Abstract
Macrophages are innate immune cells that derive from circulating monocytes, reside in all tissues, and participate in many states of pathology. Macrophages play a dichotomous role in cancer, where they promote tumor growth but also serve as critical immune effectors of therapeutic antibodies. Macrophages express all classes of Fcγ receptors, and they have immense potential to destroy tumors via the process of antibody-dependent phagocytosis. A number of studies have demonstrated that macrophage phagocytosis is a major mechanism of action of many antibodies approved to treat cancer. Consequently, a number of approaches to augment macrophage responses to therapeutic antibodies are under investigation, including the exploration of new targets and development of antibodies with enhanced functions. For example, the interaction of CD47 with signal-regulatory protein α (SIRPα) serves as a myeloid-specific immune checkpoint that limits the response of macrophages to antibody therapies, and CD47-blocking agents overcome this barrier to augment phagocytosis. The response of macrophages to antibody therapies can also be enhanced with engineered Fc variants, bispecific antibodies, or antibody-drug conjugates. Macrophages have demonstrated success as effectors of cancer immunotherapy, and further investigation will unlock their full potential for the benefit of patients.
Keywords: ADCP, antibodies, cancer, CD47, Fc receptor, immune checkpoint, immunotherapy, macrophages, phagocytosis, SIRPα
Abbreviations
- Fc
fragment crystallizable
- FcγR
Fcγ receptors
- CD
cluster of differentiation
- SIRPα
signal-regulatory protein α
- ADCC
antibody-dependent cell-mediated cytotoxicity
- ADCP
antibody-dependent cellular phagocytosis
- NK
natural killer
- M-CSF
macrophage colony stimulating factor
- IgG
immunoglobulin G
- HER2
human epidermal growth factor receptor 2
- EGFR
epidermal growth factor receptor
- GM-CSF
granulocyte-macrophage colony stimulating factor
- HSC
haematopoietic stem cell
- AML
acute myelogenous leukemia
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- SHP
Src homology 2 domain-containing phosphatase
- ITAM
immunoreceptor tyrosine-based activation motif
- CLL
chronic lymphocytic leukemia
- BTK
Bruton's tyrosine kinase
- ADC
antibody-drug conjugate
Macrophages and cancer immunotherapy
Cancer immunotherapy is emerging as one of the most promising areas of cancer research and treatment.1,2 Overall, the goal of cancer immunotherapy is to stimulate a patient's immune system to recognize cancer cells as foreign and attack them. A number of recent advances have sparked an unprecedented interest in the field. In particular, breakthroughs have been made using therapies that augment T cell responses to tumors. These include chimeric antigen receptor (CAR) T cells and immune checkpoint inhibitors, such as antibodies targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) or the programmed death (PD)-1/PD-ligand 1 axis.3,4 Three immune checkpoint inhibitors (ipilimumab, pembrolizumab, nivolumab) have recently been approved for melanoma, and studies applying them to other cancers are advancing rapidly.
In contrast to efforts targeting the adaptive immune system, few therapies have been aimed at stimulating the myeloid arm of the immune system to attack cancer. The myeloid immune lineage consists primarily of granulocytes and monocytes, the latter of which can differentiate into macrophages or dendritic cells. Macrophages in particular are poised to be tremendous effectors of cancer immunotherapy. These innate immune cells reside in tissues throughout the body,5 and specialized tissue-specific macrophage populations exist, e.g., Kupffer cells in the liver, microglia in the brain, osteoclasts in bone, and alveolar macrophages in the lungs. Macrophages are capable of performing phagocytosis, a process that involves the engulfment and degradation of material such as debris, dead cells, or pathogens. They recognize material for engulfment by pattern recognition receptors, scavenger receptors, and antibody fragment crystallizable (Fc) receptors.6 Macrophages participate in many states of pathology, including infection, inflammatory disease, wound healing, and cancer.7
The complex relationship between macrophages and tumors obscures the potential that macrophages have to act as immune effectors. Macrophages are often found in high numbers within tumors, and a number of studies have found the degree of macrophage infiltration correlates with poor prognosis across many different types of cancer.8-13 At baseline, macrophages may promote tumor growth and dissemination by supporting angiogenesis, performing matrix remodeling, and secreting growth factors and immunosuppressive cytokines.14 These “tumor-associated macrophages” have been contrasted with pro-inflammatory or “classically activated” macrophages that attack pathogens.15 As a result, some therapies have been designed to deplete macrophages in tumors.16
Natural killer (NK) cells have classically been described as the primary immune effectors of antibodies therapies due to their involvement in the process of antibody-dependent cell-mediated cytotoxicity (ADCC). However, macrophages are crucial to the efficacy of many antibodies because they perform antibody-dependent cellular phagocytosis (ADCP) (Fig. 1A). Macrophages express all classes of Fcγ receptors (FcγR), in contrast to NK cells which primarily express FcγRIIIa.17,18 The contribution of macrophages has been marginalized in the past because they are more difficult to study compared to NK cells or other peripheral blood leukocytes. Macrophages do not circulate in the bloodstream; hence they cannot be purified expediently in large quantities. Instead, macrophages must be differentiated ex vivo from circulating monocytes by culturing for a week or longer in the presence of human serum or growth factors such as macrophage colony stimulating factor (M-CSF).19,20 Moreover, macrophage-mediated cytotoxicity occurs primarily via phagocytosis,21,22 which is technically challenging to assay and requires microscopic visualization or flow cytometry to quantify cellular engulfment. Chromium release assays, the gold standard for measuring ADCC, are insufficient for evaluating cytotoxicity by macrophages because macrophages retain the radioactive probe after phagocytosis.21 For these reasons, macrophages are underappreciated as effector cells that can target cancer.
Figure 1.
