Abstract
Strigolactones (SLs) play significant role in shaping root architecture whereby auxin-SL crosstalk has been observed in SL-mediated responses of primary root elongation, lateral root formation and adventitious root (AR) initiation. Whereas GR24 (a synthetic strigolactone) inhibits LR and AR formation, the effect of SL biosynthesis inhibitor (fluridone) is just the opposite (root proliferation). Naphthylphthalamic acid (NPA) leads to LR proliferation but completely inhibits AR development. The diffusive distribution of PIN1 in the provascular cells in the differentiating zone of the roots in response to GR24, fluridone or NPA treatments further indicates the involvement of localized auxin accumulation in LR development responses. Inhibition of LR formation by GR24 treatment coincides with inhibition of ACC synthase activity. Profuse LR development by fluridone and NPA treatments correlates with enhanced [Ca2+]cyt in the apical region and differentiating zones of LR, indicating a critical role of [Ca2+] in LR development in response to the coordinated action of auxins, ethylene and SLs. Significant enhancement of carotenoid cleavage dioxygenase (CCD) activity (enzyme responsible for SL biosynthesis) in tissue homogenates in presence of cPTIO (NO scavenger) indicates the role of endogenous NO as a negative modulator of CCD activity. Differences in the spatial distribution of NO in the primary and lateral roots further highlight the involvement of NO in SL-modulated root morphogenesis in sunflower seedlings. Present work provides new report on the negative modulation of SL biosynthesis through modulation of CCD activity by endogenous nitric oxide during SL-modulated LR development.
Keywords: adventitious root formation, auxin, ethylene, lateral root formation, nitric oxide, strigolactone biosynthesis
Abbreviations
- AR
adventitious root
- AVG
aminoethoxyvinylglycine
- CCD
carotenoid cleavage dioxygenase
- cPTIO
carboxy-2-phenyl-4,4,5,5-tetramethyllimidazoline-1-oxyl-3-oxide
- DAF-2DA
4,5-Diaminofluorescein diacetate
- Fl
fluridone
- LR
lateral root
- NPA
1-napthylphthalamic acid
- PR
primary root
- SL
strigolactone.
Introduction
Plants produce a variety of strigolactones (SLs). Till date, more than 15 SLs have been identified and characterized from the root exudates of higher plants. SLs are plastidial in origin and are derived from carotenoid biosynthetic pathway, involving 2 carotenoid cleavage dioxygenases (CCDs) [CCD7 (MAX3, RMS5, D17, DAD3) and CCD8 (MAX4, RMS1, D10, DAD1)], a cytochrome P450 monooxygenase (MAX1), and a carotenoid isomerase (D27).1-3 The carlactone derived from the action of CCD8 on cis-β-carotene moves into cytosol and cyclizes to form 5-Deoxystrigol in presence of cytochrome P450 monooxygenase. 5-Deoxystrigol is thought to be the common precursor of other natural SLs. CCD7 and CCD8 are key enzymes in SL biosynthetic pathway. The 3D structure of CCD for the first time was obtained from apocarotenoid cleavage oxygenase (ACO), a cyanobacterial enzyme which synthesizes the C20 apocarotenoid retinal.4 Structurally all CCDs characterized to date, from bacteria to animals to plants, share a common structure.5 They contain a Fe2+ ion in the active site which is coordinated by 4 conserved His residues. Fe2+ has been found to activate oxygen for the cleavage of carotenoids.6,7
SLs are now established as phytohormones with various physiological roles.8 SL-auxin interaction in controlling shoot branching is one of the established interactions of SLs with phytohormones but whether auxins act upstream or downstream of SLs, is still controversial.9-13 Using shoot branching mutants of rice and pea, which are impaired in genes encoding (CCDs), role of SLs in the inhibition of shoot branching has now been revealed.14,15 SLs play significant role in shaping root architecture whereby auxin-SL crosstalk has been observed in SL-mediated responses of primary root elongation, lateral root formation, adventitious root initiation and secondary growth.13 An upregulation of the SL biosynthetic genes [CCD7, CCD8 and D27] by auxin has also been reported.16 Lateral roots are post-embryonic roots which emerge as lateral root primordia (LRP) from pericycle cells in the primary root through series of divisions. LRP furher differentiates to form mature lateral roots through formation of lateral root apical meristem and a vascular system.17 Various environmental and endogenous factors such as temperature, light, hormones, sugar, mineral salts act as cues for lateral root initiation and emergence. Exogenous application of IAA induces lateral root formation and increases the production of lateral roots in transgenic plants which overexpress bacterial IAA biosynthetic genes, suggesting the role of IAA as a signal molecule in lateral root initiation in plant.18-21 Auxin-signaling plays an important role in pericycle priming, initiation of cell division, and LRP formation to LR emergence.22 Recent work in Arabidopsis thaliana mutant has suggested the role of IAA in the initiation of cell division in the pericycle and promotion of cell division and maintenance of cell viability in the developing lateral root.23 Other hormones like ethylene, ABA, cytokinin, gibberellic acids, brassinosteroids and SLs act during LR formation via interference with auxin synthesis, transport and sensitivity indicating auxin as a central player in LR formation.24-33
