Abstract
The first indication of the aluminum (Al) toxicity in plants growing in acidic soils is the cessation of root growth, but the detailed mechanism of Al effect is unknown. Here we examined the impact of Al stress on the activity of non-specific phospholipase C (NPC) in the connection with the processes related to the plasma membrane using fluorescently labeled phosphatidylcholine. We observed a rapid and significant decrease of labeled diacylglycerol (DAG), product of NPC activity, in Arabidopsis seedlings treated with AlCl3. Interestingly, an application of the membrane fluidizer, benzyl alcohol, restored the level of DAG during Al treatment. Our observations suggest that the activity of NPC is affected by Al-induced changes in plasma membrane physical properties.
Keywords: aluminum toxicity, Arabidopsis thaliana, benzyl alcohol, BODIPY, diacylglycerol, membrane fluidity, non-specific phospholipase C
List of abbreviations
- BA
benzyl alcohol
- BODIPY
4, 4-difluoro-4-bora-3a, 4a-diaza-s-indacene
- BY-2
Bright Yellow 2
- DAG
diacylglycerol
- HP-TLC
high-performance thin-layer chromatography
- MS
Murashige-Skoog
- NPC
non-specific phospholipase C
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PC-PLC
phosphatidylcholine-specific phospholipase C
- PIP2
phosphatidylinositol 4, 5-bisphosphate
- PI-PLC
phosphatidylinositol-specific phospholipase C
- PLD
phospholipase D
- PM
plasma membrane
Low pH of soil enables release of toxic aluminum (Al) ions from its insoluble forms fixed in soil minerals causing that Al is a major growth–limiting factor in the regions with acid soils. Prolonged exposure to Al ions leads to changes in root morphology, e.g. root thickening, bursting, changes in the cell wall architecture, and even cell death. However, the first indication of the Al toxicity in plants is rapid cessation of root growth. The root tip has been found to be the most Al-responsive part of roots.1 Although molecular mechanisms of the prompt Al-mediated root growth inhibition are largely unclear, it is known that a number of physiologically important processes connected with plasma membrane (PM) are affected by Al.2-9 The rapid response of root growth suggests that signaling pathways are a part of the mechanism participating in Al toxicity.
Phospholipid-signaling pathway is now considered to be one of the important plant signaling mechanisms involved in many different reactions of plants to environmental factors such as drought, cold, salinity or pathogen attack (for a review see refs.10,11). Al has been shown to affect the phospholipid-signaling pathway as well. Changes of phospholipase A2 activity in vitro,12 phospholipase D (PLD) activity13,14 and phosphatidylinositol-specific phospholipase C (PI-PLC) activity15-17 after Al treatment were demonstrated. In addition to PI-PLC, non-specific phospholipase C (NPC, PC-PLC in prokaryotes and animals), an enzyme that is able to hydrolyse phosphatidylcholine (PC) instead of phosphatidylinositol 4,5-bisphosphate (PIP2), was characterized in plants18 in relation with a number of different cell processes or stress condition18-26 including Al stress.27,28
Studies of the target of Al action in plants have demonstrated that Al binds to the apoplast29 and changes the properties of the PM.8,9 Recent study introduced by Krtková et al.30 revealed that Al treatment induced cessation of Arabidopsis root growth and loss of membrane fluidity. When a membrane fluidizer benzyl alcohol was applied in the presence of Al, membrane fluidity was restored. Moreover, partial restoration of root growth was observed. This suggests that the physical state of PM could play a role in morphological changes caused by Al toxicity. We previously described that the formation of diacylglycerol (DAG) generated by NPC is rapidly inhibited by Al in tobacco cell line BY-2 and in tobacco pollen tubes, that Al inhibits growth of tobacco pollen tubes and that this growth, arrested by Al, can be rescued by externally added DAG.27 This raises question: what is the role of the NPC/DAG in aluminum toxicity? Here we report that NPC activity in Arabidopsis seedlings is influenced by the physical state of PM.
