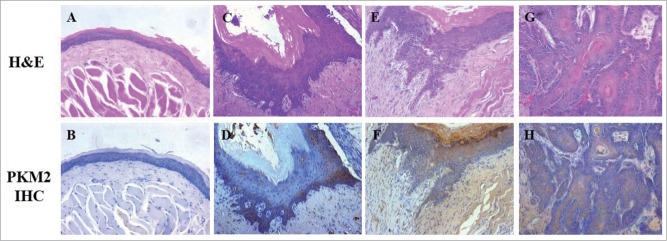
PKM2 expression in DMBA-induced OSCC animal model. (A and B) H&E and PKM2 immunohistochemical staining of normal buccal epithelial in control animals (×200); (C and D) H&E and PKM2 immunohistochemical staining of epithelial hyperplasia in experimental animals (×200); (E and F) H&E and PKM2 immunohistochemical staining of epithelial dysplasia/carcinoma in situ in experimental animals (×200); (G and H) H&E and PKM2 immunohistochemical staining of squamous cell carcinoma in experimental animals (×200).
Table 1.
PKM2 expression in the DMBA-induced buccal pouch squamous cell carcinogenesis model
| lesion/total | PKM2 expression |
||||
|---|---|---|---|---|---|
| Negative | Low | High | p-values | ||
| Normal mucosa | 10/10 | 8 | 2 | 0 | 0.0266 |
| Hyperplasia | 9/10 | 5 | 3 | 1 | |
| Dysplasia/carcinoma in situ | 9/10 | 4 | 3 | 2 | |
| Squamous cell carcinoma | 10/10 | 1 | 3 | 6 | |
Clinicopathological characteristics and PKM2 expression in OSCC patients
To further examine PKM2 expression in clinical specimens and its associations with clinicopathological parameters, we next evaluated the expression level of PKM2 by immunohistochemical staining in a retrospective cohort of 111 primary OSCC patients. The detailed information and clinicopathological features regarding these patients were summarized in Table 2. In brief, 60 males and 61 females with mean age 52.8 y were enrolled. The follow-up durations ranged from 3 months to 78 months with average 51.4 months. Based on our immunohistochemistry scoring method, PKM2 abundance in these primary OSCC was further determined. As shown in Table 2, PKM2 levels in these OSCC sample can be graded as low (48) or high expression group (63), indicating PKM2 was overexpressed in a significant fraction of oral cancers. Representative labeling of low and high PKM2 in 2 OSCC samples was shown in Figure 2. Noticeably, PKM2 staining can be identified both in cell cytoplasm and nucleus in the cancerous cells, while no obvious staining can be found in the nearby non-cancerous cells. The detailed relationships between PKM2 abundance and clinicopathological variables were further shown in Table 2. Notably, the PKM2 expression level was found to be associated with tumor size, cervical nodes metastasis and clinical stages with P-value 0.027, 0.004, 0.000, respectively.
Table 2.
Associations between PKM2 expression and multiple clinicopathological parameters in OSCC
| Parameter | Patient No. | PKM2 |
||
|---|---|---|---|---|
| Low | High | p-value | ||
| Gender | 111 | 48 | 63 | |
| Male | 60 | 24 | 26 | 0.442 |
| Female | 61 | 24 | 37 | |
| Age | ||||
| ≤60 | 57 | 26 | 31 | 0.702 |
| >60 | 54 | 22 | 32 | |
| Primary sites | ||||
| Tongue | 40 | 20 | 20 | 0.498 |
| Buccal | 30 | 14 | 16 | |
| Gingiva | 23 | 7 | 16 | |
| Others(palate, mouth floor, et al) | 18 | 7 | 11 | |
| Tumor size | ||||
| T1 | 10 | 5 | 10 | 0.027 |
| T2 | 29 | 37 | 29 | |
| T3-T4 | 9 | 21 | 9 | |
| Pathological grade | ||||
| I | 64 | 33 | 31 | 0.343 |
| II | 40 | 26 | 14 | |
| III | 7 | 4 | 3 | |
| Local Recurrence | ||||
| No | 85 | 41 | 44 | 0.071 |
| Yes | 26 | 7 | 19 | |
| Cervical nodal metastasis | ||||
| N(0) | 63 | 35 | 28 | 0.004 |
| N(+) | 48 | 13 | 35 | |
| Distant metastasis (bone/lung) | ||||
| No | 100 | 46 | 54 | 0.110 |
| Yes | 11 | 2 | 9 | |
| Clinical stage | ||||
| I-II | 53 | 32 | 21 | 0.000 |
| III-IV | 58 | 16 | 42 | |
Figure 2.

