Abstract
Since the commercialization of the first therapeutic monoclonal antibody product in 1986, this class of biopharmaceutical products has grown significantly so that, as of November 10, 2014, forty-seven monoclonal antibody products have been approved in the US or Europe for the treatment of a variety of diseases, and many of these products have also been approved for other global markets. At the current approval rate of ∼ four new products per year, ∼70 monoclonal antibody products will be on the market by 2020, and combined world-wide sales will be nearly $125 billion.
Introduction
The commercial development of therapeutic monoclonal antibodies commenced in the early 1980s, and by 1986 the first therapeutic monoclonal antibody, Orthoclone OKT3, was approved for prevention of kidney transplant rejection. Since the approval of OKT3, therapeutic monoclonal antibodies and antibody-related products such as Fc-fusion proteins, antibody fragments, and antibody-drug conjugates (collectively referred to hereafter as monoclonal antibody products) have grown to become the dominant product class within the biopharmaceutical market. Monoclonal antibody products today are approved for the treatment of a variety of diseases, ranging from those that treat patient populations of a few thousand or less for such orphan indications as paroxysmal nocturnal hemoglobinuria or the cryopyrin-associated periodic syndromes to those treating hundreds of thousands of patients for some cancers and multiple sclerosis or even millions of patients for diseases such as asthma and rheumatoid arthritis.
Growth of the Monoclonal Antibody Market
Following the approval of the first monoclonal antibody product in 1986, sales growth and approval of additional products was slow until the late 1990s when the first chimeric monoclonal antibodies were approved. With the approval of these products, followed by the approval of humanized and then fully human monoclonal antibodies, the rate of product approvals and sales of monoclonal antibody products has increased dramatically so that in 2013, global sales revenue for all monoclonal antibody products was nearly $75 billion,1 representing approximately half of the total sales of all biopharmaceutical products. The continued growth in sales of the currently approved monoclonal antibody products, along with the over 300 monoclonal antibody product candidates currently in development, many for multiple indications,1,2 will result in continued growth in sales of monoclonal antibody products in the coming years and will continue to drive the overall sales of all biopharmaceutical products.
As shown in Figure 1, the number of monoclonal antibody products approved for commercial sale in the US and Europe has grown steadily, with three to five new products approved per year for the last several years. While a total of fifty-eight monoclonal antibody products have been approved in Europe and/or the US since 1986, eleven of these products have been withdrawn for various reasons, leaving forty-four approved monoclonal antibody products currently on the market (Table 1). Of the forty-seven monoclonal antibody products approved and marketed in the United States and Europe as of November 10, 2014,1,3,4 three are produced in E. coli while all of the other products are produced in mammalian cells. Of the products produced in mammalian cell culture, thirty-one are full-length naked monoclonal antibodies; one is a bispecific antibody, two are antibody-drug conjugates, one is a radio-labelled antibody conjugate, one is an antigen-binding fragment (Fab), and eight are Fc‑fusion proteins containing the antibody constant region fused to another non-antibody-related protein domain. Two of the three products produced in E. coli are Fabs while the third is an Fc-fusion protein. Of the full length monoclonal antibodies, we consider Prolia and Xgeva as two separate products even though they are manufactured from the same biologically active substance. This decision is based on the fact that Prolia and Xgeva are presented in different formulations and container/closure systems, and separate Biological License Applications (not Supplemental Applications) were filed in the US for each. The list of forty-seven approved monoclonal antibody products also includes the first biosimilar monoclonal antibodies approved in Europe, Inflectra and Remsima. The bulk monoclonal antibody used to produce these biosimilars is manufactured by a single supplier (Celltrion), however, we consider them as separate products since the final drug product for each is manufactured by a separate entity and two separate manufacturers are responsible for final batch release of the products. Furthermore, separate European Marketing Authorization Applications were submitted for each product.
Figure 1.