Adventitious roots (ARs) are post-embryonic roots known to originate from hypocotyl, stem, leaf petiole and non-pericycle tissues of old roots. In young stem, AR commonly arise from the interfascicular parenchyma while in older stem they appear from vascular rays near the cambium. AR formation begins with redifferentiation of predetermined cells which switch from their morphogenetic path to act as mother cells for the initiation of root primordium.34 The process of AR formation consists of 3 physiologically interdependent phases – induction, initiation and extension.34,35 AR formation involves the interaction of environmental and endogenous factors among which the phytohormone auxin plays an important role. The three phases of AR formation that is induction, initiation and extension are regulated by alterations in the endogenous level of auxin suggesting its central role.36 Auxins appear to evoke dose-dependent AR response. A transient increase in auxin concentration has been reported during the induction phase, which is followed by a decrease and again an increase during extension phase.23 Mutants or treatments resulting in disturbed polar auxin transport show reduced AR formation.37,38 While auxins and auxin signaling are essential for all stages of LR development,39-42 exogenous auxin stimulates during the first stage and inhibits later developmental stages of AR formation,43-45 thus indicating different sensitivity of both root-types to exogenous auxin. Besides exogenous or newly biosynthesized auxin, auxin transport, through the ABC-type multi-drug-resistance ABCB19 transporter, is also essential for AR induction in hypocotyls.38 Earlier reports on auxin-SL interaction in regulating adventitious root initiation have revealed the role of SL in reducing adventitious root initiation through its negative impact on auxin levels in the cells of pericycle.46,47 SL crosstalk with cytokinins and auxin in regulating AR formation has also been established.46 Recent reports in Arabidopsis and pea have suggested that SLs negatively regulate AR initiation by interfering with polar auxin transport (efflux), thus reducing the optimal concentration of IAA required for AR formation at the hypocotyl base (zone of AR formation).46 Several mutant studies examining the role of SL in photomorphogenesis in Arabidopsis,48-50 rice51 and pea52 have revealed significant differences in regulation of developmental processes by SLs between these species. Recent work in pea showed that unlike in adventitious roots formed by cuttings in light where SL plays a positive role,46 in the dark in intact peas SL appears to inhibit rooting.52 Ethylene has been found to affect LR development in a dose-dependent manner. Low concentrations of the ethylene precursor, 1-aminocyclopropane- 1-carboxylic acid (ACC) promotes LR initiation. Higher doses strongly inhibit LR primordia (LRP) initiation but promote the emergence of existing LRPs.53 Recent work in Arabidopsis mutants has suggested that ethylene-induced auxin transport through PIN3 and PIN7 prevents formation of local auxin maxima and hence suppression of LR.26 Genetic studies in Arabidopsis and tomato have further shown that ethylene inhibits LR formation while enhancing auxin transport and reducing auxin optima.24,25,53 SLs and ethylene also modulate root hair elongation via a common regulatory pathway in which ethylene is epistatic to SLs.32 Ethylene is found to have both positive and negative effect on AR development and emergence depending upon the species. For e.g. in mung bean or sunflower hypocotyls cuttings, Rumex, maize, rice and tomato prolonged exposure to exogenous 1-aminocyclopropane-1-carboxylic acid (ACC), the precursor of ethylene, results in increased root numbers.54-57 However, in Arabidopsis thaliana, stimulatory role of ethylene on AR formation is not observed.
Nitric oxide is known to play a crucial role in root development.58 Using various pharmacological agents, a probable signaling cascade (linking NO, cyclic GMP and mitogen-activated protein kinases) for auxin-induced AR formation has been proposed in cucumber seedlings.59-61 Yadav et al.,35 reported NO modulation during initiation and extension phases of auxin-induced AR formation in sunflower seedlings. Exogenous supply of NO donors-sodium nitroprusside (SNP) and S-nitroso N-acetyl penicillamine (SNAP) to cucumber explants mimicks the effect of IAA, leading to AR formation.59 SNP elicits its effect in a dose-dependent manner, with maximal biological response at a particular concentration, which is species-dependent. Employing the inhibitor for guanylate cyclase (GC), it has been demonstrated that NO operates downstream of IAA, promoting AR development through the GC-catalyzed synthesis of cGMP that further induces the Ca2+-dependent protein kinase (CDPK).60,62 Alternatively, MAPK signaling cascade is activated by IAA via a NO-mediated but cGMP-independent pathway.63 NO regulates cell divisions and organogenic processes in a calcium-dependent way.64 NO is emerging as a player in LR development due to its signling role in conversion of local peroxisomal IBA to IAA, which is important for LR formation.64-66
In the present work, attempts have been made to examine the critical role of SLs in LR formation in seedling roots and AR development from the basal region of hypocotyls cut ends in context with a crosstalk of SL action with auxin and ethylene. Analysis of ACC synthase activity and PIN1 protein distribution in seedling roots and their regulation by SL and associated biomolecules (Fl, SL biosynthesis inhibitor; NPA, PIN blocker) has also been undertaken. Role of GR24 (a synthetic strigolactone) and other biomolecules on intracellular free calcium ion [Ca2+]cyt localization in LR development has also been examined by CLSM. Present work puts forward new evidence for the involvement of NO in SL-modulated LR development.
