Abstract
The subcellular localization of aquaporins belonging to the plasma membrane intrinsic protein (PIP) subfamily is highly regulated. In maize (Zea mays), ZmPIP1s are retained in the endoplasmic reticulum (ER) whereas ZmPIP2s are able to reach the plasma membrane (PM). We recently identified a new sorting determinant which is buried within the third transmembrane domain (TM3) of ZmPIP2;5. The Leu127 and Ala131 are required for the localization of ZmPIP2;5 in the PM and for its exit from the ER. However, when inserted into ZmPIP1;2, these amino acids were not sufficient to export the protein out of the ER. Here, we show that, when inserted into a truncated version of ZmPIP1;2 consisting only of its TM3 region, Leu127 and Ala131 of ZmPIP2;5 are able to partially bring the protein to the PM, demonstrating the active anterograde sorting function of this motif.
Keywords: Aquaporin, Plasma membrane intrinsic protein, Sorting Motif, Secretory Pathway, Trafficking, Transmembrane Domain, Zea mays
Abbreviations
- PIP
plasma membrane intrinsic protein
- ER
endoplasmic reticulum
- PM
plasma membrane
- TM
transmembrane domain
- Zm
Zea mays
- mYFP
monomeric yellow fluorescent protein
Aquaporins are channels that facilitate the diffusion of water and small neutral solutes and play important roles in plants.1-3 Aquaporins of the plasma membrane intrinsic protein (PIP) subfamily have classically been described as localized in the plasma membrane (PM).4,5 However, when expressed alone in maize (Zea mays) cells, PIP proteins belonging to the ZmPIP1 and ZmPIP2 groups display distinct subcellular localization patterns.6 Confocal microscopy experiments have shown that fluorescently-tagged ZmPIP2s are able to reach the PM, whereas ZmPIP1s remain in the endoplasmic reticulum (ER; Fig. 2A, panels 1–6). Only coexpression of ZmPIP2s with ZmPIP1s allows ZmPIP1s to reach the PM as a result of a physical interaction with ZmPIP2s. A diacidic motif in the N-terminus of some PIP2s is required for their export out of the ER.7,8 However, not all PM-localized PIPs contain this motif, and ER-retained ZmPIP1s contain diacidic sequences in their N-terminal tail.6,7 Thus, additional sorting signals must exist to account for the general discrimination between ZmPIP2s and ZmPIP1s along the secretory pathway. In accordance with this, we recently identified a novel trafficking signal required for the correct PM localization of ZmPIP2s.9 The Leu127 and Ala131 residues of ZmPIP2;5 appeared to be critical for the protein to reach the PM, and particularly to exit the ER. Surprisingly, this LxxxA motif is localized in the transmembrane domain (TM) 3. Therefore, contrary to most trafficking signals identified to date, the newly identified sorting motif is not directly accessible to cytosolic trafficking machineries. To link this new trafficking signal with well-established secretory transport mechanisms, interaction with a receptor protein or with specific lipids has been hypothesized.9-11 However, despite their critical role in the anterograde routing of ZmPIP2;5 toward the PM, Leu127 and Ala131 were not sufficient to bring ZmPIP1;2 out of the ER. Here, we present data supporting an active role of Leu127 and Ala131 in the routing of ZmPIP2;5 to the PM.
Figure 2.