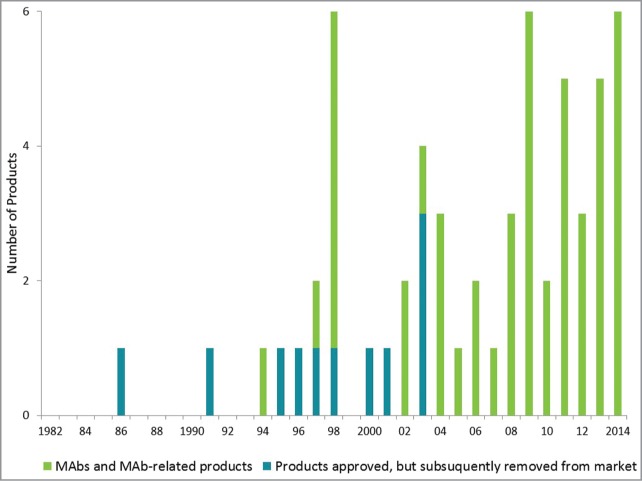
Annual approvals of monoclonal antibody products.3,4 The number of monoclonal antibody products first approved for commercial sale in the US or Europe each year since 1982 is shown. The totals include all monoclonal antibody and antibody-related products. Products approved but subsequently removed from the market are denoted in blue; products currently marketed are denoted in green. 2014 total is as of November 10, 2014.
Table 1.
Marketed therapeutic monoclonal antibody products
| Brand name (INN) | Original BLA/MAA Applicant | Company Reporting US Sales | Company Reporting EU Sales | Year of First Approval | 2013 Global Sales ($M)a |
|---|---|---|---|---|---|
| Abthrax (raxibacumab) | Human Genome Sciences | GlaxoSmithKline | N/Ab | 2012 | 23 |
| Actemra (tocilizumab) | Roche | Roche | Roche | 2009 | 1,119 |
| Adcetrisc (brentuximab vedotin) | Seattle Genetics | Seattle Genetics | Takeda Pharmaceutical Co. | 2011 | 253 |
| AlprolIXd (Factor IX Fc fusion protein) | Biogen Idec | Biogen Idec | N/A | 2014 | NoMe |
| Arcalystf (rilonacept) | Regeneron Pharmaceuticals | Regeneron Pharmaceuticals | N/A | 2008 | 17 |
| Arzerra (ofatumumab) | GlaxoSmithKline | GlaxoSmithKline | GlaxoSmithKline | 2009 | 117 |
| Avastin (bevacizumab) | Genentech | Roche | Roche | 2004 | 6,748 |
| Benlysta (belimumab) | Human Genome Sciences | GlaxoSmithKline | GlaxoSmithKline | 2011 | 228 |
| Cimziag (certolizumab pegol) | UCB | UCB | UCB | 2008 | 789 |
| Cyramza (ramucirumab) | Eli Lilly and Co. | Eli Lilly and Co. | N/A | 2014 | NoMe |
| Eloctateh (Factor VIII Fc fusion protein) | Biogen Idec | Biogen Idec | N/A | 2014 | NoMe |
| Enbreli (etanercept) | Immunex | Amgen | Pfizer | 1998 | 8,325 |
| Entyvio (vedolizumab) | Takeda Pharmaceuticals U.S.A., Inc | Takeda Pharmaceutical Co. | Takeda Pharmaceutical Co. | 2014 | NoMe |
| Erbitux (cetuximab) | ImClone Systems | Bristol-Myers Squibb | Merck KGaA | 2004 | 1,926 |
| Eyleaj (aflibercept) | Regeneron Pharmaceuticals | Regeneron Pharmaceuticals | Bayer Healthcare Pharmaceuticals | 2011 | 1,851 |
| Gazyva (obinutuzumab) | Genentech | Roche | Roche | 2013 | 3 |
| Herceptin (trastuzumab) | Genentech | Roche | Roche | 1998 | 6,559 |
| Humira (adalimumab) | Abbott Laboratories | AbbVie | AbbVie | 2002 | 10,659 |
| Ilaris (canakinumab) | Novartis Pharmaceuticals | Novartis Pharmaceuticals | Novartis Pharmaceuticals | 2009 | 119 |
| Inflectrak l (infliximab [biosimilar]) | Hospira | N/A | Hospira | 2013 | <1m |
| Kadcylan (ado-trastuzumab emtansine) | Genentech | Roche | Roche | 2013 | 252 |