PKM2 expression in human OSCC samples measured by immunohistochemical staining. (A) Representative low expression of PKM2 in a primary human OSCC (×200); (B) Representative high expression of PKM2 in a primary human OSCC (×200). Nuclei are counterstained with hematoxylin.
PKM2 expression levels associated with OSCC patients' survival
To determine relationship between PKM2 expression and OSCC patients' prognosis, we next evaluated the relationship between its expression and clinical outcomes. Until the last follow-up, 70 of 111 (63.1%) patients remained alive and disease-free, 8 (7.2%) patients alive but with recurrences and/or cervical nodal metastases, while 33 (29.7%) died as a result of local recurrence, metastases or other unrelated diseases. The Kaplan-Meier survival analyses revealed that high PKM2 had adverse prognostic impact for patients' outcomes. As indicated in Figure 3, high PKM2 expression in OSCC was significantly associated with short overall survival (Log-rank, P = 0.010) and disease-free survival (Log-rank, P = 0.008). Moreover, as indicated in Table 3, multivariate survival analysis further revealed that PKM2 expression can serve a critical independent prognostic factor for patients' overall survival (P = 0.028) in addition to cervical node metastasis (P = 0.034) and distant metastasis (P = 0.043).
Figure 3.
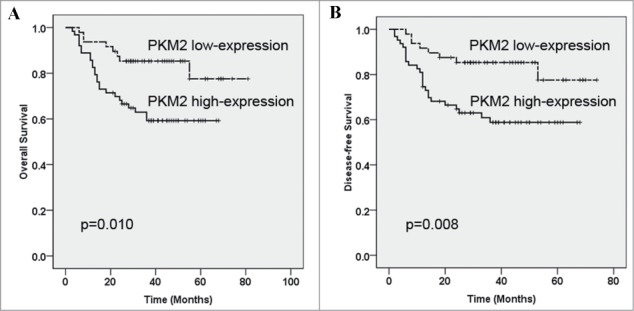
Kaplan-Meier graphs representing the probability of cumulative overall and disease-free survival in OSCC patients based on PKM2 expression. (A) High PKM2 expression significantly associated with reduced overall survival in OSCC patients. (B) High PKM2 expression associated with reduced disease-free survival in OSCC patients. These survival analyses were estimated by Kaplan-Meier method and compared with log-rank test.
Table 3.
Multivariate survival analyses (proportional hazards method) for patients with OSCC
| Variable | Multivariate survival analysis |
||
|---|---|---|---|
| Hazard ratio | 95% CI | p-value | |
| Gender (male, female) | 1.627 | (0.591, 4.479) | 0.346 |
| Age (≤60, >60) | 0.998 | (0.383, 2.611) | 0.699 |
| Primary site | 1.033 | (0.244, 4.366) | 0.565 |
| Tumor size (T1,T2, T3-T4) | 1.380 | (0.672, 2.834) | 0.380 |
| Pathological grade (I, II, III) | 1.150 | (0.613, 2.160) | 0.363 |
| Local recurrence (No, Yes) | 0.953 | (0.573, 1.652) | 0.532 |
| Cervical nodal metastasis (N0, N+) | 2.412 | (1.431, 4.562) | 0.034 |
| Distant metastasis (NO, Yes) | 2.536 | (1.843, 5.231) | 0.043 |
| Clinical stage (I-II, III-IV) | 1.504 | (0.649, 3.227) | 0.611 |
| PKM2 expression (low, high) | 3.118 | (1.445, 5.082) | 0.028 |
Note: The numbers in bold indicate statistical significance.