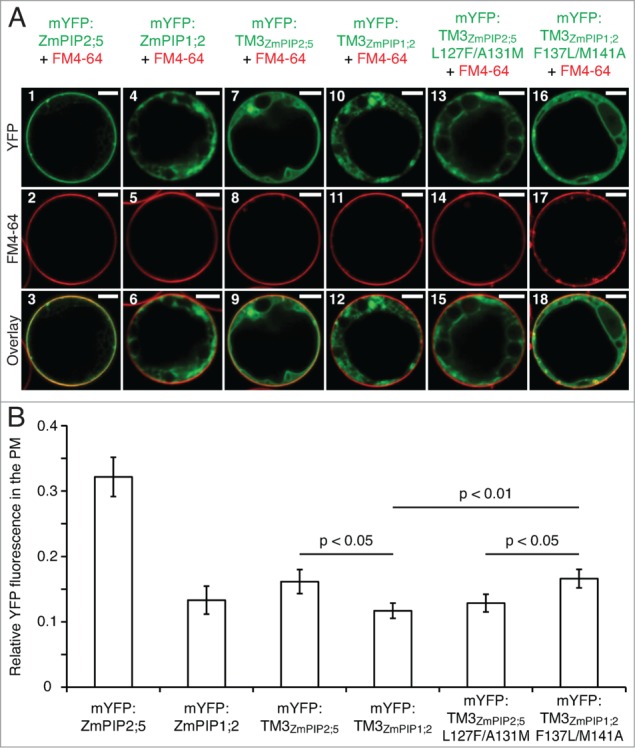
The Leu127 and Ala131 residues of ZmPIP2;5 induce a partial PM localization of mYFP:TM3ZmPIP1;2 in maize mesophyll protoplasts. (A) Protoplasts expressing mYFP:ZmPIP2;5, mYFP:ZmPIP1;2, the TM3 of ZmPIP2;5 fused to the mYFP (mYFP:TM3ZmPIP2;5), the TM3 of ZmPIP1;2 fused to the mYFP (mYFP:TM3ZmPIP1;2), the TM3 of ZmPIP2;5 with the Leu127 and Ala131 residues mutated into their ZmPIP1;2 counterparts fused to the mYFP (mYFP:TM3ZmPIP2;5L127F/A131M), or the TM3 of ZmPIP1;2 in which the Leu127 and Ala131 of ZmPIP2;5 have been inserted fused to the mYFP (mYFP:TM3ZmPIP1;2F137L/M141A) (in green). The protoplasts have been treated with FM4–64 to label the PM (Red). Scale bars = 5 μm. (B) Quantification of the effect of the LxxxA motif on the PM localization of mYFP:TM3ZmPIP1;2. Relative YFP fluorescence intensity in the PM of maize mesophyll protoplasts transiently expressing mYFP:ZmPIP2;5, mYFP:ZmPIP1;2, mYFP:TM3ZmPIP2;5, mYFP:TM3ZmPIP1;2, mYFP:TM3ZmPIP2;5L127F/A131M or mYFP:TM3ZmPIP1;2F137L/M141A. The Y-axis shows the ratio between the fluorescence originating from the PM and the fluorescence originating from the whole cell. Error bars are confidence intervals (a = 0.05). Statistically significant differences and their associated p-values are indicated. The intensity of the PM fluorescence was significantly higher for mYFP:ZmPIP2;5 than for all other fusion proteins, but these p-values are not indicated on the graph for clarity reasons. The localization patterns of the proteins of interest (A) are representative of at least 23 cells. The PM fluorescence calculations (B) have been performed on the same dataset.
In our previous study, we showed that, when fused to the monomeric fluorescent protein (mYFP) the TM3 of ZmPIP1;2 (mYFP:TM3ZmPIP1;2; Fig. 1) and ZmPIP2;5 (mYFP:TM3ZmPIP2;5) are localized in the ER and the PM of leaf epidermal cells, respectively, reflecting the localization of the full-length proteins.9 Here, to test whether Leu127 and Ala131 of ZmPIP2;5 were able to confer their anterograde trafficking function to another protein, these amino acid residues were inserted into the single-TM reporter mYFP:TM3ZmPIP1;2 (mYFP:TM3ZmPIP1;2F137L/M141A; the numbering of the amino acids refers to their position in the full-length proteins; Fig. 1). The ZmPIP2;5 counterpart (mYFP:TM3ZmPIP2;5L127F/A131M) was generated as well. The subcellular localization of these proteins was analyzed in maize mesophyll protoplasts (Fig. 2). The YFP-fused TM3 region of ZmPIP1;2 (mYFP:TM3ZmPIP1;2) was fully blocked in intracellular membranes, while the ZmPIP2;5-derived reporter mYFP:TM3ZmPIP2;5 could reach the PM (Fig. 2A, panels 7–12). But mYFP:TM3ZmPIP2;5 was also partially localized in intracellular structures in addition to the PM. As expected, mutation of the critical L and A residues (mYFP:TM3ZmPIP2;5L127F/A131M) completely prevented the protein to reach the PM (Fig. 2A, panels 13–15). Interestingly, insertion of the L and A residues into the TM3 of ZmPIP1;2 (mYFP:TM3ZmPIP1;2F137L/M141A) allowed the protein to partially reach the PM (Fig. 2A, panels 16–18). Even though only a fraction of the signal originated from the PM of protoplasts expressing mYFP:TM3ZmPIP2;5 or mYFP:TM3ZmPIP1;2F137L/M141A, there was a clear difference compared to the localization of mYFP:TM3ZmPIP1;2 and mYFP:TM3ZmPIP2;5L127F/A131M. While