Results
Lateral root (LR) development in sunflower seedlings and adventitious root (AR) initiation from the basal cut ends of hypocotyls are modulated by the application of a synthetic strigolactone (GR24), SL biosynthesis inhibitor (Fluridone; Fl) and auxin efflux blocker (naphthylphthalamic acid; NPA) (Fig 1A–D). Whereas GR24 significantly inhibits LR and AR formation, the effect of SL biosynthesis inhibitor is just the opposite (proliferation). NPA, through its action on inhibiting auxin efflux (and consequent localized accumulation), leads to LR proliferation but completely inhibits AR development. These observations indicate the role of SLs in AR and LR development to be modulated by auxins and ethylene.
Figure 1.
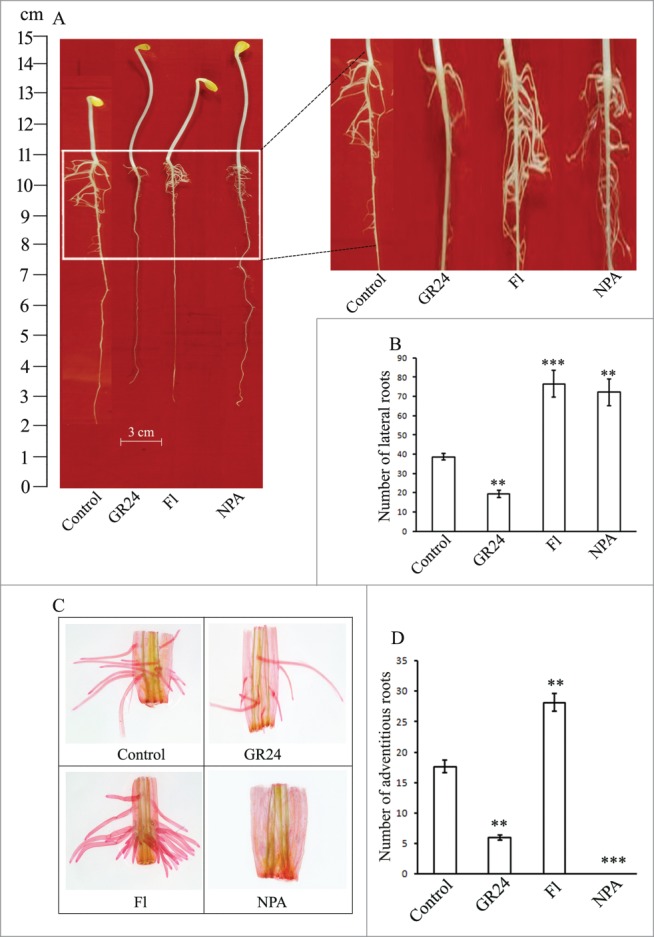
(A) Regulation of lateral root (LR) development by GR24 (a synthetic strigolactone), fluridone (inhibitor of SL biosynthesis) and NPA (auxin efflux blocker). (B) Quantitative analyses of the number of lateral roots formed in the above-stated treatments. (C) Effect of GR24 and related pharmacological agents on adventitious rooting in hypocotyl explants derived from 6 d old, dark-grown seedlings. Evaluation of the number of endogenous root initials and their elongation was undertaken in explants cleared after staining with safranin. (D) Quantitative analyses of AR initiation. Each datum indicates mean ± SE from at least 3 replicates, showing changes to be significant at different levels (*P < .05, **P < .01, ***P <.001) from the control, analyzed by one-way ANOVA.
Inhibition of LR formation by GR24 treatment coincides with inhibition of ACC synthase activity. With reference to control seedlings, both fluridone (SL biosynthesis inhibitor) and NPA (auxin efflux blocker) treatments also lower ACC synthase activity by 92 % of control (Fig. 2A). These observations indicate low ethylene production in seedling roots being triggered by these treatments irrespective of their influence on LR development. The diffusive distribution of PIN1 in the provascular cells in the differentiating zone of the roots in response to GR24, fluridone or NPA treatments further indicates the involvement of localized auxin accumulation in LR development responses (Fig. 2B).
Figure 2.