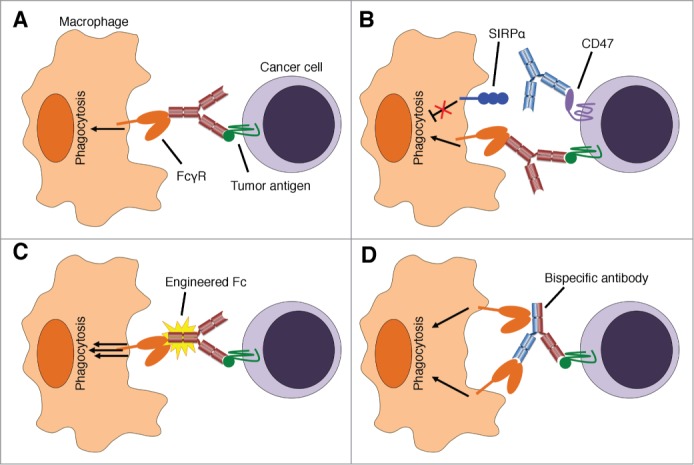
Augmenting macrophage responses to therapeutic antibodies. (A) Tumor-binding antibodies stimulate macrophage phagocytosis via Fcγ receptors (FcγR), which is a major mechanism of action of many therapeutic antibodies. (B) The CD47-SIRPα interaction inhibits maximal antibody-dependent phagocytosis. CD47-blocking therapies (blue antibody) prevent inhibitory signaling from SIRPα to augment macrophage activation. (C) Tumor-binding antibodies with engineered Fc fragments exhibit enhanced binding to Fc receptors and potently stimulate phagocytosis. (D) Bispecific antibodies that have dual specificity for tumor antigens and receptors on macrophages can augment phagocytosis and direct macrophage responses against tumors. “Trifunctional” antibodies have intact Fc fragments that can engage additional Fc receptors as depicted. Antibody-drug conjugates with immunostimulatory properties (not depicted) also deliver activating stimuli to macrophages.
Evidence supporting macrophages as effectors of therapeutic antibodies for cancer
Nonetheless, macrophage phagocytosis has been found to contribute to the efficacy of monoclonal antibodies for as long as they have been investigated as therapeutics. In studies published in the early 1980s, monoclonal antibodies against tumor antigens were found to stimulate phagocytosis of cancer cells in vitro, induce macrophage infiltration into tumors, and elicit macrophage-mediated destruction of tumors in mice.23-25 More recent studies have examined phagocytosis in response to therapeutic antibodies, such as the anti-CD20 antibody rituximab. In vitro, macrophage phagocytosis of lymphoma and leukemia cells in response to rituximab has been demonstrated in a number of studies using human macrophages.26-30 Interestingly, macrophages polarized toward a tumor-associated state with M-CSF and IL-10 exhibited greater phagocytosis of rituximab-opsonized lymphoma cells than those polarized toward a pro-inflammatory state.31 Polarization resulted in upregulation of multiple Fcγ receptors on macrophages, correlating with their phagocytic response. Furthermore, all subclasses of human immunoglobulin G (IgG) are able to induce human macrophage phagocytosis, as demonstrated using a panel of rituximab variants with identical variable regions but differing heavy chain isotypes.32 Even human IgG4, which exhibits less ADCC,33 has the potential to stimulate macrophage phagocytosis. This is likely mediated by its ability to engage Fc receptors that are present on macrophages but not NK cells. This finding suggests the majority of tumor-binding antibodies approved for therapy have the ability to stimulate macrophage phagocytosis. Antibody-dependent phagocytosis of solid tumors has also been demonstrated in vitro using anti-human epidermal growth factor receptor (HER) 2 antibodies against breast cancer and anti-epidermal growth factor receptor (EGFR) antibodies for colon cancer.32,34 As described by Overdijk et al. in this issue of mAbs, daratumumab, an anti-CD38 antibody, was found to induce macrophage phagocytosis of multiple myeloma cells. Phagocytosis in response to a number of other investigational antibodies, such as anti-KIT antibodies for gastrointestinal stromal tumors,35 has also been observed.
In vivo findings have also demonstrated a crucial role for macrophages as effectors of antibodies therapies. In studies using anti-CD20 antibodies, macrophage depletion with liposomal clodronate abrogated the ability of the antibodies to deplete normal and malignant B cells.36,37 Similarly, CSF-1op mice, which have defects in macrophage number and development, also had impaired responses to anti-CD20 antibodies.36 In contrast, the antibodies remained effective in mice deficient in T and B cells or NK cells, suggesting macrophages are the main effectors of the antibodies in vivo.36 Studies with transgenic mice expressing human CD20 have demonstrated that depletion of circulating cells opsonized by anti-CD20 antibodies occurs rapidly in the liver.37 New efforts using intravital imaging have elegantly demonstrated that these effects are mediated by Kupffer cells, which immobilize and engulf the opsonized cells soon after administration of the antibodies.38 Similarly, Kupffer cells eliminated circulating tumor cells and prevented liver metastases when antibodies were used in models of colon cancer and melanoma.22,39 Investigations of anti-CD142 antibodies for breast cancer showed that although macrophages supported tumor growth, they were also essential for the anti-tumor effects of the antibodies.40 Therefore, macrophages are key effectors to the efficacy of antibodies in vivo, and the reticuloendothelial system likely plays a major role in elimination of circulating tumor cells that are bound by therapeutic antibodies.