PKM2 is involved in OSCC cell proliferation, apoptosis and migration
To get in-depth understanding of PKM2 oncogenic roles in OSCC, we next exploited the siRNA-mediated knockdown to assess the phenotypic changes upon endogenous PKM2 depletion in OSCC cell lines. Two siRNAs targeting human PKM2 were designed and transfected into cells in the initial experiments (data not shown). The one siRNA with more knockdown efficiency was selected in the following experiments. The OSCC cell lines (HN4 and HN12) with relatively high endogenous PKM2 were utilized in siRNA experiments.15 As displayed in Figure 4A-B, siPKM2 treatment significantly decreased PKM2 abundance in both mRNA and protein levels as compared with negative control (siNC). Twenty-four hours after siPKM2 transfection, cells were detached and seeded for functional assays. Data from MTT assay revealed that PKM2 silencing remarkably impaired cell proliferation in both cells (Fig. 4C and data not shown). On the other hand, in relative to siNC-treated cells, the apoptotic cell percentage in siPKM2-treated cells were markedly increased from 5.12% to 16.21% (HN4) and from 5.32% to 14.82% (HN12), respectively (Fig. 4D). Furthermore, the results from the migration assay indicated that the migratory property was pronouncedly impaired in PKM2-depleted cells as compared with control cells (Fig. 4E).
Figure 4.
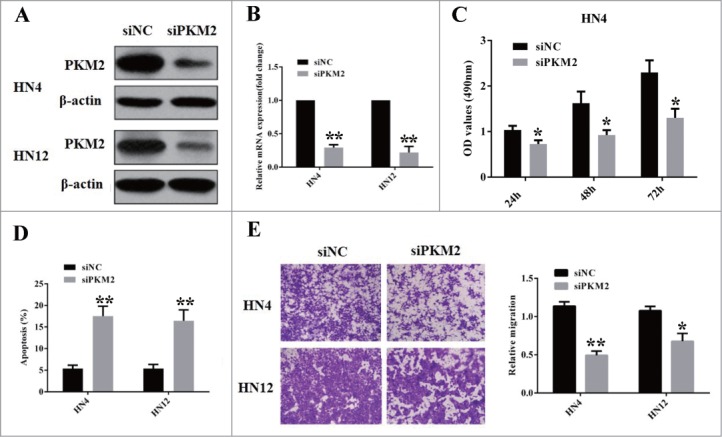
PKM2 is involved in OSCC cell proliferation, apoptosis and migration. (A) Western blot analyses of PKM2 in lysates from HN4 and HN6 cells transfected siRNA targeting PKM2 (100 nM, 48 h). β-actin serves as loading control. A representative image of WB is shown. (B) Real-time RT-PCR analyses of PKM2 mRNA in HN4 and HN6 cells transfected siRNA targeting PKM2 (100 nM, 48 h). **P < 0.01, student-t test. (C) Cell proliferation assay (MTT) was performed at 24, 48 and 72 h after cells were transfected with siPKM2 and siNC 24 h later. Data showed here are mean ± SD from 3 independent experiments. *P < 0.05, student-t test. (D) Cell apoptotic assay was measured after cells were transfected with siPKM2 and siNC 48 h later. Data showed here are mean ± SD from 3 independent experiments. **P < 0.01, student-t test. (E) Cell migratory property was determined via chamber migration assay at 12 h after seeding in cells transfected with siPKM2 and siNC (48 h). Data showed here are mean ± SD from 2 independent experiments, *P < 0.05, **P < 0.01, Student-t test.
Discussion
Aerobic glycolysis represent a pivotal metabolic hallmark of cancer, which is characterized by aberrant glucose consumption to fuel oncogenic growth.5,18 This can be a consequence of oncogenic alternations, as well as glycolytic enzymes or metabolite transporters hyperactivation in cancer cells.18 In particular, among them, PKM2, one of the essential enzymes mediating this process, is aberrantly overexpressed in diverse cancers.9 Genetic or pharmacological depletion of PKM2 impaired tumor growth in several cancer animal models.12,13 These findings support that PKM2 is an bona fide oncogene that might be exploited as cancer biomarker and therapeutic target. In the present study, we analyzed the expression patterns of PKM2 in both chemical-induced OSCC animal model and human primary OSCC and further determined its clinicopathological significance and biological roles. Our findings indicate that PKM2 involves in OSCC initiation and progression likely by modulating cell proliferation, apoptosis and migration. Its overexpression in a significant fraction of OSCC associates with aggressive clinicopathological parameters and unfavorable patients' prognosis.
The human OSCC is characterized by multiple and consecutive pathological stages from hyperplasia to squamous cell carcinoma driven by key oncogenes.19 This feature has been elegantly reproduced in several chemical-induced animal models, such as DMBA-induced buccal pouch SCC in hamster and 4-nitroquinoline 1-oxide(4NQO)-induced tongue SCC in rodents, et al.17,20,21 Here we selected the former model and determined the PKM2 expression during buccal carcinogenesis by DMBA topical application. As anticipated, based on this model, our findings established that PKM2 expression was almost negative in normal epithelial, but gradually became more positive in dysplasia and squamous cell carcinoma, suggesting that PKM2 served as an oncogene critically involved in OSCC pathogenesis.