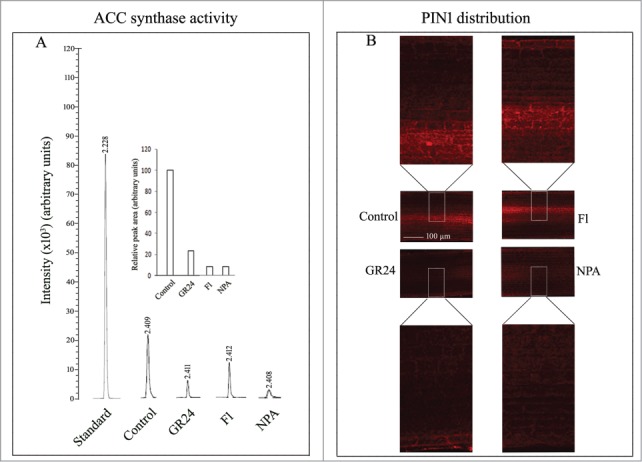
(A) GC-MS analysis of ACC synthase activity in 6 d old whole roots from etiolated sunflower seedlings grown in presence of distilled water (control) or 3 µM each of GR24, Fl, NPA. (B) CLSM imaging of PIN1 protein distribution in the seedling roots and their regulation by SL (GR24), fluridone (Fl) and NPA. Magnification 20X; scale bar = 100 µm.
Intracellular free calcium [Ca2+]cyt distribution is highest in the lateral roots of control seedlings and is significantly lowered by GR24 - a treatment which significantly inhibits LR development (Fig. 3). Profuse LR development by fluridone and NPA treatments correlates with enhanced [Ca2+]cyt both in the apical region and differentiating zones, indicating a critical role of intracellular free calcium in LR development in response to the coordinated action of auxins, ethylene and strigolactones.
Figure 3.
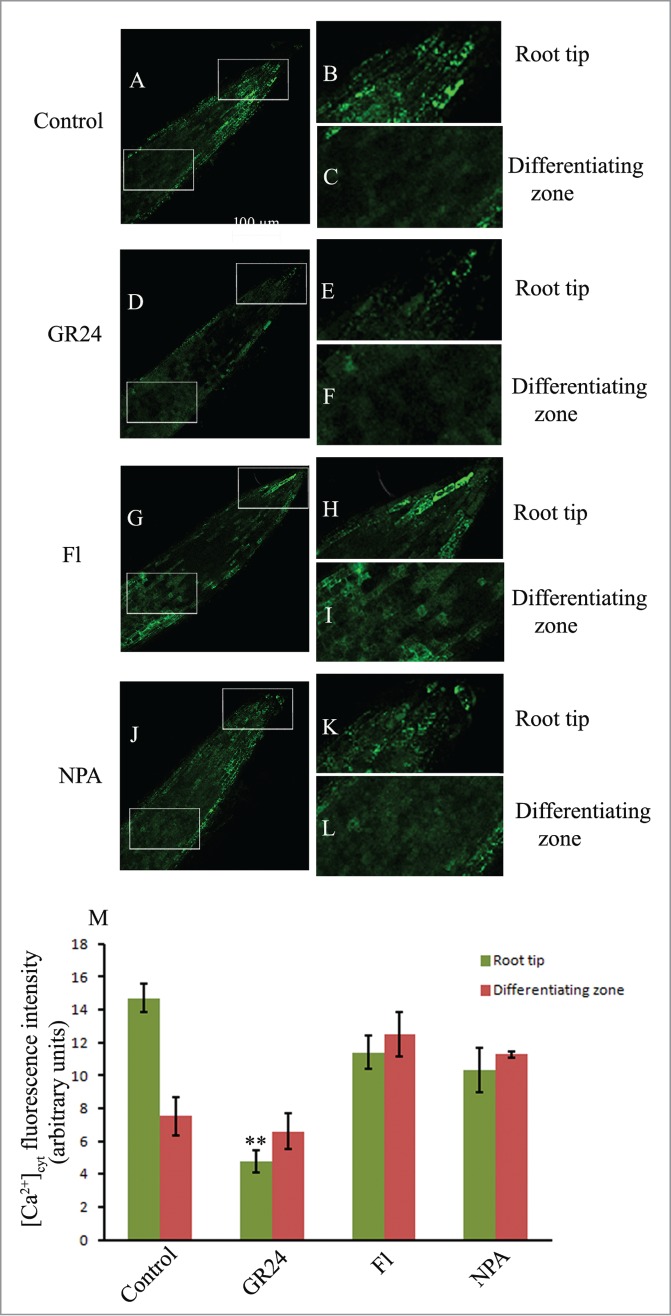
CLSM analysis of cytosolic calcium ion [Ca2+]cyt distribution using Oregon green BAPTA-1 in the lateral roots of 6d old seedling grown in the distilled water (control; A–C) and other pharmacological agents (GR24, Fl, NPA; D–L). Magnification 20X; scale bar = 100 µm. Histogram shows relative fluorescence intensity of calcium ion distribution in the respective samples (M). Each datum indicates mean ± SE from at least 3 replicates, showing changes to be significant at different levels (*P < .05, **P < .01, ***P < .001) from the control, analyzed by one-way ANOVA.