In clinical investigations, macrophages are commonly found in tumors in high numbers.8-13 Studies on Fc receptor polymorphisms suggest antibodies have Fc-dependent mechanisms of action in patients. In particular, lymphoma patients with polymorphisms in FcγRIIIa that confer high affinity binding to antibodies exhibited greater therapeutic responses to rituximab.41 While this receptor is expressed on both NK cells and macrophages, polymorphisms in FcγRIIa, a major mediator of phagocytosis,42 also correlated with the therapeutic efficacy of rituximab for lymphoma, as well as cetuximab for colon cancer and trastuzumab for breast cancer.41,43,44 Moreover, in lymphoma patients treated with conventional therapy, the degree of macrophage infiltration correlates with poor prognosis;11 however, macrophage infiltration appears to be a favorable prognostic indicator when rituximab is added to conventional therapy.45 These studies further implicate macrophages as important effectors for the therapeutic benefit of antibodies in patients. Other studies have examined combinations of antibody therapies with cytokines. Treatment with granulocyte-macrophage colony stimulating factor (GM-CSF), which activates macrophages and other myeloid cells, enhanced the efficacy of rituximab for follicular lymphoma and anti-GD2 antibodies for neuroblastoma.46,47 As further evidence of the anti-tumor potential of macrophages in response to antibody therapies, a Phase 1 clinical trial of agonistic anti-CD40 antibodies demonstrated efficacy against pancreatic cancer primarily by macrophage effector functions.48
The CD47- signal-regulatory protein α axis: The myeloid-specific immune checkpoint
A key molecule that governs macrophage phagocytosis is CD47, a transmembrane protein that is widely expressed on the surface of many cell types throughout the body. Oldenborg et al. first identified a role for CD47 in regulating phagocytosis.49 When the authors purified red blood cells from CD47-/- mice and transfused them into wild-type mice, they found that the CD47-/- red blood cells were rapidly cleared from the circulation.49 The method of red blood cell removal was determined to be phagocytosis by macrophages in the spleen. This study demonstrated that CD47 serves as a “marker of self” to prevent macrophage phagocytosis. A role for CD47 in cancer was first identified from studies of haematopoietic stem cells (HSCs) and leukemia. HSCs occasionally exit their niches in the bone marrow and circulate through the peripheral blood. To avoid phagocytosis by macrophages in the spleen, these circulating HSCs upregulate CD47 on the cell surface.50 Similarly, acute myeloid leukemia (AML) stem cells also upregulate CD47, presumably to avoid phagocytosis by splenic macrophages similar to normal HSCs.51 CD47 was evaluated as a putative therapeutic target on AML using anti-CD47 antibodies that block the interaction between CD47 and signal-regulatory protein (SIRP) α, an inhibitory receptor on macrophages. These antibodies were able to stimulate macrophage phagocytosis of AML cells in vitro and exhibit therapeutic efficacy against AML in mouse models.51 A broader role for CD47 in cancer was appreciated when CD47 expression was examined on solid tumors such as ovarian cancer, bladder cancer, breast cancer, and leiomyosarcoma.52,53 Again, CD47 was highly expressed on these cancers and treatment with anti-CD47 antibodies induced macrophage phagocytosis and stimulated anti-tumor responses in vivo, showing the broad promise of targeting the CD47/SIRPα interaction in cancer. Based on these results, a humanized anti-CD47 antibody (Hu5F9-G4) was developed at Stanford University, and it is now undergoing evaluation in a Phase 1 clinical study of patients with solid tumors (www.clinicaltrials.gov identifier: NCT02216409).
CD47 inhibits macrophage responses to therapeutic antibodies
CD47 acts by sending inhibitory signals through SIRPα, a transmembrane receptor that is expressed on macrophages and other myeloid cells.54-56 SIRPα contains immunoreceptor tyrosine-based inhibition motifs (ITIMs) in its cytoplasmic tail. When CD47 binds to SIRPα, it causes phosphorylation of the ITIMs that activate the Src homology 2 domain-containing phosphatases SHP-1 and SHP-2.57 The SHP phosphatases in turn cleave phosphate groups from proteins containing immunoreceptor tyrosine-based activation motifs (ITAMs) and myosin light chains, thereby inhibiting pro-phagocytic signaling and preventing rearrangements to the cytoskeleton that are necessary for phagocytosis to occur.57-59 Fc receptors are transmembrane proteins with extracellular domains that bind the Fc region of antibodies and cytoplasmic tails that contain ITAMs.17 Upon binding to target-bound antibodies, conformational changes induce phosphorylation of the Fc receptor ITAMs, thereby initiating a signaling cascade that promotes phagocytosis. Phosphorylation of Fc receptor ITAMs is balanced by inhibitory signaling from the CD47-SIRPα axis.60,61 The balance is likely mediated by SHP-1 and SHP-2 phosphatases that cleave phosphate groups from ITAMs of the Fc receptors as described above. In this manner, the CD47-SIRPα axis serves as a barrier to antibody-dependent phagocytosis. Based on these findings, CD47-blocking therapies were hypothesized to synergize with anticancer antibodies (Fig. 1B). Indeed, the combination of CD47-blocking antibodies with rituximab exhibited synergy in vitro and in vivo against lymphoma.29 Furthermore, engineered SIRPα variants, 14 kDa proteins that potently block CD47 but lack the pro-phagocytic stimulus of an Fc were evaluated against cancer.32 They synergized with rituximab, cetuximab, trastuzumab, and alemtuzumab by augmenting macrophage activity. Therefore, CD47 is a key regulator of macrophage phagocytosis, particularly when induced by therapeutic antibodies, and reagents that target the CD47-SIRPα axis may act as universal adjuvants to anticancer antibodies.
Conventional therapies and macrophage effector functions
Antibody therapies are typically used in unison with chemotherapeutic agents, and the effects of chemotherapy on macrophage effector functions are not fully understood. Agents that kill cancer cells with limited specificity may interfere with the ability of macrophages and other immune cells to act as therapeutic effectors. For example, vinca alkaloids may inhibit phagocytosis due to their effects on cytoskeletal rearrangement.62 Even targeted therapies can have unanticipated effects on immune cell functions. Ibrutinib, a small molecule inhibitor used for the treatment of chronic lymphocytic leukemia (CLL) and mantle cell lymphoma, acts by disabling signals from Bruton's tyrosine kinase (BTK). While BTK promotes growth of B cell malignancies, it also transduces signals downstream of Fc receptors. As a consequence, ibrutinib inhibits ADCC and phagocytosis.63,64 Although the addition of ibrutinib to rituximab regimens seems promising in clinical trials,65 the inhibition of Fc receptor signaling suggests additional mechanisms to increase NK cell or macrophage functions may be beneficial. When combining these types of therapies with antibodies, it may be best to optimize the timing of treatments to avoid unfavorable interactions.