Because we worked with different plant model organism than in our previous work,27 first we tested the reaction of Arabidopsis seedlings to Al stress in the view of NPC activity. We utilized the fluorescent derivative of PC (BODIPY-PC) as a phospholipase substrate. For NPC activity measurements, 7-day-old seedlings were treated with different concentrations of AlCl3 in the presence of BODIPY-PC for 2 h. HP-TLC analysis of the labeled products showed significantly lower production of BODIPY-DAG in the seedlings treated with AlCl3 (Fig. 1). The effect of Al on DAG formation was concentration-dependent. Lower DAG production (74% of control, non-treated seedlings) was detected already for 1 µM AlCl3 and was more pronounced for 10 µM and 100 µM AlCl3 (Fig. 1). Based on these results, 10 µM AlCl3 was chosen as a working concentration for in situ measurements of NPC activity in following experiments. In contrast to NPC activity in situ in Arabidopsis seedlings under salt stress,25 here we observed negative effect of Al on DAG production. Thus, the possible influence of Al ions on BODIPY-PC incorporation into the roots of Arabidopsis seedlings exposed to Al treatment was examined by laser scanning confocal microscopy. Rapid incorporation for both Al-treated and non-treated seedlings with no effect of Al on this process was observed (Fig. 2). This makes in situ method usable also for Al-treated Arabidopsis seedlings.
Figure 1.
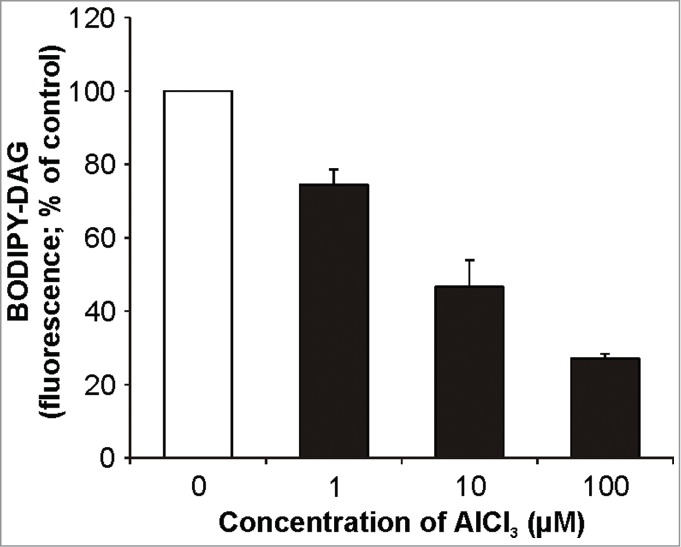
Concentration dependence of BODIPY-DAG production in Arabidopsis seedlings treated with Al. NPC activity in Arabidopsis seedlings was measured according to Kocourková et al.25 Seven-day-old Arabidopsis seedlings (5 seedlings for each sample) were grown in MS medium, transferred into 1/8 MS medium (pH 4) containing different concentrations in the range 0–100 µM AlCl3 and 0.66 µg ml−1 of fluorescent phosphatidylcholine (BODIPY-PC, D-3771, Invitrogen, USA) and were incubated on an orbital shaker at 23°C for 2 h. Lipids were extracted, separated by high-performance thin layer chromatography and quantified. Identification of the BODIPY-DAG corresponding spot was based on comparison with the BODIPY-DAG standard prepared as described earlier.31 Each value is related to the control non-treated cells (100%). The plotted values are the means + SD from 3 independent experiments run in duplicates. DAG; diacylglycerol, NPC; non-specific phospholipase C, PC; phosphatidylcholine.
Figure 2.
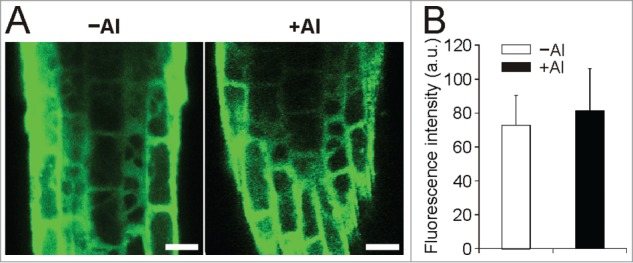
Incorporation of BODIPY-PC into the Arabidopsis roots is not affected by Al. A) Seven-day-old Arabidopsis seedlings were grown, fluorescently labeled and treated with 10 µM AlCl3 for 15 min as described in Figure 1. Incorporation of BODIPY-PC was observed by Zeiss LSM 5 DUO confocal laser scanning microscope. Bar = 20 µm. B) Quantification of BODIPY-PC incorporation. Mean pixel intensity in the meristematic zone of root tip was measured using ImageJ software. The plotted values are the means + SD. PC; phosphatidylcholine.