| Keytruda (pembrolizumab) | Merck & Co. | Merck & Co. | N/A | 2014 | NoMe |
| Lemtrada (alemtuzumab) | Genzyme Therapeutics | N/A | Sanofi | 2013 | 3 |
| Lucentiso (ranibizumab) | Genentech | Roche | Novartis Pharmaceuticals | 2006 | 4,205 |
| Nplatep (romiplostim) | Amgen | Amgen | Amgen | 2008 | 427 |
| Nulojixq (belatacept) | Bristol-Myers Squibb | Bristol-Myers Squibb | Bristol-Myers Squibb | 2011 | 26 |
| Orenciar (abatacept) | Bristol-Myers Squibb | Bristol-Myers Squibb | Bristol-Myers Squibb | 2005 | 1,444 |
| Perjeta(pertuzumab) | Genentech | Roche | Roche | 2012 | 352 |
| Prolias (denosumab) | Amgen | Amgen | GlaxoSmithKline | 2011 | 824 |
| Remicade (infliximab) | Centocor | Johnson & Johnson | Merck & Co. | 1998 | 8,944 |
| Removabt (catumaxomab) | Fresenius Biotech | N/A | NeoPharm Group | 2009 | 5 |
| Remsimak l (infliximab [biosimilar]) | Celltrion | N/A | Celltrion | 2013 | <1m |
| ReoProu (abciximab) | Centocor | Lilly | N/A | 1994 | 127 |
| Rituxan (rituximab) | Genentech | Roche | Roche | 1997 | 7,500 |
| Simponi/ Simponi Aria (golimumab) | Centocor Ortho Biotech | Johnson & Johnson | Merck & Co. | 2009 | 1,432 |
| Simulect (basiliximab) | Novartis Pharmaceuticals | Novartis Pharmaceuticals | Novartis Pharmaceuticals | 1998 | 30v |
| Soliris (eculizumab) | Alexion Pharmaceuticals | Alexion Pharmaceuticals | Alexion Pharmaceuticals | 2007 | 1,551 |
| Stelara (ustekinumab) | Janssen-Cilag International | Johnson & Johnson | Johnson & Johnson | 2009 | 1,504 |
| Sylvant (siltuximab) | Janssen Biotech | Johnson & Johnson | Johnson & Johnson | 2014 | NoMe |
| Synagis (palivizumab) | Abbott Laboratories | AstraZeneca | Abbvie | 1998 | 1,887 |
| Tysabri (natalizumab) | Biogen Idec | Biogen Idec | Biogen Idec | 2004 | 1,527 |
| Vectibix (panitumumab) | Amgen | Amgen | Amgen | 2006 | 389 |
| Xgevas (denosumab) | Amgen | Amgen | Amgen | 2010 | 1,030 |
| Xolair (omalizumab) | Genentech | Roche | Novartis | 2003 | 1,465 |
| Yervoy (ipilimumab) | Bristol-Myers Squibb | Bristol-Myers Squibb | Bristol-Myers Squibb | 2011 | 960 |
| Zaltrapw (ziv-aflibercept) | Sanofi Aventis | Sanofi | Sanofi | 2012 | 70 |
| Zevalinx (ibritumomab tiuxetan) | IDEC Pharmaceuticals | Spectrum Pharmaceuticals | Spectrum Pharmaceuticals | 2002 | 29 |
a Sales information obtained from company annual reports and other publically available sources.
b N/A denote product not available in this region.
c Antibody-Drug Conjugate, MMAE.
d Fc Fusion Protein, Fc-Factor IX.
e Product approval in 2014; no sales in 2013.
f Fc Fusion Protein, Fc-IL1R.