mYFP:TM3ZmPIP1;2 and mYFP:TM3ZmPIP2;5L127F/A131M were fully absent from the PM and localized only in a structure reminiscent of the ER, mYFP:TM3ZmPIP2;5 and mYFP:TM3ZmPIP1;2F137L/M141A partially reached the PM, but were also localized in an intracellular membrane surrounding a large intracellular compartment, tentatively identified as the tonoplast, and in the ER. This partial PM localization pattern was seen in 50% and 69% of the cells expressing mYFP:TM3ZmPIP2;5 and mYFP:TM3ZmPIP1;2F137L/M141A, respectively.
Figure 1.
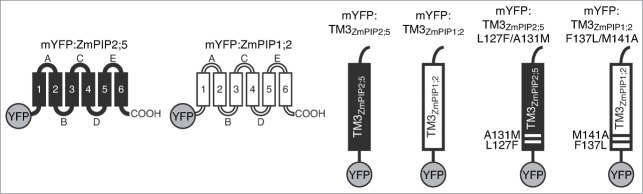
Cartoon representation of mYFP-tagged proteins analyzed in this study. ZmPIP2;5 portions are shown in black and ZmPIP1;2 portions are shown in white. The mYFP is represented as a gray sphere. Site-directed mutations, relative to their respective position in full-length ZmPIP2;5 and ZmPIP1;2, are indicated.
To assess these results, the relative signal in the protoplast PM was quantified as described in Besserer et al.12 (Fig. 2B). With the exception of mYFP:ZmPIP2;5, all proteins displayed a relatively weak signal in the PM, but statistically significant differences could be observed. As expected, insertion of the critical L and A residues into the TM3 of ZmPIP1;2 (mYFP:TM3ZmPIP1;2F137L/M141A) induced a significant increase of the PM signal compared to mYFP:TM3ZmPIP1;2. The PM fluorescence observed for mYFP:TM3ZmPIP1;2F137L/M141A and mYFP:TM3ZmPIP2;5 was similar, indicating that the trafficking function of Leu127 and Ala131 of ZmPIP2;5 could indeed be transferred to mYFP:TM3ZmPIP1;2. In support of this, mYFP:TM3ZmPIP1;2F137L/M141A was significantly more present in the PM than mYFP:TM3ZmPIP2;5L127F/A131M, while the non-mutated TM3 reporters showed the opposite behavior. Finally, despites its partial intracellular localization, mYFP:TM3ZmPIP2;5 was significantly more targeted to the PM than mYFP:TM3ZmPIP1;2, confirming the qualitative data obtained in epidermal cells.9
Even though they were statistically significant, the differences between ER-retained (mYFP:TM3ZmPIP1;2 and mYFP:TM3ZmPIP2;5L127F/A131M) and PM-localized (mYFP:TM3ZmPIP2;5 and mYFP:TM3ZmPIP1;2F137L/M141A) TM3 fusions were relatively weak. The fluorescent signal in the PM might have been underestimated for the latter proteins as a result of their partial ER and tonoplast localization. The observed presence of two reporters (mYFP:TM3ZmPIP2;5 and mYFP:TM3ZmPIP1;2F137L/M141A) in a structure reminiscent of the tonoplast, in addition to the ER and the PM, is surprising. The fact that these proteins reach post-ER compartments shows that the TM3-based LxxxA motif of ZmPIP2;5 indeed confers to these reporter proteins a partial ability to leave the ER. However, compared to ZmPIP2;5 full length, a fraction of the pool of mYFP:TM3ZmPIP2;5 and mYFP:TM3ZmPIP1;2F137L/M141A proteins seems to be misrouted at the TGN toward the vacuolar membrane. This suggests that the correct localization of ZmPIP2;5 in the PM relies on both the diacidic and LxxxA motifs for ER export, but likely also on other trafficking determinants localized in regions of the protein different than the TM3 for PM targeting from the TGN. This observation underlines the complexity of the subcellular sorting of PIP aquaporins toward the PM, and suggests the existence of even other, yet unidentified, trafficking motifs in these proteins.