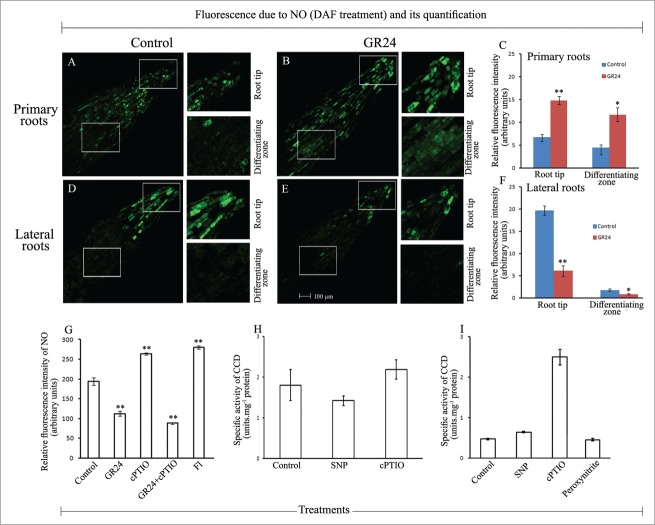
In our attempts to find a possible correlation between SL-inhibited LR formation, CCD activity and nitric oxide (NO), interesting differences have been observed in the spatial distribution of endogenous NO in the apical and differentiating zones of primary roots (PRs) (Fig. 4A–C) and lateral roots (LRs) (Fig. 4D–F) in response to GR24 treatment. The tip region of primary roots exhibits enhanced NO accumulation in presence of GR24 in contrast to root tips of lateral roots with reference to their respective controls. The differentiating zone of the primary roots shows similar pattern of NO accumulation in response to GR24 treatment as in the root tips, though at a relatively reduced level. In contrast, the differentiating zone of lateral roots does not exhibit any significant accumulation of NO both in control and seedling roots subjected to GR24 treatment. These observations indicate an inverse correlation between the extension growth of lateral roots and apical accumulation of nitric oxide during the phase of LR extension growth.
Figure 4.
Spatial distribution of NO in the primary roots (A–C) and lateral roots (D–F) in sunflower seedlings raised in control conditions or in presence of syntheic strigolactone - GR24. (G) Whole root NO content. (H) CCD activity in the roots of 2 d old seedlings raised in presence of SNP (100 µM) or cPTIO (500 µM). (I) Modulation of CCD activity in root homogenates (20,800 g) from 2 d old control seedlings subjected to SNP / cPTIO / peroxynitrite (250 µM each) treatments. Data indicate mean ± SE of at least 3 replicates, showing changes to be significant at different levels (*P < .05, **P < .01, ***P < .001) from the control, analyzed by one-way ANOVA.
A significant lowering of whole root NO content has been observed in response to GR24 (and its combination with cPTIO) treatments (Fig. 4G). Enhanced NO accumulation is accordingly evident with fluridone (Fl, SL biosynthetic inhibitor) and also with cPTIO treatments, probably due to the well known dual role of cPTIO as a nitric oxide scavenger and indirect N2O3 producer (which binds with DAF), depending on the tissue type and its physiological state. So, the analysis of NO content data from the whole roots also indicates a correlation with the influence of GR24 on the growth and differentiation pattern of roots in sunflower seedlings.
Investigations on examining the possible role of NO in modulating a biochemical pathway routinely employ sodium nitroprusside (SNP) which releases NO, cPTIO, an established NO scavenger and peroxynitrite, which causes nitration of proteins. Whether NO acts on SL-modulated LR formation through its effect on CCD activity in the seedling roots, is made evident from the variations in CCD activity in seedling roots exposed to SNP and cPTIO treatments (Fig. 4H). This was also very significantly evident when the root homogenates derived from control seedling roots were subjected to SNP, cPTIO or peroxynitrite treatments prior to CCD activity estimation (Fig. 4I). Several-fold (about 2-fold) enhancement of CCD activity in tissue homogenates in presence of cPTIO indicates the critical role of endogenous NO as a negative modulator of CCD activity. NO depletion enhances CCD activity. SNP (NO donor) treatment does not alter CCD activity confirming the negative role of nitric oxide on CCD activity. Peroxynitrite treatment does not alter CCD activity indicating its no negative role or any direct influence on CCD activity. These interesting observations together with noteworthy differences in the spatial distribution of NO in the primary and lateral roots highlight the involvement of NO in SL-modulated root morphogenesis in sunflower seedlings. It is possible that NO produced in the tissue system inhibits CCD activity either directly or through inhibition of some other enzymes associated with the production of precursors for CCD.
Discussion
SLs have earlier been reported to promote rhizoid elongation in liverworts67 and stimulate mycorrhizal association with the roots of higher plants.68 Among higher plants, SLs regulate plant architecture through a modulation of shoot branching and root growth and development.69 They are known to be synthesized both in root and stem and are transported across xylem.70 In Petunia, ATP binding cassette (ABC transporter) PDR1 has been reported to be critical for SL transport.71 Adventitious roots (ARs) can be formed from the non-root tissues such as hypocotyls or from the divisions of vascular cambial cells. AR initiation has earlier been reported to be inhibited by SLs by reducing the cambial activity in the cells of the cut surface.46 The mechanism of regulation of AR development is, however, not yet clear. In contrast to SL action, auxins promote LR formation.