Based on observations concerning Al-induced changes of membrane fluidity30 and as we detected the most NPC activity in the PM-enriched fraction,27 we examined the possible connection between PM physical state and NPC activity during Al stress. We utilized benzyl alcohol to change PM fluidity and we measured DAG formation in Arabidopsis wt seedlings. Seven-day-old seedlings were treated with 20 mM benzyl alcohol with or without 10 µM AlCl3 in the presence of BODIPY-PC for 2 h. HP-TLC analysis of the labeled products showed that BODIPY-DAG production is decreased in seedlings treated with AlCl3. In contrast, higher production of BODIPY-DAG was observed when seedlings were treated with benzyl alcohol alone compared to non-treated seedlings. Intriguingly, strong effect on DAG formation was observed when the seedlings were treated with benzyl alcohol and AlCl3 together. In that case, benzyl alcohol was able to restore the inhibition of BODIPY-DAG formation induced by Al-treatment alone (Fig. 3) and thus to prevent inhibiting effect of Al on NPC activity. Similar results were observed for the tobacco cell line BY-2 and pollen tubes (data not shown). These results indicate that the physical state of PM influences NPC activity that leads to decrease in the DAG level during Al stress.
Figure 3.
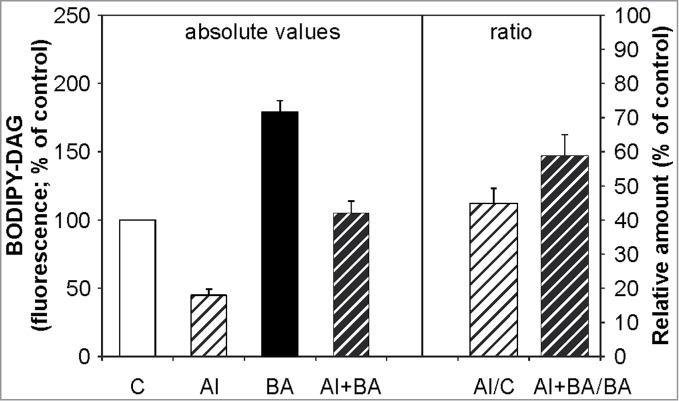
Effect of benzyl alcohol on BODIPY-diacylglycerol formation in Al-treated Arabidopsis seedlings. Seven-day-old Arabidopsis seedlings were grown, fluorescently labeled, treated with 20 mM benzyl alcohol and 10 µM AlCl3 for 2 h and processed as described in Figure 1. Each value is related to the control non-treated cells (100%). The plotted values are the means + SD from 5 independent experiments run in duplicates. BA; benzyl alcohol, C; non-treated control, DAG; diacylglycerol.
Interestingly, some components of prokaryotic (gram-positive bacteria Clostridium perfringens and Bacillus cereus) phospholipid-signaling pathway have been shown to be affected by Al-induced changes in membrane properties.32,33 Al3+-mediated changes on membrane fluidity did not affect PC-PLC-mediated hydrolysis of PC while PI-PLC-mediated hydrolysis of PIP2 was decreased in liposomes. Authors suggest that it could be partially due to a higher lipid packing induced by Al3+, which could affect the interaction between the enzyme and its substrate.32 However, we showed previously that the activity of NPC, an enzyme that is able to hydrolyse PC, is affected by Al in tobacco27 and we confirmed such finding in this study also for Arabidopsis. The possible explanation of this difference is that plant NPCs, a gene family that consist of 6 members denoted NPC1-NPC6 in Arabidopsis, are similar to gram-negative bacterial PC-PLCs, not to gram-positive ones.34 This could result in a slightly different mechanism of response to the Al-induced changes in membrane properties. However, both affected enzymatic activities produce DAG. This may suggest that DAG is an important molecule in Al3+-mediated changes on membrane fluidity rather than the type of DAG-producing enzyme.