Finally, the mutated and non-mutated single-TM reporter proteins have been expressed in leaf epidermal cells by microparticle-mediated DNA delivery (Fig. 3). This confirmed the results obtained in the protoplast system. Mutation of the Leu127 and Ala131 residues in the TM3 of ZmPIP2;5 (mYFP:TM3ZmPIP2;5L127F/A131M) dramatically retained the protein in intracellular structures. On the other hand, insertion of ZmPIP2;5 Leu127 and Ala131 into the TM3 of ZmPIP1;2 (mYFP:TM3ZmPIP1;2F137L/M141A) allowed the protein to partially reach the PM. However, some unidentified intracellular punctate structures were labeled in addition to the PM.
Figure 3.
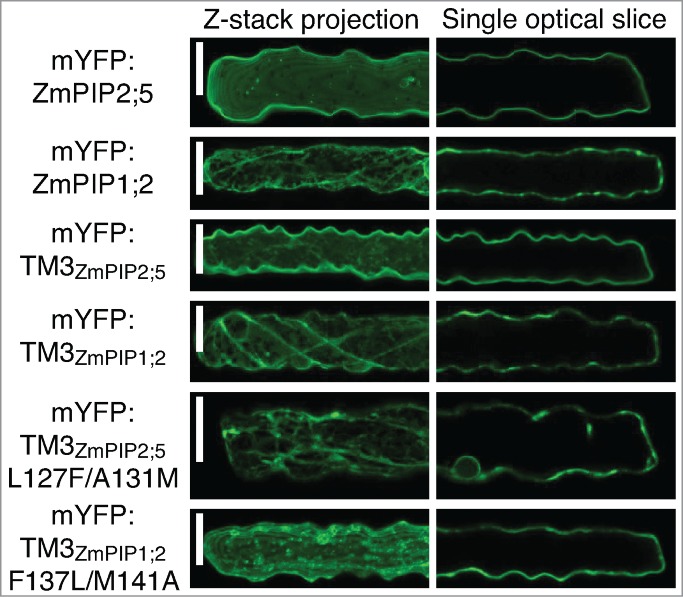
The LxxxA motif of ZmPIP2;5 partially brings mYFP:TM3ZmPIP1;2 to the PM in maize leaf epidermal cells. Maize leaf epidermal cells expressing mYFP:ZmPIP2;5, mYFP:ZmPIP1;2, mYFP:TM3ZmPIP2;5, mYFP:TM3ZmPIP1;2, mYFP:TM3ZmPIP2;5L127F/A131M or mYFP:TM3ZmPIP1;2F137L/M141A. The left half of each cell is shown as a maximum projection of a Z-stack to visualize intracellular structures. The localization patterns of the proteins of interest are representative of a total of at least 13 cells coming from a minimum of 2 independent experiments. Scale bars = 20 μm.
In this short report, the use of artificial single-TM reporter proteins allowed to highlight the active anterograde sorting function of the Leu127 and Ala131 residues of ZmPIP2;5. This signal also functions when transferred into the single TM3 of ZmPIP1;2 whereas its effect was masked when full-length ZmPIP1;2 was used, likely as a consequence of the presence of other sorting signals.7,9
Acknowledgments
The authors would like to thank Pierre Morsomme, Henri Batoko, Catherine Navarre and Marie C. Berny for helpful discussions, and the IMABIOL imaging platform. This work was supported by grants from the Belgian National Fund for Scientific Research, the Interuniversity Attraction Poles Program-Belgian Science Policy, the Communauté française de Belgique-Actions de Recherches Concertées, the Francqui Foundation, and the Bauchau Award. A.S.C. was a research fellow at the Fonds de Formation à la Recherche dans l'Industrie et l'Agriculture (FNRS, grant number FC89796).