Since auxin transport and its consequent accumulation play crucial roles in root development, SLs are expected to play a role in regulating auxin fluxes. SLs may bring about these changes in the localized auxin level either by altering auxin biosynthesis or its polar transport.69 Role of SLs in reducing polar auxin transport has also been made evident through its effect on PIN distribution. Recently, a depletion of PIN1 from the xylem parenchyma cells was observed in response to SL.11 In an analysis of the primary root growth in SL-deficient and insensitive Arabidopsis plants, root growth has been found to be less than that in wild type plants. This is nullified by the application of GR24. The intensity of PIN proteins in the provascular tissue of the primary root tip also decreases. GR24 application suppresses LR primordia development.33 Similar observations have been made in the present work as well. These results suggest that SLs are able to modulate localized auxin concentration and SL action is dependent on the auxin status of the plant. A negative influence of SL on LR formation has recently been reported elsewhere as well.32,33 This coincides with the observation of localized application of auxin in the pericycle by the PIN-dependent transport mechanism.72 Inhibition of LR primordia formation by GR24, has been reported to be due to SL-concentration dependent modulation of initiation of LR (rather than elongation or outgrowth) has also been suggested.32 Part of SL regulation of LR formation is likely to result from the changes in auxin transport capacity through reduction in PIN1 expression.33
Root development has also been shown to be regulated by ethylene, and ethylene signaling is believed to be involved in SL response, particularly under sufficient Pi in the growth medium. There is marked reduction in SL response in ethylene signaling mutants - etr and ein. Moreover, the negative effect of AVG (aminoethoxyvinylglycine; an ethylene synthesis inhibitor) on root hair development due to SL and the ability of SL to induce ACC synthase activity, highlight a correlation between SL action and ethylene availability.32 SLs have also been shown to induce ethylene biosynthesis in the parasitic plant – Striga, leading to their seed germination.73 In the present work, however, an inverse correlation has been observed between SL-induced LR development and ACC synthase activity. Auxin and ethylene signaling pathways are differentially activated and these regulate different aspects of root development under diverse Pi growth conditions. SLs may be one of the signals responsible for sensitizing plant roots to auxin and ethylene. Thus, SLs are likely to adjust the balance between auxin and ethylene signaling pathways to activate various developmental programmes responsible for root development.74 Auxin application has also been shown to promote the expression of SL synthesis gene in Arabidopsis like other plant systems.75 It is apparent from the findings so far that SLs signaling affects the tissue specificity of auxin. Additionally, SLs may reduce localized availability of free auxin, thereby altering AR/LR development response.69
The present work reports for the first time a negative modulation of CCD activity by endogenous nitric oxide, since NO depletion by cPTIO treatment significantly enhances CCD activity. The basic molecular structure of CCDs shows that they contain a Fe2+ in the active site, which is coordinated by 4 conserved histidine residues.5,76 NO can reversibly bind Fe2+ linked with histidine residues, as in case of guanilate cyclase, cytochrome oxidase or catalase.77-80 Based on this affinity of NO for Fe2+, it is expected that Fe2+ in CCD binds with available NO and inhibit its activity. Thus, it is proposed that the SL-modulated sensitization of auxin-ethylene regulated LR development in plants is being controlled through a reversible inhibition of CCD activity through its NO binding / release with Fe2+. Present work has thus opened a new front for further work on understanding the mechanism of LR development through NO signaling.
To sum up, present work highlights the critical role of strigolactones (SLs) in LR formation in seedling roots and AR development from the basal region of hypocotyl cut ends. Auxin transport and its consequent accumulation play crucial roles in root development and SLs are expected to play a role in regulating auxin fluxes. SLs may bring about these changes in the localized auxin level by altering its polar transport. Present results suggest that SLs are able to modulate localized auxin concentration. Auxin and ethylene signaling pathways are differentially activated and they regulate different aspects of root development. SLs are likely to adjust the balance between auxin and ethylene signaling pathways to activate various developmental programmes responsible for root development. Present work indicates an inverse correlation between SL-induced LR development and ACC synthase activity. It is also evident that NO produced in the tissue system inhibits CCD activity, thereby lowering SL biosynthesis. It is proposed that the SL-modulated sensitization of auxin-ethylene regulated LR development in plants is being controlled through a reversible inhibition of CCD activity through its NO binding / release with Fe2+.
Materials and Methods
Pharmacological treatments
Soon after germination (radicle emergence), dark-grown sunflower seedlings were subjected to various pharmacological treatments (3 µM each), namely GR24 (rac-GR24, from Chiralix, Netherlands), fluridone (Fl; SL biosynthesis inhibitor) (Sigma-Aldrich, USA) and 1-napthylphthalamic acid (NPA; auxin efflux blocker) (Sigma-Aldrich, USA). Seedlings grown in distilled water served as control. LR development was evident in control seedlings 2 days after growth. Following six days of growth, the number of lateral roots from 10 seedlings per treatment was counted and the response was photographed.