On the other hand, chemotherapeutic agents may stimulate inflammatory responses that enable the immune system to respond more effectively to anticancer antibodies. For example, the efficacy of doxorubicin was reduced when macrophages were inhibited, suggesting this agent acts in part by stimulating macrophage effector functions.66 More recently, one study examined human leukemia xenografts that were refractory to treatment with alemtuzumab, a humanized anti-CD52 antibody.67 The authors found that cyclophosphamide, a nitrogen mustard chemotherapeutic, stimulated secretion of inflammatory cytokines within the tumor microenvironment and produced synergy by increasing antibody-dependent phagocytosis. It will be important to evaluate which chemotherapeutic agents aid or hinder macrophage phagocytosis in order to tailor treatment regimens to maximize efficacy and specificity against tumors.
Engineering antibodies to engage macrophages
Based on the importance of macrophages as effector cells, additional efforts to enhance macrophage responses to antibodies are warranted. One approach is to alter the binding of antibody Fc fragments to Fc receptors via molecular engineering (Fig. 1C). Antibodies have been glycoengineered to lack fucosylation, which results in greater binding to Fcγ receptors. Consequently, these antibodies exhibit greater ADCC and phagocytosis, as evidenced by studies on obinutuzumab, a glycoengineered anti-CD20 antibody approved for the treatment of CLL.68 Other protein engineering efforts have been aimed at developing Fc variants with enhanced binding to Fc receptors. Lazar et al. generated variants of human IgG1 with increased affinity for FcγRIIIa.69 They found that these variants improved ADCC and macrophage phagocytosis in response to trastuzumab and rituximab. In another study, an anti-CD19 antibody with the same modifications improved ADCC and phagocytosis in vitro and enhanced efficacy in xenograft models of B cell malignancies.70,71 This approach demonstrated safety and efficacy in a Phase 1 clinical study.72 Additional engineering efforts identified variants of human IgG1 with increased binding to FcγRIIa, a major mediator of phagocytosis, leading to increased macrophage-mediated destruction.42 Another interesting approach created hybrids of IgG and IgA Fc chains, termed “cross-isotype” antibodies, that engage both FcαR and Fcγ receptors for enhanced myeloid effector functions including phagocytosis.73 Conversely, when developing therapies for which immune effector functions are not desired, the response of macrophages must also be considered since they express all classes of Fcγ receptors and respond to all subclasses of human IgG. Mutant Fc variants that abolish binding to all Fcγ receptors have been described.74,75
An alternative strategy to engaging macrophages has focused on engineering bispecific antibodies that simultaneously bind antigens on tumor cells and receptors on macrophages (Fig. 1D). In this sense, they cross-link macrophages to cancer cells for enhanced efficacy and anti-tumor specificity. Many of these agents have targeted FcγRIIIa, expressed on NK cells as well as macrophages. An early attempt at this approach tested an antibody with dual specificity for HER2 and FcγRIIIa in a clinical study of patients with HER2+ adenocarcinoma.76 Some signs of efficacy were observed, but the development of cytokine storm reactions with low dose administration precluded further investigation. New bispecifics targeting FcγRIIIa and CD30 are currently under development for Hodgkin lymphoma.77 Chemically linked bispecific Fab fragments targeting FcγRI and HER2 have also been evaluated. This type of therapeutic was able to induce phagocytosis by macrophages in vitro and exhibited mild benefit in clinical trials.34,78 A similar bispecific antibody targeting FcγRI and EGFR was also tested in clinical trials for solid tumors with minimal success.79 The limited success in these studies targeting FcγRI may be due to the lack of an appropriate Fc to stimulate macrophages fully. Although bispecific antibodies targeting macrophages and tumors have not yet demonstrated sufficient efficacy in clinical trials, this approach holds much promise. Additional receptors on macrophages should be tested to determine the safest and most effective way to engage these immune cells for the benefit of patients.
Macrophage responses to antibody-drug conjugates
Antibody-drug conjugates (ADCs), which are tumor-binding antibodies conjugated to small molecules, are also emerging as novel anticancer agents. These therapeutics function by binding to tumor antigens and delivering a cytotoxic payload upon antigen internalization. However, since the antibodies can engage macrophages and other immune cells via Fc receptors, the collateral effects on immune cells must also be considered. In particular, ADCs that result in phagocytosis may in fact deliver their cytoxic payload to macrophages attacking tumors. The anti-CD30 antibody brentuximab, when tested as a naked antibody, was capable of stimulating phagocytosis and macrophage functions in vivo.37 When brentuximab is conjugated to the cytotoxin vedotin, the resulting ADC could incapacitate macrophages and limit their function. Alternatively, ADCs could be designed to augment macrophage phagocytosis. These could include conjugates to immunostimulatory agents such as Toll-like receptor agonists or scavenger receptor ligands. Antibody conjugation to cytokines or chemokines that increase macrophage infiltration or activity could also be conceived. In one example, an anti-HER2 antibody fused with GM-CSF exhibited greater in vivo efficacy than the unmodified antibody.80
Conclusions
Macrophages are important mediators of the efficacy of many therapeutic antibodies for cancer. Macrophages are often present in high numbers within the tumor microenvironment, and tumor-associated macrophages may promote tumor growth in the absence of therapeutic intervention. Nonetheless, these macrophages can mount robust responses against cancer when given the appropriate antibody stimulus.31,40,48 Macrophages fail to recognize tumor cells as foreign due at least in part to the CD47-SIRPα interaction, a myeloid-specific immune checkpoint. Studies with CD47-blocking therapies demonstrate the potential of macrophages in tumors, particularly in combination with tumor-binding antibodies. CD47-blockade lowers the threshold for macrophage phagocytosis, while tumor-binding antibodies direct macrophage attack against tumors for greater specificity. Furthermore, macrophage phagocytosis in response to antibodies may lead to antigen presentation that initiates long-lasting adaptive immune responses against tumors.81 Additional approaches to engage macrophages in tumors include engineering Fc fragments for greater binding to Fc receptors, and the use of either bispecific antibodies that cross link macrophages and cancer cells or ADCs conjugated with immunostimulatory agents. By designing therapies that better engage macrophages, the full potential of the innate immune system can be realized for the benefit of patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Jens-Peter Volkmer, Aaron Ring, Dr. Roy Maute, Peter J. Schnorr, Nan Guo, Dr. Nathaniel Fernhoff, Dr. Anne Volkmer, Dr. Geoffrey Krampitz, Dr. Stephen Willingham, Dr. Badreddin Edris, members of the Weissman lab, Majeti lab, and the CD47 Disease Team for support and helpful discussions. We thank Dr. K. Christopher Garcia, Dr. Ravi Majeti, Dr. Ronald Levy, Dr. Holbrook Kohrt, Dr. Beverely Mitchell, and Dr. Judith Shizuru, Dr. Matt van De Rijn for advice and discussions.