References
- 1. Maurel C, Verdoucq L, Luu DT, Santoni V. Plant aquaporins: Membrane channels with multiple integrated functions. Ann Rev Plant Biol 2008; 59:595-624; PMID:18444909; http://dx.doi.org/ 10.1146/annurev.arplant.59.032607.092734 [DOI] [PubMed] [Google Scholar]
- 2. Chaumont F, Tyerman SD. Aquaporins: highly regulated channels controlling plant water relations. Plant Physiol 2014; 164:1600-18; PMID:24449709; http://dx.doi.org/ 10.1104/pp.113.233791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li G, Santoni V, Maurel C. Plant aquaporins: roles in plant physiology. Biochim Biophys Acta 2014; 1840:1574-82; PMID:24246957; http://dx.doi.org/ 10.1016/j.bbagen.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 4. Hachez C, Besserer A, Chevalier AS, Chaumont F. Insights into plant plasma membrane aquaporin trafficking. Trends Plant Sci 2013; 18:344-52; PMID:23291163; http://dx.doi.org/ 10.1016/j.tplants.2012.12.003 [DOI] [PubMed] [Google Scholar]
- 5. Luu DT, Maurel C. Aquaporin trafficking in plant cells: an emerging membrane-protein model. Traffic 2013; 14:629-35; PMID:23425337; http://dx.doi.org/ 10.1111/tra.12062 [DOI] [PubMed] [Google Scholar]
- 6. Zelazny E, Borst JW, Muylaert M, Batoko H, Hemminga MA, Chaumont F. FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proc Natl Acad Sci U S A 2007; 104:12359-64; PMID:17636130; http://dx.doi.org/ 10.1073/pnas.0701180104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zelazny E, Miecielica U, Borst JW, Hemminga MA, Chaumont F. An N-terminal diacidic motif is required for the trafficking of maize aquaporins ZmPIP2;4 and ZmPIP2;5 to the plasma membrane. Plant J 2009; 57:346-55; PMID:18808456; http://dx.doi.org/ 10.1111/j.1365-313X.2008.03691.x [DOI] [PubMed] [Google Scholar]
- 8. Sorieul M, Santoni V, Maurel C, Luu DT. Mechanisms and effects of retention of over-expressed aquaporin AtPIP2;1 in the endoplasmic reticulum. Traffic 2011; 12:473-82; PMID:21182578; http://dx.doi.org/ 10.1111/j.1600-0854.2010.01154.x [DOI] [PubMed] [Google Scholar]
- 9. Chevalier AS, Bienert GP, Chaumont F. A New LxxxA Motif in the Transmembrane Helix3 of Maize Aquaporins Belonging to the Plasma Membrane Intrinsic Protein PIP2 Group Is Required for Their Trafficking to the Plasma Membrane. Plant Physiol 2014; 166:125-38; PMID:24989232; http://dx.doi.org/ 10.1104/pp.114.240945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nebenfuhr A. Targeting of polytopic proteins to the plasma membrane. Plant Physiol 2014; 166:3-4; PMID:25174046; http://dx.doi.org/ 10.1104/pp.114.247080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cosson P, Perrin J, Bonifacino JS. Anchors aweigh: protein localization and transport mediated by transmembrane domains. Trends Cell Biol 2013; 23:511-7; PMID:23806646; http://dx.doi.org/ 10.1016/j.tcb.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Besserer A, Burnotte E, Bienert GP, Chevalier AS, Errachid A, Grefen C, Blatt MR, Chaumont F. Selective regulation of maize plasma membrane aquaporin trafficking and activity by the SNARE SYP121. Plant Cell 2012; 24:3463-81; PMID:22942383; http://dx.doi.org/ 10.1105/tpc.112.101758 [DOI] [PMC free article] [PubMed] [Google Scholar]