Previous reports have indicated that PKM2 is commonly overexpressed in a broad spectrum of cancers and required for unchecked tumor overgrowth.7 Overexpression of PKM2 significantly associated with advanced tumor stages and lymph node metastasis and poor prognosis in diverse cancers including head neck squamous cell carcinoma.15,16 Our previous findings also revealed essential roles of PKM2 with clinical significance in tongue squamous cell carcinoma, which was the most common subtype of OSCC. Consistently, we extended our previous findings and further measured PKM2 abundance in another independent cohort of primary OSCCs. Our immunohistochemical data revealed that PKM2 was overexpressed in a major faction of these samples examined. Noticeably, elevated PKM2 associated with tumor size, cervical lymph node metastasis as well as advanced clinical stages. These data indicated that PKM2 overexpression might be a novel diagnostic biomarkers for OSCC. Consistent with our previous findings and others, our results further indicate that high PKM2 significantly associates with decreased patients' overall and disease-free survival in OSCC.8,15,16 Its overexpression served as an independent prognostic factor for patients' overall survival. Therefore, PKM2 measurement in OSCC samples might offer valuable clues about patients' prognostic prediction.
It has been well established that PKM2 couples with other oncogenic metabolism enzymes to confer cancer cells with glycolytic phenotype and growth advantages.6,7 However, the accurate and detailed oncogenic roles of PKM2, especially in OSCC, remains largely unexplored thus far. Herein, we employed the siRNA-mediated knockdown strategy to unravel the biological roles of PKM2 in OSCC cell lines. As anticipated, our data from in vitro experiments revealed that PKM2 is critically involved in cancer cell proliferation and apoptosis. These findings is not very surprising given the well-established roles of PKM2 to fuel oncogenic growth via providing metabolic intermediates in cancers.7 The pro-proliferative functions of PKM2 in OSCC cells was also in accordance with positive association between PKM2 expression and tumor size in our human samples. Interestingly, we also found that cell migration was remarkably impaired upon PKM2 silencing. We reason that PKM2 may have some other unexplored roles during cancer initiation and propagation besides the recognized metabolic regulation. Indeed, recent reports have provided valuable evidence that PKM2 can translocate into nucleus and function as a transcriptional cofactor to activate key oncogenic drivers including c-MYC and HIF-1α in response to extracellular signals.22,23 Moreover, very recently, PKM2 has been identified as a key mediator of epithelial-mesenchymal transition via inhibiting E-cadherin and critical for cancer cells to acquire invasive potential.24 Thus, our data suggest that PKM2 might serve as a pivotal oncogenic driver with multiple roles during OSCC development. Further studies are warranted to further in-depth understand the detailed functions and relevant mechanisms regarding PKM2 underlying oral carcinogenesis.
In conclusion, our findings reveal that PKM2 is critically involved in OSCC initiation and progression probably by promoting cell proliferation and migration as well as reducing apoptosis. Its overexpression correlates with aggressive clinicopathological features and unfavorable patients' outcome. This glycolytic enzyme might serve as a novel biomarker and therapeutic target for OSCC.
Materials and Methods
Cell lines, chemicals, siRNA and transfection
Two human OSCC cell lines HN4 and HN12 were used in the present study. These cells were maintained in a humidified incubator with 5% CO2 at 37°C, grown in DMEM (Invitrogen) added with 10% FBS (Gibco) and penicillin/streptomycin. The carcinogenic chemical polycyclic aromatic hydrocarbon 7,12-dimethyl-1,2-bezan-tracene (DMBA) was purchased from Sigma Aldrich (D3254) and dissolved in mineral oil.
Two small interference RNAs (siRNA) against human PKM2 were designed, synthesized and purchased from Shanghai GenePharma Company. The sequences for siPKM2 were as follows: siPKM2-1: GAGGCTTCTTATAAGTGTTTA; siPKM2-2: CGTGGATGATGGGCTTATTTC. The sequence without targeting any known human genes was used as negative control (siNC). These nucleotides were further transfected into cancer cells at final concentration (100 nM) using Lipofectamine 2000 (Invitrogen) per manufactures' instructions. After 24 or 48 hours following transfection, the cells were harvested for further analyses.