Funding
The authors are supported in part by funding from the National Cancer Institute (F30CA168059), the Stanford University SPARK Program, the Joanna M. Nicolay Melanoma Foundation, and the Virginia and D.K. Ludwig Fund for Cancer Research.
References
- 1. Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol 2011; 29:4828-36; PMID:22042955; http://dx.doi.org/ 10.1200/JCO.2011.38.0899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480:480-9; PMID:22193102; http://dx.doi.org/ 10.1038/nature10673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annu Rev Med 2014; 65:333-47; PMID:24274181; http://dx.doi.org/ 10.1146/annurev-med-060512-150254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu Rev Med 2014; 65:185-202; PMID:24188664; http://dx.doi.org/ 10.1146/annurev-med-092012-112807 [DOI] [PubMed] [Google Scholar]
- 5. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science 2010; 327:656-61; PMID:20133564; http://dx.doi.org/ 10.1126/science.1178331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell 2002; 111:927-30; PMID:12507420; http://dx.doi.org/ 10.1016/S0092-8674(02)01201-1 [DOI] [PubMed] [Google Scholar]
- 7. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011; 11:723-37; PMID:21997792; http://dx.doi.org/ 10.1038/nri3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 1996; 56:4625-9; PMID:8840975 [PubMed] [Google Scholar]
- 9. Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 2002; 196:254-65; PMID:11857487; http://dx.doi.org/ 10.1002/path.1027 [DOI] [PubMed] [Google Scholar]
- 10. Chen JJ, Lin YC, Yao PL, Yuan A, Chen HY, Shun CT, Tsai MF, Chen CH, Yang PC. Tumor-associated macrophages: the double-edged sword in cancer progression. J Clin Oncol 2005; 23:953-64; PMID:15598976; http://dx.doi.org/ 10.1200/JCO.2005.12.172 [DOI] [PubMed] [Google Scholar]
- 11. Farinha P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, Gill K, Klasa R, Voss N, Connors JM, Gascoyne RD. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood 2005; 106:2169-74; PMID:15933054; http://dx.doi.org/ 10.1182/blood-2005-04-1565 [DOI] [PubMed] [Google Scholar]
- 12. Lee CH, Espinosa I, Vrijaldenhoven S, Subramanian S, Montgomery KD, Zhu S, Marinelli RJ, Peterse JL, Poulin N, Nielsen TO, et al. Prognostic significance of macrophage infiltration in leiomyosarcomas. Clin Cancer Res 2008; 14:1423-30; PMID:18316565; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-1712 [DOI] [PubMed] [Google Scholar]
- 13. Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med 2010; 362:875-85; PMID:20220182; http://dx.doi.org/ 10.1056/NEJMoa0905680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010; 11:889-96; PMID:20856220; http://dx.doi.org/ 10.1038/ni.1937 [DOI] [PubMed] [Google Scholar]
- 15. Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 2009; 86:1065-73; PMID:19741157; http://dx.doi.org/ 10.1189/jlb.0609385 [DOI] [PubMed] [Google Scholar]
- 16. Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 2014; 25:846-59; PMID:24898549; http://dx.doi.org/ 10.1016/j.ccr.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 17. Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 2008; 8:34-47; PMID:18064051; http://dx.doi.org/ 10.1038/nri2206 [DOI] [PubMed] [Google Scholar]
- 18. Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 2012; 119:5640-9; PMID:22535666; http://dx.doi.org/ 10.1182/blood-2012-01-380121 [DOI] [PubMed] [Google Scholar]
- 19. Johnson WD, Jr., Mei B, Cohn ZA. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med 1977; 146:1613-26; PMID:925613; http://dx.doi.org/ 10.1084/jem.146.6.1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Becker S, Warren MK, Haskill S. Colony-stimulating factor-induced monocyte survival and differentiation into macrophages in serum-free cultures. J Immunol 1987; 139:3703-9; PMID:2824612 [PubMed] [Google Scholar]
- 21. Munn DH, Cheung NK. Phagocytosis of tumor cells by human monocytes cultured in recombinant macrophage colony-stimulating factor. J Exp Med 1990; 172:231-7; PMID:2193096; http://dx.doi.org/ 10.1084/jem.172.1.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gul N, Babes L, Siegmund K, Korthouwer R, Bogels M, Braster R, Vidarsson G, ten Hagen TL, Kubes P, van Egmond M. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J Clin Invest 2014; 124:812-23; PMID:24430180; http://dx.doi.org/ 10.1172/JCI66776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herlyn D, Koprowski H. IgG2a monoclonal antibodies inhibit human tumor growth through interaction with effector cells. Proc Natl Acad Sci U S A 1982; 79:4761-5; PMID:6289317; http://dx.doi.org/ 10.1073/pnas.79.15.4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steplewski Z, Lubeck MD, Koprowski H. Human macrophages armed with murine immunoglobulin G2a antibodies to tumors destroy human cancer cells. Science 1983; 221:865-7; PMID:6879183; http://dx.doi.org/ 10.1126/science.6879183 [DOI] [PubMed] [Google Scholar]
- 25. Adams DO, Hall T, Steplewski Z, Koprowski H. Tumors undergoing rejection induced by monoclonal antibodies of the IgG2a isotype contain increased numbers of macrophages activated for a distinctive form of antibody-dependent cytolysis. Proc Natl Acad Sci U S A 1984; 81:3506-10; PMID:6587365; http://dx.doi.org/ 10.1073/pnas.81.11.3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaperot L, Chokri M, Jacob MC, Drillat P, Garban F, Egelhofer H, Molens JP, Sotto JJ, Bensa JC, Plumas J. Differentiation of antigen-presenting cells (dendritic cells and macrophages) for therapeutic application in patients with lymphoma. Leukemia 2000; 14:1667-77; PMID:10995015; http://dx.doi.org/ 10.1038/sj.leu.2401888 [DOI] [PubMed] [Google Scholar]
