Abstract
The symbiotic association between the legume Lotus japonicus and the nitrogen-fixing bacterium Mesorhizobium loti results in the formation of root nodules. This process begins with the recognition of the rhizobial nodulation factor (NF) by the NF receptors (NFR) at the cell surface of the host roots. The downstream signaling cascades after NFR recognition have not been fully characterized. We recently identified a clathrin heavy chain 1 (CHC1) from L. japonicus as a potential target of the NF signaling cascades. CHC is a known central component in the clathrin-mediated endocytosis (CME) in eukaryotic cells. The CHC1 gene was highly expressed in Rhizobium-infected root hairs and the CHC1 protein was present in cytoplasmic punctate structures near the infection pockets and along the infection thread membrane. Furthermore, expression of a dominant-negative variant of CHC1 or treatment with a chemical inhibitor of CME resulted in impaired phenotypes in the NF signaling, rhizobial infection and nodulation. These findings open a new avenue for future work aiming at understanding the role of endocytosis in NF signaling pathway and rhizobial infection.
Keywords: clathrin-mediated endocytosis, clathrin heavy chain, infection thread, Lotus japonicus, NFR5, ROP GTPase
Abbreviations
- NF
nod factor
- NFR
nod factor receptor
- CHC
clathrin heavy chain
- CME
clathrin-mediated endocytosis
- IT
infection thread
- PM
plasma membrane
- RNS
root nodule symbiosis
- AP
adaptor protein
To establish a symbiotic association with legumes, rhizobial cells invade the roots from root hairs and penetrate through root epidermal cells using specialized plant-derived membrane structures referred to as infection threads (IT). Rhizobial cells are released from the ITs to the cytoplasm of root cortical cells, where the bacteria are enclosed by the symbiosome membrane, a lipid bilayer derived from the host plasma membrane (PM). Eventually, a new root organ, the nodule, is developed to house the rhizobia at the infected locations. Inside the nodule, the 2 partners execute reciprocal symbiosis and exchange nitrogen and carbon sources. At the earliest step of this process, the nodulation factor (NF) from rhizobia is recognized by NF receptors (NFR1 and NFR5 in L. japonicus). A ROP GTPase, LjROP6, was previously identified in our laboratory as an interaction partner of NFR5 and shown to act as a regulator of IT formation and nodulation.1 In the subsequent study, we have recently identified a clathrin heavy chain 1 (CHC1, Fig. 1) as an interaction partner of ROP6 and a potential target of the NF signaling pathway.2
Figure 1.
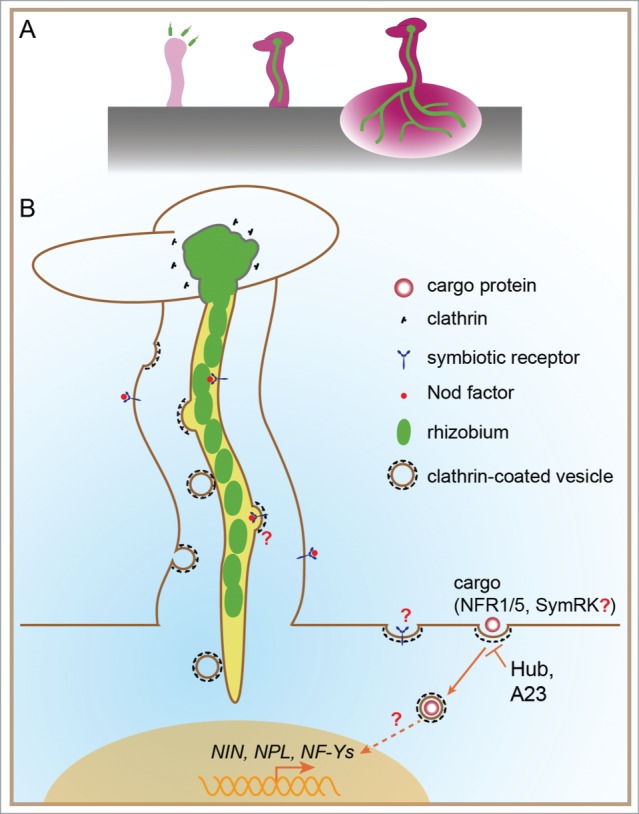
(A) Expression patterns of the CHC1 gene in L. japonicus. Pink color represents the locations and strength of CHC1 expression. Green color represents rhizobia and rhizobia-filled infection threads. (B) Proposed locations of clathrin-mediated endocytosis (CME) and clathrin-coated vesicles during rhizobial infection. The CHC1 gene is highly expressed in ITs, and the CHC1 protein is present abundantly near the infection pockets and along the IT membrane. The experimental data was described in a recent report2.