Pharmacological treatments to hypocotyl explants
Hypocotyl explants with intact apical meristem but excised cotyledons, were selected from 6-d-old, dark-grown seedlings. Such explants maintained an apical source of endogenous auxin from the shoot meristem. Freshly harvested explants were put upright in glass vials with their proximal cut ends dipped in 1 mL of different pharmacological treatments, thus bathing the hypocotyl segments up to 6 mm of their lower ends. Explants were maintained in dark during the course of experiments. The number of adventitious roots (ARs) visible on the basal surface of hypocotyls was recorded daily up to 6 days of incubation. Various test solutions, namely GR24, Fl and NPA (3 µM each) were used to investigate their effects on adventitious rooting. Hypocotyl explants incubated in distilled water served as control. Detailed evaluation of root initiation within the hypocotyl tissue was undertaken after clearing by immersing the explants in a 3:1 solution of ethanol: acetic acid overnight. They were then transferred to 2N NaOH solution, left overnight, washed once with distilled water and stained with safranin solution for 2–3 min. Excess stain was removed by repeated washing in distilled water. The lower 2 cm region of hypocotyl explants was then cut and mounted on a glass slide to examine and photograph endogenous root initials.
GC-MS analysis of ACC synthase activity
Samples were prepared according to Boller et al.81 Six days old roots from etiolated sunflower seedlings grown in the presence of distilled water and other pharmachological agents - GR24, Fl and NPA, were ground in liquid nitrogen. The powder was suspended in extraction buffer (100 mmolL−1 HEPES buffer, pH 8.5) containing 0.5 µmolL−1 pyridoxal phosphate (PLP), 10 mmolL−1 EDTA and 4 mmolL−1 DTT, vortexed at 4°C for 30 min and centrifuged twice at 10,000 g at 4°C for 20 min. The supernatant was filtered through Sep-Pak cartridge (Waters Corporation, USA) and used for enzyme assay.
ACC synthase activity assay was carried out in a reaction mixture consisting of 0.5 mL of 60 µmolL−1 adenosylmethionine and 1 mL of enzyme extract incubated at 30°C for 2 h in sealed vial. The amount of ACC formed was determined by the standard protocol of Lizada and Yang.82 Following incubation, 1 µM HgCl2 was added to the reaction mixture through syringe and the vial was sealed and kept on ice for 10 min. Thereafter, 100 µL of a cold mixture of 5% NaOCl and saturated NaOH (2:1, v/v), which contained about 45 µM NaOCl, was injected into the sealed vial containing the reaction mixture. The reaction mixture was agitated on a shaker for 2.5 min. Gas samples were withdrawn using the 100 µL syringe (SGE Analytical Science, Louisiana, USA) and injected in GC-MS. ACC synthase activity is directly related to the amount of ethylene produced. Endogenous ethylene was detected in various samples by running ethylene standard as a reference.
Immunofluorescence (CLSM) localization of PIN 1 distribution in seedling roots
Apical root segments were selected from the primary root of 2 d old, etiolated seedlings grown in the presence of distilled water (control), GR24, Fl and NPA. Root segments were fixed for 15 min in a mixture of 4% p-formaldehyde and 0.05% glutaraldehyde prepared in 0.5X stabilizing buffer (MTSB, 50 mM PIPES, 5 mM MgSO4.7H2O, 5 mM EGTA, pH 6.9) and samples were washed for 20 min in stabilizing buffer containing 0.1% Triton-X 100 according to Truernit et al.83 Samples were then dehydrated for 15 min at room temperature in 80% methanol diluted in phosphate buffer saline (PBS, 0.14 M NaCl, 2.7 mM KCl, 6.5 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.3) followed by washing in PBS for 5 min. Samples were then digested for 15 min in digestion buffer (MES 50 mM pH 5.5, CaCl2 20 mM, mannitol 700 mM, 0.1% cellulose, 0.02% pectinase) at 37°C, followed by washing in PBS for 5 min. Samples were then incubated in blocking solution containing 0.1% BSA dissolved in PBS for 30 min. Prior to immunolabeling, samples were washed in PBS for 5 min, followed by incubation for 16 h at 4°C in anti-PIN1 (Santa Cruz Biotechnology, USA) at a dilution of 1:100 in PBS. Incubation with primary antibodies was terminated by washing in PBS twice for 5 min each. Samples were subsequently incubated with secondary antibodies dissolved in PBS for 2 h [Donkey anti-goat Alexafluor (1:200) for PIN and anti-mouse FITC (1:50) for actin; Invitrogen Bioservices Pvt. Ltd, USA] at room temperature. Samples were then washed in PBS containg 50 mM glycine and mounted in glycerine. Imaging was performed using CLSM (Leica, TCS SP5, Germany) at an excitation of 555 nm for Alexafluor (for PIN 1). Images of radial longitudinal section (RLS) from the differentiating zone of root segments were obtained at a magnification of 20X. Z-series was obtained at a thickness of 2 µm (pinhole 1).