DAG is known as a key intermediate of glycerolipid metabolism in plants.35 Additionally, NPC family in plants was first characterized in relation to phospholipid-to-galactosyl diacylglycerol exchange.18 In contrast, the signaling role of DAG in plants is under debate but a number of studies imply that DAG is likely to act as a signaling molecule in some systems36 including Al stress.27,28 However, DAG seems to be important also for the structure and dynamics of biological membranes. DAG can modify membrane curvature and can induce unstable asymmetric regions in membrane bilayers.37,38 Moreover, molecular dynamics simulations revealed that DAG decreases lateral diffusion of molecules, produces a strong “condensing effect” in PC bilayers and increases the spacing between PC headgroups with the largest spacing usually occurring between the first and the second nearest-neighbor PC headgroups from a DAG, due to the umbrella effect.39 Compared to other lipids, DAG has also increased translocation across membrane leaflets (flip-flop).40 These instabilities are important in many physiological processes, such as exocytosis, endocytosis, membrane biogenesis and cell division. Moreover, it has been found that mechanism of the rapid Al-mediated inhibition of the root growth is related to the inhibition of endocytosis9,30 and the root growth was described to be controlled through the local auxin biosynthesis and signaling.41,42 Intriguingly, the overexpression of auxin transporter gene OsPIN2 alleviated Al-mediated membrane rigidity43 and NPC3 and NPC4, members of the Arabidopsis NPC gene family, were shown to be affected by auxin.24 Here we show for the first time that plant NPC activity is altered by physical state of plasma membrane. We hypothesize that Al-mediated membrane rigidity decreases NPC activity. This leads to the decrease of DAG production and to the modification of plasma membrane that could result in the inhibition of the processes mentioned above.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Daniela Kocourková and Kateřina Raková for their excellent technical assistance.
Funding
This work was supported by the Czech Science Foundation grants no. P501/12/P950, P501/12/1942 and 13–19073S.
References
- 1.Panda SK, Baluška F, Matsumoto H. Aluminum stress signaling in plants. Plant Signal Behav 2009; 4:592-7; PMID:19820334; http://dx.doi.org/ 10.4161/psb.4.7.8903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boscolo PRS, Menossi M, Jorge RA. Aluminum-induced oxidative stress in maize. Phytochemistry 2003; 62:181-9; PMID:12482454; http://dx.doi.org/ 10.1016/S0031-9422(02)00491-0 [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto H. Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol 2000; 200:1-46; PMID:10965465; http://dx.doi.org/ 10.1016/S0074-7696(00)00001-2 [DOI] [PubMed] [Google Scholar]
- 4.Rengel Z, Zhang WH. Role of dynamics of intracellular calcium in aluminium-toxicity syndrome. New Phytol 2003; 159:295-314; http://dx.doi.org/ 10.1046/j.1469-8137.2003.00821.x [DOI] [PubMed] [Google Scholar]
- 5.Tian Q-Y, Sun D-H, Zhao M-G, Zhang W-H. Inhibition of nitric oxide synthase (NOS) underlies aluminum-induced inhibition of root elongation in Hibiscus moscheutos. New Phytol 2007; 174:322-31; PMID:17388895; http://dx.doi.org/ 10.1111/j.1469-8137.2007.02005.x [DOI] [PubMed] [Google Scholar]
- 6.Schwarzerová K, Zelenková S, Nick P, Opatrný Z. Aluminum-induced rapid changes in the microtubular cytoskeleton of tobacco cell lines. Plant Cell Physiol 2002; 43:207-16; PMID:11867700; http://dx.doi.org/ 10.1093/pcp/pcf028 [DOI] [PubMed] [Google Scholar]