Our results suggested a strong association between ROP6 and CHC proteins (CHC1 and CHC2).2 ROP6 may regulate clathrin-mediated endocytosis (CME) through direct interactions with CHCs. It is interesting to note that the binding site of CHC1 for the clathrin light chain (CLC) overlaps with the interaction region of CHC1 with ROP6. Because the assembly of CHCs with CLC is known to be essential for CME, the observation of competitive bindings implies that ROP6 may play a negative regulation role in CME in plant cells. This observation was supported by a recent report on Arabidopsis thaliana ROP6, in which ROP6 is found to regulate the CME of auxin efflux carriers PIN-FORMED (PIN) proteins, although a direct interaction between CHC and ROP6 has not been tested.3
ROPs are molecular switches that control complex cellular processes.4 ROP GTPases have 2 conformational states: GTP-bound and GDP-bound. In the active state, i.e. GTP-bound, ROP GTPases are believed to recognize target proteins and generate a response until GTP hydrolysis returns the switch to the inactive state. We have recently expressed constitutively active GTP-bound rop6CA and dominant-negative GDP-bound rop6DN mutants in yeast cells for testing interaction with CHC1 (our unpublished data). Both mutant proteins interacted with CHC1 in a similar level of strength as in the interaction between wild type ROP6 and CHC1, suggesting that the role of ROP6 in regulation of CME does not seem to be dependent on its conformational changes, whether in the GTP- or GDP-bound state. It is not known if protein abundance of ROP6 in the cell plays a role in the regulation of CME, because the expression levels of ROP genes vary in different tissues and are sensitive to environmental changes, such as rhizobial inoculation.5
In our recent work,2 expression of the CHC1-Hub domain had an inhibitory effect on CME (Fig. 1). Transgenic plants expressing the Hub domain exhibited impaired phenotypes in symbiotic gene expression, infection initiation and nodulation. More direct evidence for a role of clathrin in root nodule symbiosis (RNS) was obtained from the use of tyrphostin A23, a potent inhibitor of CME of cargo proteins from the PM. We also tested whether the subcellular localization patterns of NFR5 could be altered by expression of CHC1-Hub. NFR5-eGFP and DsRED-tagged CHC1-Hub were co-expressed in Nicotiana benthamiana leaf cells from the same plasmid. NFR5-eGFP was localized at the PM as well as the cytosolic punctate structures (Fig. 2A, upper). When co-expressed with CHC1-Hub-DsRED, NFR5-eGFP was found predominantly at the PM and only a few cytosolic punctate structures (Fig. 2A, lower). This result suggests that CHC1-Hub may serve as a dominant-negative effector for the intracellular localization of NFR5. Several lines of evidence from recent reports have been consistent, supporting a possible association of NFR5 with the clathrin-mediated process.
Figure 2.