g Fab Conjugate, PEG (produced by microbial fermentation).
h Fc Fusion Protein, Fc-Factor VIII.
i Fc Fusion Protein, Fc-TNFR (p75).
j Fc Fusion Protein, Fc-VEGFR (1,2).
k Biosimilar Antibody, Remicade Originator.
l Inflectra and Remsima are considered as two individual products; see text.
m Product approval in late 2013; no annual sales disclosed, bioTRAK® estimate of global sales.
n Antibody-Drug Conjugate, DM1.
o Fab (produced by microbial fermentation).
p Fc Fusion Protein, Fc-TPO-R binding peptide (produced by microbial fermentation).
q Fc Fusion Protein, Fc-CTLA-4 with amino acid substitutions.
r Fc Fusion Protein, Fc-CTLA-4.
s Prolia and Xgeva are considered as two individual products even though they contain the same bulk monoclonal antibody; see text.
t Bispecific, Tri-functional Antibody.
u Sales data not disclosed, small patient market, bioTRAK® estimate of global sales.
v Fab, produced by papain digestion of full length monoclonal antibody.
w Fc Fusion Protein, Fc-VEGFR.
x Antibody Conjugate, Y-90.
Based on a review of historical success and turnover rates (i.e., the length of time required for a product to move from one stage of development to the next) of biopharmaceutical product development candidates, ∼26% of the monoclonal antibody products entering Phase 2 human clinical trials in recent years will ultimately achieve market approval with an average time from the start of Phase 2 clinical trials to approval of ∼ seven years.5-7 Given the large number of monoclonal antibody candidates currently in development, we expect that the number of products approved each year for the coming years will be approximately the same or more than it has been for the last several years. In fact, as of November 10, six monoclonal antibody products were granted first approvals in 2014, with approval decisions for at least two additional products likely to be announced by the end of this year. Based on an approval rate of approximately four monoclonal antibody products per year, we anticipate that there will be 70 or more monoclonal antibody products on the market by 2020.
Along with a higher approval rate than other biopharmaceutical products, global sales of monoclonal antibody products have grown faster than these other products for the past five years. Figure 2 shows the breakdown of sales for monoclonal antibody products based on the product type (e.g., full length antibody, antibody conjugate, antibody fragment, etc.) and production system (e.g., mammalian cell culture, microbial fermentation). As seen from these data, sales of all monoclonal antibody products, regardless of the production system, have grown from ∼$39 billion in 2008 to almost $75 billion in 2013, a 90% increase. By comparison, sales of other recombinant protein therapeutics have only increased ∼26% in the same time period.
Figure 2.
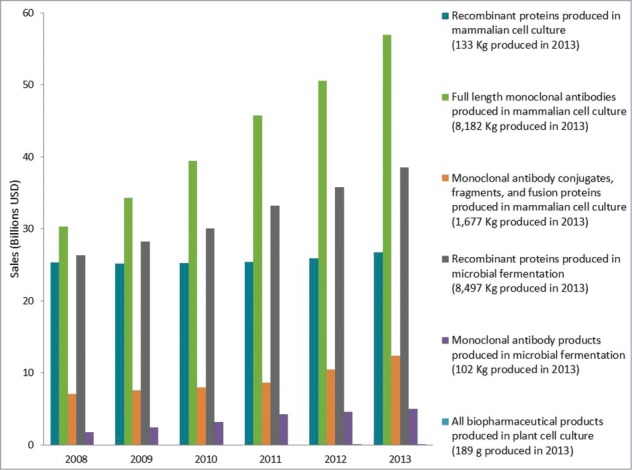
Sales of biopharmaceutical products by product type. Total annual sales of biopharmaceutical products are shown as a function of product type. Note that recombinant proteins produced by microbial fermentation include recombinant human insulin products which represent nearly 50% of the sales and >90% of the material produced in this category.