DMBA-induced OSCC animal model
The DMBA-induced hamster buccal pouch squamous cell carcinogenesis model was performed as previous reports with minor modifications.17,20 Briefly, 40 outbred Syrian golden hamsters (male, 6 weeks old, approximately 100 g weight), purchased from Shanghai Laboratory Animal Center, Chinese Academy of Sciences, were randomly divided into 3 experimental and one control groups (10 animals/group). Following acclimatization for 1 week, the left pouches of animals in the experimental groups were painted with a 0.5% DMBA solution using a No. 4 sable-hair brush on every Monday, Wednesday and Friday for consecutive 16 weeks. The control animals were received vehicle only. At 4, 10, 16 weeks, the animals were sacrificed with CO2 inhalation and left buccal pouches were harvested and processed for further histopathological analysis. The animal experimental protocol was approved by our institution and conducted in accordance with the NIH Guide for the Care and Use of Animals.
Cell proliferation and apoptotic assay
Cell proliferation and apoptosis were measured using MTT assay and Annexin V: PE Apoptosis Detection Kit (BD Bioscience) followed flow cytometer respectively, as our previously described.25
Cell migration assay
Cell migration assay was performed using transwell chambers (8-μm pore size, Corning). Cells were starved without serum overnight, then seeded in the upper chambers with serum-free medium. Complete medium containing 10% FBS was added in the lower chambers as chemoattractant. Twelve hours later, the non-invaded cells were removed and those migrated cell located on the lower side were fixed and stained with crystal violet, and counted under microscope.
RNA extraction and real time RT-PCR
Total RNA was extracted from cells with Trizol (Invitrogen) and then subjected to RT-PCR reactions using PrimeScript™ RT-PCR kit (Takara) as described previously.26 The gene-specific primers for human PKM2 and GAPDH were used as previously reported.13,26 Relative mRNA expression was calculated as compared to GAPDH using comparative CT method.
Protein extraction, subcellular fractionation and western blot analysis
Cells were lysed with ice-cold RIPA buffer containing protease inhibitor cocktail (Roche). Lysates were separated by SDS-PAGE, and proteins were transferred onto PVDF membranes (Bio-Rad). After blocking and incubation with primary antibodies (PKM2, Cell signaling, #3198, 1:1000 dilution; β-actin: Santa Cruz, sc-47778, 1:2000 dilution), the blots were detected using appropriate secondary horseradish peroxidase-conjugated secondary antibodies (Invitrogen) and visualized by enhanced chemiluminescence detection (GE healthcare). The relative levels of each protein were determined with Image J software.
Patients and tissue specimens
A total number of 111 patients with primary OSCC receiving surgical treatment at our hospital from 2005 Jan. to 2011 Dec. were included. Patient inclusion criteria were described as follows: (1) primary SCC without any prior therapy; (2) patients received extensive tumor resection and neck dissection; (3) detailed information including clinical, pathological and follow-up data. The archived H&E slides were retrieved and further analyzed to verify the previous histological diagnoses. Written informed consent was obtained from these patients in accordance with our institutional guidelines. This study protocol was reviewed and approved by the Research Ethic Committee of Nanjing Medical University.
Histopathological evaluation and immunohistochemistry
The clinicopathological parameters, such as histological grade, clinical stage, TNM classification, et al were determined as our previous studies.16,26 Immunohistochemical staining for PKM2 was performed on 4-μm formalin-fixed, paraffin-embedded tumor specimens. Briefly, tissue sections from representative paraffin blocks were deparaffinised and rehydrated. Antigens retrieval was performed in microwave heating in 10 mmol/L citrate buffer (pH 6.0) for 15 minutes. These slides were further incubated with primary antibodies (PKM2, Cell signaling, #3198, 1:250 dilution) at 4°C overnight and developed with 3.3'-diaminobenzidine and counterstained with hematoxylin. Negative controls (without primary antibody incubation) were included in each staining run. PKM2 immunoreactivity in each slide was measured independently by oral pathologists who were blinded to all relevant data.
PKM2 immunoreactivity in both animal and human samples was semi-quantitatively evaluated on the basis of staining intensity and distribution using the immunoreactive score which was calculated as intensity score × proportion score as we reported previously.16,26 Intensity score was defined as 0, negative; 1, weak; 2, moderate; 3, strong, while the proportion score was graded as 0, negative; 1, <10%; 2,11–50%; 3,51–80%; 4,>80% positive cells. Thus, the total score ranged from 0–12. Accordingly, PKM2 immunoreactivity in tissue samples was divided into 3 groups based on the final score: 0, negative; 1–4, low expression; 4–12, high expression.