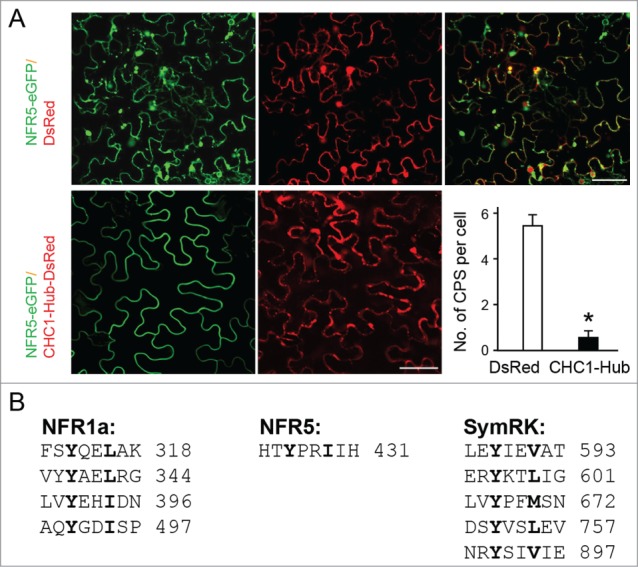
Subcellular localization patterns of NFR5. When transiently expressed in N. benthamiana leaf cells, NFR5 was localized at the PM and intracellular punctate structures. DsRed was expressed from the plasmid, p35S:NFR5-eGFP-35S:DsRed, and served as a control (A, upper). In the presence of CHC1-Hub, a dominant-negative effector of CME, NFR5 was found predominantly at the PM, suggesting possible arrest in NFR5 internalization via CME by CHC1-Hub expression. CHC1-Hub was expressed as a DsRed-tagged protein from the plasmid, p35S:NFR5-eGFP-35S:CHC1-Hub-DsRed (A, lower). Cytoplasmic punctate structures (CPS) with a diameter of over 5 μm of 60 randomly selected cells from 4 leaf samples were recorded (A, lower-right). Asterisk indicates a statistically significant difference (P<0.001) between the cells expressing DsRed and those expressing CHC1-Hub-DsRed. The experimental procedure was described recently2. (B) Presence of the YXXΦ motif in the cytoplasmic domains of symbiosis-related receptors NFR1a, NFR5 and SymRK (GenBank: CAE02589.1, CAE02597.1 and AAM67418.1). Y, tyrosine residue; X, any amino acid residue; Φ, a hydrophobic residue.
Endocytosis-mediated signaling of receptors has been implicated for several plant receptors. BRI1 has been shown to undergo constitutive endocytosis and cycling, whereas FLS2, CLV1 and LeEix2 appear to be internalized by ligand-induced endocytosis,6-8 indicating that the biological role of receptor-mediated endocytosis can vary from receptor to receptor. Recently, BRI1 has been shown to bind its ligand, the brassinosteroid, at the cell surface and is constitutively endocytosed in a manner dependent on CME.9 Whether the receptor-ligand complexes of FLS2/flg22, CLV1/CLV3 and LeEix2/EIX are internalized through CME remain unknown. Receptor endocytosis and endosomal trafficking is believed to be a means to extend the limited signaling surface through adding distribution of activated receptors in highly mobile endosomes. This mechanism ensures a robust and efficient signaling system.10 Alternatively, it mediates signal attenuation by delivering internalized receptor for lysosomal degradation.11 For NF signaling, there has been lack of direct evidence for internalization of NFR5 from the PM by endocytosis. Such evidence could come from colocalization of NFR5 with clathrin-coated vesicles or other endocytic vesicles. Early reports have suggested that NF is internalized in plant cells.12,13 It would be interesting to test if NF is internalized together with the receptor complex NFR1/NFR5, and what other proteins are required for this process.
Other symbiosis-related receptors, such as SymRK and NFR1 in L. japonicus, might also be internalized from the PM. Sorting of internalized PM proteins to endosomes is often mediated by a consensus YXXΦ (Φ represents a hydrophobic residue and X can be any residue) motif presenting within the cytosolic domain of PM proteins.11 This signal motif is recognized specifically and interacts with the μ subunit of the adaptor protein-2 (AP-2) complex that directly links to clathrin.14 YXXΦ is also the target site of tyrphostin A23, which disrupts the interaction of μ subunit with cargo proteins.10 Bioinformatic analysis revealed that this motif is present 4, one and 6 times in NFR1, NFR5 and SymRK, respectively (Fig. 2B). A putative μ subunit of AP-2 (Chr2.CM20021.610.r2.m, http://www.kazusa.or.jp/lotus/) from L. japonicus was chosen and tested for potential interactions with the cytosolic domains of these 3 receptors by the yeast-2 hybrid system. Our preliminary data have not indicated any interaction between the putative μ subunit of AP-2 and the 3 receptors. As the L. japonicus genome sequencing and annotation continue to be improved, more AP complex components will be identified, which will facilitates in-depth studies of endocytosis in plants.