- 27. Manches O, Lui G, Chaperot L, Gressin R, Molens JP, Jacob MC, Sotto JJ, Leroux D, Bensa JC, Plumas J. In vitro mechanisms of action of rituximab on primary non-Hodgkin lymphomas. Blood 2003; 101:949-54; PMID:12393572; http://dx.doi.org/ 10.1182/blood-2002-02-0469 [DOI] [PubMed] [Google Scholar]
- 28. Lefebvre ML, Krause SW, Salcedo M, Nardin A. Ex vivo-activated human macrophages kill chronic lymphocytic leukemia cells in the presence of rituximab: mechanism of antibody-dependent cellular cytotoxicity and impact of human serum. J Immunother 2006; 29:388-97; PMID:16799334; http://dx.doi.org/ 10.1097/01.cji.0000203081.43235.d7 [DOI] [PubMed] [Google Scholar]
- 29. Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, Jan M, Cha AC, Chan CK, Tan BT, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 2010; 142:699-713; PMID:20813259; http://dx.doi.org/ 10.1016/j.cell.2010.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bologna L, Gotti E, Manganini M, Rambaldi A, Intermesoli T, Introna M, Golay J. Mechanism of action of type II, glycoengineered, anti-CD20 monoclonal antibody GA101 in B-chronic lymphocytic leukemia whole blood assays in comparison with rituximab and alemtuzumab. J Immunol 2011; 186:3762-9; PMID:21296976; http://dx.doi.org/ 10.4049/jimmunol.1000303 [DOI] [PubMed] [Google Scholar]
- 31. Leidi M, Gotti E, Bologna L, Miranda E, Rimoldi M, Sica A, Roncalli M, Palumbo GA, Introna M, Golay J. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than m1 cells in vitro. J Immunol 2009; 182:4415-22; PMID:19299742; http://dx.doi.org/ 10.4049/jimmunol.0713732 [DOI] [PubMed] [Google Scholar]
- 32. Weiskopf K, Ring AM, Ho CC, Volkmer JP, Levin AM, Volkmer AK, Ozkan E, Fernhoff NB, van de Rijn M, Weissman IL, et al. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science 2013; 341:88-91; PMID:23722425; http://dx.doi.org/ 10.1126/science.1238856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bruggemann M, Williams GT, Bindon CI, Clark MR, Walker MR, Jefferis R, Waldmann H, Neuberger MS. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med 1987; 166:1351-61; PMID:3500259; http://dx.doi.org/ 10.1084/jem.166.5.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Watanabe M, Wallace PK, Keler T, Deo YM, Akewanlop C, Hayes DF. Antibody dependent cellular phagocytosis (ADCP) and antibody dependent cellular cytotoxicity (ADCC) of breast cancer cells mediated by bispecific antibody, MDX-210. Breast Cancer Res Treat 1999; 53:199-207; PMID:10369066; http://dx.doi.org/ 10.1023/A:1006145507567 [DOI] [PubMed] [Google Scholar]
- 35. Edris B, Willingham SB, Weiskopf K, Volkmer AK, Volkmer JP, Muhlenberg T, Montgomery KD, Contreras-Trujillo H, Czechowicz A, Fletcher JA, et al. Anti-KIT monoclonal antibody inhibits imatinib-resistant gastrointestinal stromal tumor growth. Proc Natl Acad Sci U S A 2013; 110:3501-6; PMID:23382202; http://dx.doi.org/ 10.1073/pnas.1222893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med 2004; 199:1659-69; PMID:15210744; http://dx.doi.org/ 10.1084/jem.20040119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, Lin WY, Hu Z, Lu Y, Chen Y, et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol 2005; 174:817-26; PMID:15634903; http://dx.doi.org/ 10.4049/jimmunol.174.2.817 [DOI] [PubMed] [Google Scholar]
- 38. Montalvao F, Garcia Z, Celli S, Breart B, Deguine J, Van Rooijen N, Bousso P. The mechanism of anti-CD20-mediated B cell depletion revealed by intravital imaging. J Clin Invest 2013; 123:5098-103; PMID:24177426; http://dx.doi.org/ 10.1172/JCI70972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van der Bij GJ, Bogels M, Otten MA, Oosterling SJ, Kuppen PJ, Meijer S, Beelen RH, van Egmond M. Experimentally induced liver metastases from colorectal cancer can be prevented by mononuclear phagocyte-mediated monoclonal antibody therapy. J Hepatol 2010; 53:677-85; PMID:20619916; http://dx.doi.org/ 10.1016/j.jhep.2010.04.023 [DOI] [PubMed] [Google Scholar]
- 40. Grugan Kd, McCabe FL, Kinder M, Greenplate AR, Harman BC, Ekert JE, van Rooijen N, Anderson GM, Nemeth JA, Strohl WR, et al. Tumor-associated macrophages promote invasion while retaining Fc-dependent anti-tumor function. J Immunol 2012; 189:5457-66; PMID:23105143; http://dx.doi.org/ 10.4049/jimmunol.1201889 [DOI] [PubMed] [Google Scholar]
- 41. Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol 2003; 21:3940-7; PMID:12975461; http://dx.doi.org/ 10.1200/JCO.2003.05.013 [DOI] [PubMed] [Google Scholar]
- 42. Richards JO, Karki S, Lazar GA, Chen H, Dang W, Desjarlais JR. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol Cancer Ther 2008; 7:2517-27; PMID:18723496; http://dx.doi.org/ 10.1158/1535-7163.MCT-08-0201 [DOI] [PubMed] [Google Scholar]
- 43. Zhang W, Gordon M, Schultheis AM, Yang DY, Nagashima F, Azuma M, Chang HM, Borucka E, Lurje G, Sherrod AE, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol 2007; 25:3712-8; PMID:17704420; http://dx.doi.org/ 10.1200/JCO.2006.08.8021 [DOI] [PubMed] [Google Scholar]