- 7.Sivaguru M, Baluška F, Volkmann D, Felle HH, Horst WJ. Impacts of aluminum on the cytoskeleton of the maize root apex. Short-term effects on the distal part of the transition zone. Plant Physiol 1999; 119:1073-82; PMID:10069846; http://dx.doi.org/ 10.1104/pp.119.3.1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sivaguru M, Pike S, Gassmann W, Baskin TI. Aluminum rapidly depolymerizes cortical microtubules and depolarizes the plasma membrane:Evidence that these responses are mediated by a glutamate receptor. Plant Cell Physiol 2003; 44:667-75; PMID:12881494; http://dx.doi.org/ 10.1093/pcp/pcg094 [DOI] [PubMed] [Google Scholar]
- 9.Illéš P, Schlicht M, Pavlovkin J, Lichtscheidl I, Baluška F, Ovečka M. Aluminium toxicity in plants:internalization of aluminium into cells of the transition zone in Arabidopsis root apices related to changes in plasma membrane potential, endosomal behaviour, and nitric oxide production. J Exp Bot 2006; 57:4201-13; PMID:17085753; http://dx.doi.org/ 10.1093/jxb/erl197 [DOI] [PubMed] [Google Scholar]
- 10.Munnik T, ed. Lipid Signaling in Plants. Berlin:Springer, 2010 [Google Scholar]
- 11.Wang X, ed. Phospholipases in Plant Signaling. Berlin:Springer-Verlag, 2014 [Google Scholar]
- 12.Jones DL, Kochian LV. Aluminum interaction with plasma membrane lipids and enzyme metal binding sites and its potential role in Al cytotoxicity. FEBS Lett 1997; 400:51-7; PMID:9000512; http://dx.doi.org/ 10.1016/S0014-5793(96)01319-1 [DOI] [PubMed] [Google Scholar]
- 13.Pejchar P, Pleskot R, Schwarzerová K, Martinec J, Valentová O, Novotná Z. Aluminum ions inhibit phospholipase D in a microtubule-dependent manner. Cell Biol Int 2008; 32:554-6; PMID:18164219; http://dx.doi.org/ 10.1016/j.cellbi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Wang C, Bedair M, Welti R, Sumner LW, Baxter I, Wang X. Suppression of phospholipase Dγs confers increased aluminum resistance in Arabidopsis thaliana. PLoS One 2011; 6:e28086; PMID:22163277; http://dx.doi.org/ 10.1371/journal.pone.0028086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones DL, Kochian LV. Aluminium inhibition of the inositol 1,4,5-trisphosphate signal transduction pathway in wheat roots:A role in aluminium toxicity? Plant Cell 1995; 7:1913-22; PMID:12242363; http://dx.doi.org/ 10.1105/tpc.7.11.1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez-Estévez M, Racagni-Di , Palma G, Muñoz-Sánchez JA, Brito-Argáez L, Loyola-Vargas VM, Hernández-Sotomayor SMT. Aluminium differentially modifies lipid metabolism from the phosphoinositide pathway in Coffea arabica cells. J Plant Physiol 2003; 160:1297-303; PMID:14658381; http://dx.doi.org/ 10.1078/0176-1617-01168 [DOI] [PubMed] [Google Scholar]
- 17.Ramos-Díaz A, Brito-Argáez L, Munnik T, Hernández-Sotomayor S. Aluminum inhibits phosphatidic acid formation by blocking the phospholipase C pathway. Planta 2007; 225:393-401; PMID:16821040; http://dx.doi.org/ 10.1007/s00425-006-0348-3 [DOI] [PubMed] [Google Scholar]
- 18.Nakamura Y, Awai K, Masuda T, Yoshioka Y, Takamiya K, Ohta H. A novel phosphatidylcholine-hydrolyzing phospholipase C induced by phosphate starvation in Arabidopsis. J Biol Chem 2005; 280:7469-76; PMID:15618226; http://dx.doi.org/ 10.1074/jbc.M408799200 [DOI] [PubMed] [Google Scholar]
- 19.Andersson MX, Larsson KE, Tjellström H, Liljenberg C, Sandelius AS. Phosphate-limited oat. the plasma membrane and the tonoplast as major targets for phospholipid-to-glycolipid replacement and stimulation of phospholipases in the plasma membrane. J Biol Chem 2005; 280:27578-86; PMID:15927962; http://dx.doi.org/ 10.1074/jbc.M503273200 [DOI] [PubMed] [Google Scholar]