Endocytosis and CME have been studied extensively in yeast and animal cells that internalize exogenous materials or signaling molecules at the cell surface. If NFRs are internalized via endocytosis, one would expect that interfering with endocytosis or CME would also affect rhizobial infection and nodulation. Our recent work2 has provided new experimental evidence for the involvement of endocytosis in the NF signaling pathway. Moreover, our observation also revealed a role of ROP in the regulation of CME during nodule organogenesis.
Funding
This work was supported by the National Natural Science Foundation of China (grant.31170224).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Nan Zhou for technical assistance in figure preparation.
References
- 1.Ke D, Fang Q, Chen C, Zhu H, Chen T, Chang X, Yuan S, Kang H, Ma L, Hong Z, et al.. The small GTPase ROP6 interacts with NFR5 and is involved in nodule formation in Lotus japonicus. Plant Physiol 2012; 159:131-43; PMID:22434040; http://dx.doi.org/ 10.1104/pp.112.197269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C, Zhu M, Duan L, Yu H, Chang X, Li L, Kang H, Feng Y, Zhu H, Hong Z, et al.. Lotus japonicus clathrin heavy chain 1 is associated with ROP6 GTPase and involved in nodule formation. Plant Physiol 2015. (submitted); 167(4):1497-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Naramoto S, Robert S, Tejos R, Lofke C, Lin D, Yang Z, Friml J, et al.. ABP1 and ROP6 GTPase signaling regulate clathrin-mediated endocytosis in Arabidopsis roots. Curr Biol 2012; 22:1326-32; http://dx.doi.org/ 10.1016/j.cub.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420:629-35; PMID:12478284; http://dx.doi.org/ 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 5.Liu W, Chen AM, Luo L, Sun J, Cao LP, Yu GQ, Zhu JB, Wang YZ, et al.. Characterization and expression analysis of Medicago truncatula ROP GTPase family during the early stage of symbiosis. J Integr Plant Bio 2010; 52:639-52. [DOI] [PubMed] [Google Scholar]
- 6.Robatzek S, Chinchilla D, Boller T. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Gene Dev 2006; 20:537-42; PMID:16510871; http://dx.doi.org/ 10.1101/gad.366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bar M, Avni A. EHD2 inhibits ligand-induced endocytosis and signaling of the leucine-rich repeat receptor-like protein LeEix2. Plant J 2009; 59:600-11; PMID:19392695; http://dx.doi.org/ 10.1111/j.1365-313X.2009.03897.x. [DOI] [PubMed] [Google Scholar]
- 8.Nimchuk ZL, Tarr PT, Ohno C, Qu X, Meyerowitz EM. Plant stem cell signaling involves ligand-dependent trafficking of the CLAVATA1 receptor kinase. Curr Bio 2011; 21:345-52 ; http://dx.doi.org/ 10.1016/j.cub.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Rubbo S, Irani NG, Kim SY, Xu ZY, Gadeyne A, Dejonghe W, Vanhoutte I, Persiau G, Eeckhout D, Simon S, et al.. The clathrin adaptor complex AP-2 mediates endocytosis of brassinosteroid insensitive1 in Arabidopsis. Plant Cell 2013; 25:2986-97; PMID:23975899; http://dx.doi.org/ 10.1105/tpc.113.114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geldner N, Robatzek S. Plant receptors go endosomal: a moving view on signal transduction. Plant Physiol 2008; 147:1565-74; PMID:18678748; http://dx.doi.org/ 10.1104/pp.108.120287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 2003; 72:395-447; PMID:12651740; http://dx.doi.org/ 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 12.Philip-Hollingsworth S, Dazzo FB, Hollingsworth RI. Structural requirements of Rhizobium chitolipooligosaccharides for uptake and bioactivity in legume roots as revealed by synthetic analogs and fluorescent probes. J Lipid Res 1997; 38:1229-41; PMID:9215550. [PubMed] [Google Scholar]
- 13.Timmers AC, Auriac MC, de Billy F, Truchet G. Nod factor internalization and microtubular cytoskeleton changes occur concomitantly during nodule differentiation in alfalfa. Development 1998; 125:339-49; PMID:9425130. [DOI] [PubMed] [Google Scholar]
- 14.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Bio 2011; 12:517-33; http://dx.doi.org/ 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]