Corresponding to the increasing sales of monoclonal antibody products, there has been an increase in the total quantities of these products produced annually to meet the market demand. As shown in Figure 2, nearly 10 metric tons of monoclonal antibody products were produced in 2013 compared to ∼8.6 metric tons of all other recombinant protein products. The demand for monoclonal antibody products has resulted in a significant amount of global manufacturing capacity devoted to their production as well as to significant improvements in methods and approaches to monoclonal antibody manufacturing process design and optimization.8-12
The forty-seven monoclonal antibody products on the market in the US and Europe as of November 10, 2014 are listed in Table 1. In 2013, eighteen of these monoclonal antibody products (Humira, Remicade, Enbrel, Rituxan, Avastin, Herceptin, Lucentis, Erbitux, Synagis, Eylea, Soliris, Tysabri, Stelara, Xolair, Orencia, Simponi, Actemra, and Xgeva) achieved annual sales of over $1 billion, while six of these products (Humira, Remicade, Enbrel, Rituxan, Avastin, Herceptin) had sales of greater than $6 billion. Humira, recorded sales of nearly $11 billion, the highest sales figure ever recorded for a biopharmaceutical product.
To further highlight the growth in sales of monoclonal antibody products during the last ten years, the sales growth profiles of the top six selling monoclonal antibody products (Humira, Remicade, Enbrel, Rituxan, Avastin, Herceptin) are compared to those of the two top-selling, recombinant protein products produced in mammalian cell culture (the cytokines Avonex and Rebif) in Figure 3. The average compound annual growth rate for these six monoclonal antibody products over this period is 20% while that of the two mature recombinant protein products was essentially flat.
Figure 3.
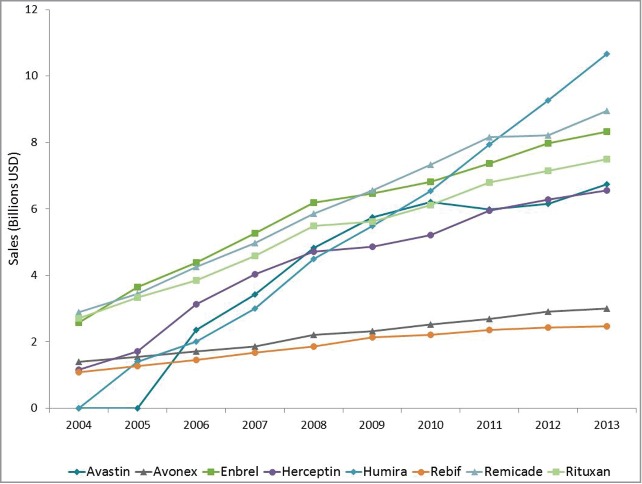
Annual sales of monoclonal antibody products. Annual sales of the top six selling monoclonal antibodies compared to the non-antibody recombinant proteins Avonex and Rebif for the period 2004 to 2013. Each monoclonal antibody product had 2013 sales of greater than $6.5 billion. Sales information was obtained from company annual reports and other publically available sources.
Based on a review of historical sales data, company annual reports, and sales projection data collected by BioProcess Technology Consultants in our proprietary bioTRAK® database, we forecast that the monoclonal antibody market will continue to grow at a CAGR of 8% or more for the next several years. At this growth rate, sales of currently approved monoclonal antibodies plus sales from new products approved in the coming years will drive the world-wide sales of monoclonal antibody products to ∼$94 billion by 2017 and nearly $125 billion by 2020. Our projections are consistent with those of others, such as a recent report from BCC Research that predicts the market for monoclonal antibody products will be nearly $90 billion by 2017.13
Factors Contributing to Growth of the Monoclonal Antibody Market
The continued interest in antibody product development is partially driven by the rapid advancement of our understanding of disease at a molecular level. Although failing to meet some observers' initial high expectations, genomics, proteomics and other systems biology tools continue, in fact, to provide important new targets for modulating disease.14 Monoclonal antibody products often provide the most rapid route to a clinical proof-of-concept for activating, inhibiting, or blocking these new targets. Since the production of most monoclonal antibody products is easily amenable to efficient platform-based approaches and antibodies are generally well-tolerated and highly specific, the risk of unexpected safety issues in human clinical trials of monoclonal antibody products is lower than with many other types of therapeutic products. Therefore, for many of these novel targets, monoclonal antibody products are often the first product candidates advancing to clinical trials. If the initial proof-of-concept studies with these products are successful, they can move rapidly towards commercialization, providing a “first-to-market” advantage.