- 44. Tamura K, Shimizu C, Hojo T, Akashi-Tanaka S, Kinoshita T, Yonemori K, Kouno T, Katsumata N, Ando M, Aogi K, et al. FcgammaR2A and 3A polymorphisms predict clinical outcome of trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann Oncol 2011; 22:1302-7; PMID:21109570; http://dx.doi.org/ 10.1093/annonc/mdq585 [DOI] [PubMed] [Google Scholar]
- 45. Taskinen M, Karjalainen-Lindsberg ML, Nyman H, Eerola LM, Leppa S. A high tumor-associated macrophage content predicts favorable outcome in follicular lymphoma patients treated with rituximab and cyclophosphamide-doxorubicin-vincristine-prednisone. Clin Cancer Res 2007; 13:5784-9; PMID:17908969; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-0778 [DOI] [PubMed] [Google Scholar]
- 46. Cartron G, Zhao-Yang L, Baudard M, Kanouni T, Rouille V, Quittet P, Klein B, Rossi JF. Granulocyte-macrophage colony-stimulating factor potentiates rituximab in patients with relapsed follicular lymphoma: results of a phase II study. J Clin Oncol 2008; 26:2725-31; PMID:18427151; http://dx.doi.org/ 10.1200/JCO.2007.13.7729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cheung NK, Cheung IY, Kramer K, Modak S, Kuk D, Pandit-Taskar N, Chamberlain E, Ostrovnaya I, Kushner BH. Key role for myeloid cells: phase II results of anti-G(D2) antibody 3F8 plus granulocyte-macrophage colony-stimulating factor for chemoresistant osteomedullary neuroblastoma. International journal of cancer Journal international du cancer 2014; 135:2199-205; PMID:24644014; http://dx.doi.org/ 10.1002/ijc.28851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011; 331:1612-6; PMID:21436454; http://dx.doi.org/ 10.1126/science.1198443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science 2000; 288:2051-4; PMID:10856220; http://dx.doi.org/ 10.1126/science.288.5473.2051 [DOI] [PubMed] [Google Scholar]
- 50. Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009; 138:271-85; PMID:19632178; http://dx.doi.org/ 10.1016/j.cell.2009.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr., van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009; 138:286-99; PMID:19632179; http://dx.doi.org/ 10.1016/j.cell.2009.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012; 109:6662-7; PMID:22451913; http://dx.doi.org/ 10.1073/pnas.1121623109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Edris B, Weiskopf K, Volkmer AK, Volkmer JP, Willingham SB, Contreras-Trujillo H, Liu J, Majeti R, West RB, Fletcher JA, et al. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci U S A 2012; 109:6656-61; PMID:22451919; http://dx.doi.org/ 10.1073/pnas.1121629109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 1997; 386:181-6; PMID:9062191; http://dx.doi.org/ 10.1038/386181a0 [DOI] [PubMed] [Google Scholar]
- 55. Adams S, van der Laan LJ, Vernon-Wilson E, Renardel de Lavalette C, Dopp EA, Dijkstra CD, Simmons DL, van den Berg TK. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J Immunol 1998; 161:1853-9; PMID:9712053 [PubMed] [Google Scholar]
- 56. Jiang P, Lagenaur CF, Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem 1999; 274:559-62; PMID:9872987; http://dx.doi.org/ 10.1074/jbc.274.2.559 [DOI] [PubMed] [Google Scholar]
- 57. Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol 2014; 32:25-50; PMID:24215318; http://dx.doi.org/ 10.1146/annurev-immunol-032713-120142 [DOI] [PubMed] [Google Scholar]
- 58. Lienard H, Bruhns P, Malbec O, Fridman WH, Daeron M. Signal regulatory proteins negatively regulate immunoreceptor-dependent cell activation. J Biol Chem 1999; 274:32493-9; PMID:10542295; http://dx.doi.org/ 10.1074/jbc.274.45.32493 [DOI] [PubMed] [Google Scholar]
- 59. Tsai RK, Discher DE. Inhibition of "self" engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol 2008; 180:989-1003; PMID:18332220; http://dx.doi.org/ 10.1083/jcb.200708043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Oldenborg PA, Gresham HD, Lindberg FP. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med 2001; 193:855-62; PMID:11283158; http://dx.doi.org/ 10.1084/jem.193.7.855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhao XW, van Beek EM, Schornagel K, Van der Maaden H, Van Houdt M, Otten MA, Finetti P, Van Egmond M, Matozaki T, Kraal G, et al. CD47-signal regulatory protein-alpha (SIRPalpha) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci U S A 2011; 108:18342-7; PMID:22042861; http://dx.doi.org/ 10.1073/pnas.1106550108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Harrison RE, Grinstein S. Phagocytosis and the microtubule cytoskeleton. Biochemistry and cell biology = Biochimie et biologie cellulaire 2002; 80:509-15; PMID:12440692; http://dx.doi.org/ 10.1139/o02-142 [DOI] [PubMed] [Google Scholar]
- 63. Kohrt HE, Sagiv-Barfi I, Rafiq S, Herman SE, Butchar JP, Cheney C, Zhang X, Buggy JJ, Muthusamy N, Levy R, et al. Ibrutinib antagonizes rituximab-dependent NK cell-mediated cytotoxicity. Blood 2014; 123:1957-60; PMID:24652965; http://dx.doi.org/ 10.1182/blood-2014-01-547869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Roit FD, Engelberts PJ, Taylor RP, Breij EC, Gritti G, Rambaldi A, Introna M, Parren PW, Beurskens FJ, Golay J. Ibrutinib interferes with the cell-mediated anti-tumor activities of therapeutic CD20 antibodies: implications for combination therapy. Haematologica 2015; 100:77-86; PMID:25344523; http://dx.doi.org/ 10.3324/haematol.2014.107011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Younes A, Thieblemont C, Morschhauser F, Flinn I, Friedberg JW, Amorim S, Hivert B, Westin J, Vermeulen J, Bandyopadhyay N, et al. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. The Lancet Oncology 2014; 15:1019-26; PMID:25042202; http://dx.doi.org/ 10.1016/S1470-2045(14)70311-0 [DOI] [PubMed] [Google Scholar]