Statistical analysis
All quantitative data in the present study was shown as mean ± SD of 2 or 3 independent experiments and compared with Student's t-test unless otherwise stated. For immunohistochemical analysis, the associations between PKM2 expression and clinicopathological parameters were evaluated using χ2-test or Fisher exact test. The overall or disease-free survival rates were estimated using Kaplan-Meier method and compared with log-rank test. A Cox proportional hazards model was applied to assess the impact of various clinicopathological parameters on patients overall survival. P values less than 0.05 were considered statistically significant.
Funding Statement
This work is financially supported by A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (grant no. 2014-37), China Postdoctoral Science Foundation (2014M560436), Jiangsu Planned Projects for Postdoctoral Research Funds (1402162C), Jiangsu Creative Training Project for College Student (201510312053X), and Jiangsu Creative Training Project for Graduates in Colleges (SJZZ_0119).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
HMW and JC designed the experiment, performed data analysis, interpretation of the results and manuscript writing. YLW, XMZ, CPY, BQ, WZ, DMW and XD collected the samples and performed IHC, animal and cellular experiments. All authors read and approved the final manuscript.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893-917; PMID:21351269; http://dx.doi.org/ 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 2.Rapidis AD, Gullane P, Langdon JD, Lefebvre JL, Scully C, Shah JP. Major advances in the knowledge and understanding of the epidemiology, aetiopathogenesis, diagnosis, management and prognosis of oral cancer. Oral Oncol 2009; 45:299-300; PMID:19411038; http://dx.doi.org/ 10.1016/j.oraloncology.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 3.Bello IO, Soini Y, Salo T. Prognostic evaluation of oral tongue cancer: means, markers and perspectives (II). Oral Oncol 2010; 46:636-43; PMID:20637679; http://dx.doi.org/ 10.1016/j.oraloncology.2010.06.008 [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/ 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 5.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 2012; 21:297-308; PMID:22439925; http://dx.doi.org/ 10.1016/j.ccr.2012.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer 2011; 11:85-95; PMID:21258394; http://dx.doi.org/ 10.1038/nrc2981 [DOI] [PubMed] [Google Scholar]
- 7.Tamada M, Suematsu M, Saya H. Pyruvate kinase M2: multiple faces for conferring benefits on cancer cells. Clin Cancer Res 2012; 18:5554-61; PMID:23071357; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-0859 [DOI] [PubMed] [Google Scholar]
- 8.Peng XC, Gong FM, Zhao YW, Zhou LX, Xie YW, Liao HL, Lin HJ, Li ZY, Tang MH, Tong AP. Comparative proteomic approach identifies PKM2 and cofilin-1 as potential diagnostic, prognostic and therapeutic targets for pulmonary adenocarcinoma. PLoS One 2011; 6:e27309; PMID:22087286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics 2004; 84:1014-20; PMID:15533718; http://dx.doi.org/ 10.1016/j.ygeno.2004.08.010 [DOI] [PubMed] [Google Scholar]
- 10.Yin L, Wang X, Luo C, Liu H, Zhang L, Zhang H, Zhang Y. The value of expression of M2-PK and VEGF in patients with advanced gastric cancer. Cell Biochem Biophys 2013; 67:1033-9; PMID:23625175; http://dx.doi.org/ 10.1007/s12013-013-9601-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene 2006; 25:4633-46; PMID:16892078; http://dx.doi.org/ 10.1038/sj.onc.1209597 [DOI] [PubMed] [Google Scholar]
- 12.Parnell KM, Foulks JM, Nix RN, Clifford A, Bullough J, Luo B, Senina A, Vollmer D, Liu J, McCarthy V, et al.. Pharmacologic activation of PKM2 slows lung tumor xenograft growth. Mol Cancer Ther 2013; 12:1453-60; PMID:23720766; http://dx.doi.org/ 10.1158/1535-7163.MCT-13-0026 [DOI] [PubMed] [Google Scholar]