- 20.Gaude N, Nakamura Y, Scheible WR, Ohta H, Dormann P. Phospholipase C5 (NPC5) is involved in galactolipid accumulation during phosphate limitation in leaves of Arabidopsis. Plant J 2008; 56:28-39; PMID:18564386; http://dx.doi.org/ 10.1111/j.1365-313X.2008.03582.x [DOI] [PubMed] [Google Scholar]
- 21.Tjellström H, Andersson MX, Larsson KL, Sandelius AS. Membrane phospholipids as a phosphate reserve:the dynamic nature of phospholipid-to-digalactosyl diacylglycerol exchange in higher plants. Plant Cell Environ 2008; 31:1388-98; PMID:18643953; http://dx.doi.org/ 10.1111/j.1365-3040.2008.01851.x [DOI] [PubMed] [Google Scholar]
- 22.Scherer GFE, Paul RU, Holk A, Martinec J. Down-regulation by elicitors of phosphatidylcholine-hydrolyzing phospholipase C and up-regulation of phospholipase A in plant cells. Biochem Biophys Res Commun 2002; 293:766-70; PMID:12054536; http://dx.doi.org/ 10.1016/S0006-291X(02)00292-9 [DOI] [PubMed] [Google Scholar]
- 23.Peters C, Li MY, Narasimhan R, Roth M, Welti R, Wang XM. Nonspecific phospholipase C NPC4 promotes responses to abscisic acid and tolerance to hyperosmotic stress in Arabidopsis. Plant Cell 2010; 22:2642-59; PMID:20699393; http://dx.doi.org/ 10.1105/tpc.109.071720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wimalasekera R, Pejchar P, Holk A, Martinec J, Scherer GFE. Plant phosphatidylcholine-hydrolyzing phospholipases C NPC3 and NPC4 with roles in root development and brassinolide signalling in Arabidopsis thaliana. Mol Plant 2010; 3:610-25; PMID:20507939; http://dx.doi.org/ 10.1093/mp/ssq005 [DOI] [PubMed] [Google Scholar]
- 25.Kocourková D, Krčková Z, Pejchar P, Veselková Š, Valentová O, Wimalasekera R, Scherer GFE, Martinec J. The phosphatidylcholine-hydrolyzing phospholipase C NPC4 plays a role in response of Arabidopsis roots to salt stress. J Exp Bot 2011; 62:3753-63; PMID:21525137; http://dx.doi.org/ 10.1093/jxb/err039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters C, Kim S-C, Devaiah S, Li M, Wang X. Non-specific phospholipase C5 and diacylglycerol promote lateral root development under mild salt stress in Arabidopsis. Plant Cell Environ 2014; 37:2002-13; PMID:24689655; http://dx.doi.org/ 10.1111/pce.12334 [DOI] [PubMed] [Google Scholar]
- 27.Pejchar P, Potocký M, Novotná Z, Veselková Š, Kocourková D, Valentová O, Schwarzerová K, Martinec J. Aluminium ions inhibit formation of diacylglycerol generated by phosphatidylcholine-hydrolysing phospholipase C in tobacco cells. New Phytol 2010; 188:150-60; PMID:20629955; http://dx.doi.org/ 10.1111/j.1469-8137.2010.03349.x [DOI] [PubMed] [Google Scholar]
- 28.Pejchar P, Potocký M, Krčková Z, Brouzdová J, Daněk M, Martinec J. Non-specific phospholipase C4 mediates response to aluminum toxicity in Arabidopsis thaliana. Front Plant Sci 2015; 6:66; PMID:25763003; http://dx.doi.org/ 10.3389/fpls.2015.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wissemeier AH, Horst WJ. Effect of calcium supply on aluminium-induced callose formation, its distribution and persistence in roots of soybean (Glycine max (L.) Merr.). J Plant Physiol 1995; 145:470-6; http://dx.doi.org/ 10.1016/S0176-1617(11)81773-6 [DOI] [Google Scholar]
- 30.Krtková J, Havelková L, Křepelová A, Fišer R, Vosolsobě S, Novotná Z, Martinec J, Schwarzerová K. Loss of membrane fluidity and endocytosis inhibition are involved in rapid aluminum-induced root growth cessation in Arabidopsis thaliana. Plant Physiol Biochem 2012; 60:88-97; PMID:22922108; http://dx.doi.org/ 10.1016/j.plaphy.2012.07.030 [DOI] [PubMed] [Google Scholar]