- 66. Mantovani A, Polentarutti N, Luini W, Peri G, Spreafico F. Role of host defense merchanisms in the antitumor activity of adriamycin and daunomycin in mice. J Natl Cancer Inst 1979; 63:61-6; PMID:286835 [PubMed] [Google Scholar]
- 67. Pallasch CP, Leskov I, Braun CJ, Vorholt D, Drake A, Soto-Feliciano YM, Bent EH, Schwamb J, Iliopoulou B, Kutsch N, et al. Sensitizing protective tumor microenvironments to antibody-mediated therapy. Cell 2014; 156:590-602; PMID:24485462; http://dx.doi.org/ 10.1016/j.cell.2013.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Herter S, Birk MC, Klein C, Gerdes C, Umana P, Bacac M. Glycoengineering of therapeutic antibodies enhances monocyte/macrophage-mediated phagocytosis and cytotoxicity. J Immunol 2014; 192:2252-60; PMID:24489098; http://dx.doi.org/ 10.4049/jimmunol.1301249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A 2006; 103:4005-10; PMID:16537476; http://dx.doi.org/ 10.1073/pnas.0508123103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Horton HM, Bernett MJ, Pong E, Peipp M, Karki S, Chu SY, Richards JO, Vostiar I, Joyce PF, Repp R, et al. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res 2008; 68:8049-57; PMID:18829563; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-2268 [DOI] [PubMed] [Google Scholar]
- 71. Awan FT, Lapalombella R, Trotta R, Butchar JP, Yu B, Benson DM, Jr., Roda JM, Cheney C, Mo X, Lehman A, et al. CD19 targeting of chronic lymphocytic leukemia with a novel Fc-domain-engineered monoclonal antibody. Blood 2010; 115:1204-13; PMID:19965644; http://dx.doi.org/ 10.1182/blood-2009-06-229039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Woyach JA, Awan F, Flinn IW, Berdeja JG, Wiley E, Mansoor S, Huang Y, Lozanski G, Foster PA, Byrd JC. A phase 1 trial of the Fc-engineered CD19 antibody XmAb5574 (MOR00208) demonstrates safety and preliminary efficacy in relapsed CLL. Blood 2014; 124:3553-60; PMID:25301708; http://dx.doi.org/ 10.1182/blood-2014-08-593269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kelton W, Mehta N, Charab W, Lee J, Lee CH, Kojima T, Kang TH, Georgiou G. IgGA: A "Cross-Isotype" Engineered Human Fc Antibody Domain that Displays Both IgG-like and IgA-like Effector Functions. Chem Biol 2014; 21:1603-9; PMID:25500223; http://dx.doi.org/ 10.1016/j.chembiol.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 74. Hezareh M, Hessell AJ, Jensen RC, van de Winkel JG, Parren PW. Effector function activities of a panel of mutants of a broadly neutralizing antibody against human immunodeficiency virus type 1. J Virol 2001; 75:12161-8; PMID:11711607; http://dx.doi.org/ 10.1128/JVI.75.24.12161-12168.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oganesyan V, Gao C, Shirinian L, Wu H, Dall'Acqua WF. Structural characterization of a human Fc fragment engineered for lack of effector functions. Acta Crystallogr D Biol Crystallogr 2008; 64:700-4; PMID:18560159; http://dx.doi.org/ 10.1107/S0907444908007877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Weiner LM, Clark JI, Davey M, Li WS, Garcia de Palazzo I, Ring DB, Alpaugh RK. Phase I trial of 2B1, a bispecific monoclonal antibody targeting c-erbB-2 and Fc gamma RIII. Cancer Res 1995; 55:4586-93; PMID:7553634 [PubMed] [Google Scholar]
- 77. Reiners KS, Kessler J, Sauer M, Rothe A, Hansen HP, Reusch U, Hucke C, Kohl U, Durkop H, Engert A, et al. Rescue of impaired NK cell activity in hodgkin lymphoma with bispecific antibodies in vitro and in patients. Molecular therapy : the journal of the American Society of Gene Therapy 2013; 21:895-903; PMID:23459515; http://dx.doi.org/ 10.1038/mt.2013.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Valone FH, Kaufman PA, Guyre PM, Lewis LD, Memoli V, Deo Y, Graziano R, Fisher JL, Meyer L, Mrozek-Orlowski M, et al. Phase Ia/Ib trial of bispecific antibody MDX-210 in patients with advanced breast or ovarian cancer that overexpresses the proto-oncogene HER-2/neu. J Clin Oncol 1995; 13:2281-92; PMID:7545221 [DOI] [PubMed] [Google Scholar]
- 79. Fury MG, Lipton A, Smith KM, Winston CB, Pfister DG. A phase-I trial of the epidermal growth factor receptor directed bispecific antibody MDX-447 without and with recombinant human granulocyte-colony stimulating factor in patients with advanced solid tumors. Cancer immunology, immunotherapy : CII 2008; 57:155-63; PMID:17602224; http://dx.doi.org/ 10.1007/s00262-007-0357-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dela Cruz JS, Trinh KR, Morrison SL, Penichet ML. Recombinant anti-human HER2/neu IgG3-(GM-CSF) fusion protein retains antigen specificity and cytokine function and demonstrates antitumor activity. J Immunol 2000; 165:5112-21; PMID:11046042; http://dx.doi.org/ 10.4049/jimmunol.165.9.5112 [DOI] [PubMed] [Google Scholar]
- 81. Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, Seita J, Inlay MA, Weiskopf K, Miyanishi M, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A 2013; 110:11103-8; PMID:23690610; http://dx.doi.org/ 10.1073/pnas.1305569110 [DOI] [PMC free article] [PubMed] [Google Scholar]