- 13.Goldberg MS, Sharp PA. Pyruvate kinase M2-specific siRNA induces apoptosis and tumor regression. J Exp Med 2012; 209:217-24; PMID:22271574; http://dx.doi.org/ 10.1084/jem.20111487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong TS, Liu XB, Chung-Wai Ho A, Po-Wing Yuen A, Wai-Man Ng R, Ignace Wei W. Identification of pyruvate kinase type M2 as potential oncoprotein in squamous cell carcinoma of tongue through microRNA profiling. Int J Cancer 2008; 123:251-7. [DOI] [PubMed] [Google Scholar]
- 15.Feng C, Gao Y, Wang C, Yu X, Zhang W, Guan H, Shan Z, Teng W. Aberrant overexpression of pyruvate kinase M2 is associated with aggressive tumor features and the BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab 2013; 98:E1524-33; PMID:23846818; http://dx.doi.org/ 10.1210/jc.2012-4258 [DOI] [PubMed] [Google Scholar]
- 16.Yuan C, Li Z, Wang Y, Qi B, Zhang W, Ye J, Wu H, Jiang H, Song LN, Yang J, et al.. Overexpression of metabolic markers PKM2 and LDH5 correlates with aggressive clinicopathological features and adverse patient prognosis in tongue cancer. Histopathology 2014; 65:595-605; PMID:24762230; http://dx.doi.org/ 10.1111/his.12441 [DOI] [PubMed] [Google Scholar]
- 17.Chen YK, Yang SH, Huang AH, Hsue SS, Lin LM. Aberrant expression in multiple components of the transforming growth factor-beta1-induced Smad signaling pathway during 7,12-dimethylbenz[a]anthracene-induced hamster buccal-pouch squamous-cell carcinogenesis. Oral Oncol 2011; 47:262-7 [DOI] [PubMed] [Google Scholar]
- 18.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324:1029-33; PMID:19460998; http://dx.doi.org/ 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhodus NL, Kerr AR, Patel K. Oral cancer: leukoplakia, premalignancy, and squamous cell carcinoma. Dent Clin North Am 2014; 58:315-40; PMID:24655525; http://dx.doi.org/ 10.1016/j.cden.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 20.Vidya Priyadarsini R, Kumar N, Khan I, Thiyagarajan P, Kondaiah P, Nagini S. Gene expression signature of DMBA-induced hamster buccal pouch carcinomas: modulation by chlorophyllin and ellagic acid. PLoS One 2012; 7:e34628; PMID:22485181; http://dx.doi.org/ 10.1371/journal.pone.0034628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Liang F, Yu D, Qing H, Yang Y. Development of a 4-nitroquinoline-1-oxide model of lymph node metastasis in oral squamous cell carcinoma. Oral Oncol 2013; 49:299-305; PMID:23187306; http://dx.doi.org/ 10.1016/j.oraloncology.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 22.Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo F, Lyssiotis CA, Aldape K, Cantley LC, Lu Z. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol 2012; 14:1295-304; PMID:23178880; http://dx.doi.org/ 10.1038/ncb2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang HJ, Hsieh YJ, Cheng WC, Lin CP, Lin YS, Yang SF, Chen CC, Izumiya Y, Yu JS, Kung HJ, et al.. JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1alpha-mediated glucose metabolism. Proc Natl Acad Sci U S A 2014; 111:279-84; PMID:24344305; http://dx.doi.org/ 10.1073/pnas.1311249111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamabe A, Konno M, Tanuma N, Shima H, Tsunekuni K, Kawamoto K, Nishida N, Koseki J, Mimori K, Gotoh N, et al.. Role of pyruvate kinase M2 in transcriptional regulation leading to epithelial-mesenchymal transition. Proc Natl Acad Sci U S A 2014; 111:15526-31; PMID:25313085; http://dx.doi.org/ 10.1073/pnas.1407717111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Wang Y, Qiu J, Li Q, Yuan C, Zhang W, Wang D, Ye J, Jiang H, Yang J, et al.. The polycomb group protein EZH2 is a novel therapeutic target in tongue cancer. Oncotarget 2013; 4:2532-49; PMID:24345883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Wang Y, Yuan C, Zhu Y, Qiu J, Zhang W, Qi B, Wu H, Ye J, Jiang H, et al.. Oncogenic roles of Bmi1 and its therapeutic inhibition by histone deacetylase inhibitor in tongue cancer. Lab Invest 2014; 94:1431-45; PMID:25286028; http://dx.doi.org/ 10.1038/labinvest.2014.123 [DOI] [PubMed] [Google Scholar]