- 31.Pejchar P, Scherer GFE, Martinec J. Assaying nonspecific phospholipase C activity. Methods Mol Biol 2013; 1009:193-203; PMID:23681535; http://dx.doi.org/ 10.1007/978-1-62703-401-2_18 [DOI] [PubMed] [Google Scholar]
- 32.Verstraeten SV, Villaverde MS, Oteiza PI. Al3+-mediated changes on membrane fluidity affects the activity of PI-PLC but not of PLC. Chem Phys Lipids 2003; 122:159-63; PMID:12598047; http://dx.doi.org/ 10.1016/S0009-3084(02)00192-5 [DOI] [PubMed] [Google Scholar]
- 33.Verstraeten SV, Oteiza PI. Al3+-mediated changes in membrane physical properties participate in the inhibition of polyphosphoinositide hydrolysis. Arch Biochem Biophys 2002; 408:263-71; PMID:12464280; http://dx.doi.org/ 10.1016/S0003-9861(02)00557-X [DOI] [PubMed] [Google Scholar]
- 34.Pokotylo I, Pejchar P, Potocký M, Kocourková D, Krčková Z, Ruelland E, Kravets V, Martinec J. The plant non-specific phospholipase C gene family. Novel competitors in lipid signalling. Prog Lipid Res 2013; 52:62-79; PMID:23089468; http://dx.doi.org/ 10.1016/j.plipres.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 35.Miège C, Maréchal É. 1,2-sn-Diacylglycerol in plant cells:Product, substrate and regulator. Plant Physiol Biochem 1999; 37:795-808; PMID:10580280; http://dx.doi.org/ 10.1016/S0981-9428(99)00118-7 [DOI] [PubMed] [Google Scholar]
- 36.Dong W, Lv H, Xia G, Wang M. Does diacylglycerol serve as a signaling molecule in plants? Plant Signal Behav 2012; 7:1-4; PMID:22301955; http://dx.doi.org/ 10.4161/psb.7.1.18574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haucke V, Di Paolo G. Lipids and lipid modifications in the regulation of membrane. Curr Opin Cell Biol 2007; 19:426-35; PMID:17651957; http://dx.doi.org/ 10.1016/j.ceb.2007.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carrasco S, Mérida I. Diacylglycerol, when simplicity becomes complex. Trends Biochem Sci 2007; 32:27-36; PMID:17157506; http://dx.doi.org/ 10.1016/j.tibs.2006.11.004 [DOI] [PubMed] [Google Scholar]
- 39.Alwarawrah M, Dai J, Huang J. Modification of lipid bilayer structure by diacylglycerol:a comparative study of diacylglycerol and cholesterol. J Chem Theory Comput 2012; 8:749-58; PMID:22389636; http://dx.doi.org/ 10.1021/ct200790q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett WFD, Tieleman DP. Molecular simulation of rapid translocation of cholesterol, diacylglycerol, and ceramide in model raft and nonraft membranes. J Lipid Res 2012; 53:421-9; PMID:22246847; http://dx.doi.org/ 10.1194/jlr.M022491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen H, Hou N, Schlicht M, Wan Y, Mancuso S, Baluška F. Aluminium toxicity targets PIN2 in Arabidopsis root apices:effects on PIN2 endocytosis, vesicular recycling, and polar auxin transport. Chin Sci Bull 2008; 53:2480-7; http://dx.doi.org/ 10.1007/s11434-008-0332-3 [DOI] [Google Scholar]
- 42.Yang ZB, Geng X, He C, Zhang F, Wang R, Horst WJ, Ding Z. TAA1-regulated local auxin biosynthesis in the root-apex transition zone mediates the aluminum-induced inhibition of root growth in Arabidopsis. Plant Cell 2014; 26:2889-904; PMID:25052716; http://dx.doi.org/ 10.1105/tpc.114.127993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu D, Shen H, Yokawa K, Baluška F. Alleviation of aluminium-induced cell rigidity by overexpression of OsPIN2 in rice roots. J Exp Bot 2014; 65:5305-15; PMID:25053643; http://dx.doi.org/ 10.1093/jxb/eru292 [DOI] [PMC free article] [PubMed] [Google Scholar]