Also fueling the growth in monoclonal antibody product sales is the global market expansion of the pharmaceutical market in general resulting from the increasing and aging worldwide population and the increasing standard of living in emerging markets.15 In addition, the continued evaluation of monoclonal antibody products in new and expanded clinical indications results in continued demand for product for clinical studies subsequent sales in newly approved indications.
As the biopharmaceutical industry matures, the number and types of diseases that will be economically treated with monoclonal antibody products will increase. Driven in part by the need for cost-effective supply of large quantities of products for such cost-sensitive indications as rheumatology and asthma,16 recent improvements in monoclonal antibody production technologies have substantially improved process yields and reduced actual manufacturing costs.17-20 As a result, there is an ever-increasing opportunity for these products to penetrate more cost-sensitive indications and markets.
As the patents providing exclusive rights to many of the high-profile and blockbuster monoclonal antibody products expire, there has been a growing interest in the development of biosimilars. In September 2013, the first biosimilar monoclonal antibodies, sold under the brand names Remsima and Inflectra, were approved for commercial sale in Europe. These biosimilar versions of the blockbuster monoclonal antibody product Remicade are the first of many biosimilar monoclonal antibodies that will undoubtedly be approved for commercial sale in the US and Europe. Although the current impact of these and other biosimilar monoclonal antibody products in US and European biopharmaceutical marketplace cannot be gauged at this early stage, we anticipate modest acceleration of the sales growth of all monoclonal antibodies as these biosimilar products gain market acceptance. There is also a surging interest in biosimilar monoclonal antibodies in the global markets of Latin America, China, Southeast Asia, India, and Russia, with several monoclonal antibody products already approved in these regions. The introduction of biosimilars in these markets is likely to have a very large impact on the global sales of monoclonal antibody products as biosimilar monoclonal antibodies are approved in geographies that are currently unable to access expensive innovator products.
Conclusion
As the development and commercialization of monoclonal antibody products continues with no limit in sight, lessons learned from the early monoclonal antibody product development, along with the use of advanced and novel technologies for their production and the increasing familiarity of global regulatory authorities with therapeutic monoclonal antibody products, will contribute to their continued dominance as the major class of biopharmaceutical products worldwide.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Bioprocess Technology Consultants [Internet] Woburn (MA): Bioprocess Technology Consultants, Inc. bioTRAK® database; 2014. [cited 2014 September 14]; [about 2 screens] Available from: http://www.bptc.com/pipeline-databases.php [Google Scholar]
- 2. Pharmaceutical Research and Manufacturers of America Medicines in development: Biologics 2013 Report [Internet]. Washington, DC: Pharmaceutical Research and Manufacturers of America; 2013 Mar. [cited 2014 September 14] 87 p. Available from: http://www.phrma.org/sites/default/files/pdf/biologics2013.pdf [Google Scholar]
- 3. Drugs@FDA [Internet] Silver Spring (MD): U.S. Food and Drug Administration, Center for Drug Evaluation and Research. 1939- [cited 2014 September 14] Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm [Google Scholar]
- 4. European public assessment reports, human medicines [Internet] London: The European Medicines Agency. 1995. [cited 2014 September 14] Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124 [Google Scholar]
- 5. Ecker DM, Ransohoff TC, Jones SD, Levine HL. The state of mammalian cell culture biomanufacturing. Woburn: (MA: ): BioProcess Technology Consultants, Inc; 2011 Dec 12; 150 p. Available from: http://www.bptc.com/ECommerce/Reports. [Google Scholar]
- 6. DiMasi JA, Feldman L, Seckler A, Wilson A. Trends in risks associated with new drug development: success rates for investigational drugs. Clin Pharmacol Ther 2010; 87(3):272-7; PMID:20130567 [DOI] [PubMed] [Google Scholar]
- 7. Pavlou AK, Reichert JM. Recombinant protein therapeutics-success rates, market trends and values to 2010. Nat Biotechnol 2004; 22(12):1513-9; PMID:15583654 [DOI] [PubMed] [Google Scholar]
- 8. Greb E, Drakulich A. Platform technologies. Pharmaceutical Technol 2012; 36(3):46-52. [Google Scholar]
- 9. Rameez S, Mostafa SS, Miller C, Shukla AA. High-throughput miniaturized bioreactors for cell culture process development: reproducibility, scalability, and control. Biotechnol Prog 2014; 30(3):718-27; PMID:24449637 [DOI] [PubMed] [Google Scholar]
- 10. Bhambure R, Kumar K, Rathore AS. High throughput process development for biopharmaceutical drug substances. Trends in Biotech 2011; 29(3):127-35; PMID:21255855 [DOI] [PubMed] [Google Scholar]
- 11. Chollangi S, et al. Accelerating purification process development of an early phase MAb with high-throughput automation. Part 1. Bioprocess Int 2014; 12(3):48-52. [Google Scholar]
- 12. Chollangi S, et al. Accelerating purification process development of an early phase MAb with high-throughput automation. Part 2. Bioprocess Int 2014; 12(4):32-41. [Google Scholar]
- 13. BCC Research Biologic therapeutic drugs: technologies and global markets. Wellesley: (MA: ): BCC Research, LLC; 2013 Jan. 142 p. Available from: http://www.bccresearch.com/market-research/biotechnology/biologic-therapeutic-drugs-markets-bio079b.html [Google Scholar]
- 14. Bilello JA. The agony and ecstasy of "OMIC" technologies in drug development. Curr Mol Med 2005; 5(1):39-52; PMID:15720269 [DOI] [PubMed] [Google Scholar]
- 15. United Nations Development Programme Human development report 2013: The rise of the South: Human progress in a diverse world [Internet]. NY (NY): United Nations Development Programme; c2013. [cited 2014 September 14] 216 p. Available from: http://hdr.undp.org/sites/default/files/reports/14/hdr2013_en_complete.pdf [Google Scholar]
- 16. Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 2004; 22(11):1393-8; PMID:15529164 [DOI] [PubMed] [Google Scholar]
- 17. Lu F, Toh PC, Burnett I, Li F, Hudson T, Amanullah A, Li J. Automated dynamic fed-batch process and media optimization for high productivity cell culture process development. Biotechnol Bioeng 2013; 110(1):191-205; PMID:22767053 [DOI] [PubMed] [Google Scholar]
- 18. Yang WC, Lu J, Kwiatkowski C, Yuan H, Kshirsagar R, Ryll T, Huang YM. Perfusion seed cultures improve biopharmaceutical fed-batch production capacity and product quality. Biotechnol Prog 2014; 30(3):616-25; PMID:24574326 [DOI] [PubMed] [Google Scholar]
- 19. Povey JF, O'Malley CJ, Root T, Martin EB, Montague GA, Feary M, Trim C, Lang DA, Alldread R, Racher AJ, Smales CM. Rapid high-throughput characterization, classification and selection of recombinant mammalian cell line phenotypes using intact cell MALDI-ToF mass spectrometry fingerprinting and PLS-DA modelling. J Biotechnol 2014; 20(184):84-93. [DOI] [PubMed] [Google Scholar]
- 20. Liu B, Spearman M, Doering J, Lattová E, Perreault H, Butler M. The availability of glucose to CHO cells affects the intracellular lipid-linked oligosaccharide distribution, site occupancy and the N-glycosylation profile of a monoclonal antibody. J Biotechnol 2014; 170:17-27; PMID:24286971 [DOI] [PubMed] [Google Scholar]