Localization of distribution of cytosolic free calcium [Ca2+]cyt in intact roots
Roots from 6 d old etiolated seedlings grown in the presence of distilled water (control) and other pharmacological agents (GR24, Fl and NPA) were incubated for 60 min in 50 µM of calcium specific fluorescent probe-Oregon Green 488 BAPTA-1 (dissolved in 100 mM Tris-HCl, pH 7.5). Following incubation, roots were washed in Tris buffer. Lateral roots were cut 4 cm from tip, mounted in slides and visualized using a confocal microscope (Leica TCS SP5, Germany) at an excitation and emission of 488 nm and 519 nm, respectively. Optical sectioning (Z-series) was performed at a thickness of 2 µm using pinhole 1. Quantification of fluorescence intensity was performed by ImageJ 1.48v (USA) fluorescence quantification software and represented graphically in terms of relative fluorescence units.
Localization of nitric oxide (NO) distribution in intact roots
Roots from 4 d old etiolated seedlings of sunflower grown in the presence and absence of GR24 were incubated for 40 min in 50 µM DAF-2DA (dissolved in 10 mM Tris-HCl, pH 7.5). Following incubation, roots were washed in Tris buffer. Both primary roots (PRs) and lateral roots (LRs) were cut 4 cm from tip, mounted on glass slides and visualized using a confocal microscope (Leica TCS SP5, Germany) at an excitation and emission of 495 nm and 515 nm, respectively. Optical sectioning was performed at a thickness of 2 µm for each section using pinhole 1. Quantification of fluorescence intensity was performed by ImageJ 1.48v (USA) fluorescence quantification software and represented graphically in terms of relative fluorescence units.
Quantification of nitric oxide distribution in root homogenates
Roots from 2 d old etiolated seedlings of sunflower grown in the presence of distilled water (control) and various pharmacological agents, including GR24, cPTIO (Invitrogen, USA), GR24 + cPTIO and Fl, were ground to powder in liquid nitrogen and the powder was dispersed in extraction buffer (50 mM Tris-HCl, 250 mM sucrose and 1 mM EDTA, pH 7.5) containing 1 mM PMSF and 5 mM DTT. The homogenates were transferred to eppendorf tubes and vortexed for 30 min. Thereafter, the homogenates were centrifuged at 10,000 g for 20 min at 4°C. The supernatants were again centrifuged at 10,000 g for 20 min at 4°C. Equal volumes (347 µL) of supernatants were incubated with 2.5 µM of DAF-2DA (Invitrogen Bioservices, USA) in the extraction buffer for 30 min at 25°C. Reaction mixtures without tissue homogenates served as control for their respective samples. Fluorescence was estimated at an excitation and emission wavelengths of 495 and 515 nm, respectively.
Estimation of carotenoid cleavage dioxygenase (CCD) activity in seedling roots
Whole tissue homogenates (20,800 g supernatants) obtained from the roots of 2 d old, dark-grown seedlings raised in presence of distilled water (control), SNP (100 µM) or cPTIO (500 µM) were used as enzyme source. Homogenization was performed using the extraction buffer consisting of Tris-sucrose (50 mM, pH 7.5), β-mercaptoethanol (0.2%) and phenylmethylsulfonyl fluoride (1 mM). Tissue homogenates were centrifuged at 20,800 g for 20 min at 4°C. Protein content of the total soluble protein (TSP) was estimated by Bradford assay.84 CCD enzyme activity was measured spectrophotometrically with lutein (Sigma Aldrich, USA) as substrate, according to Mathieu et al.,85 with minor modifications. Lutein was dissolved in 80% acetone (0.5 g.L−1) to obtain a final concentration of 35 µM. The reaction mixture consisted of dithiothreitol (1 mM), FeSO4 (50 µM), Triton X-100 (0.11%), 80% acetone (10%), lutein (35 µM), protein aliquot (50 µg). Final volume of the reaction mixture was made to 660 µL using the above stated buffer. Reaction mixture was vortexed and incubated in dark at 30°C for 30 min in a shaker with gentle agitation and decrease in absorbance due to lutein degradation was recorded at 450 nm. Reaction mixture without protein served as control. One unit of CCD activity refers to the quantity of protein required to cleave 1 nmol of lutein in 1 min under similar assay conditions and its activity was expressed as units.mg−1 protein.
Estimation of carotenoid cleavage dioxygenase (CCD) activity in root homogenates obtained from control seedlings were done in the same way as stated above, except protein was subjected to SNP / cPTIO / peroxynitrite (Calbiochem, Germany) (250 µM each) treatments for 1 h prior to CCD activity.
Statistical analyses
All experiments were performed at least thrice and data were analyzed by SPSS 16.0 statistical program (SPSS Inc., Chicago, IL) using one-way ANOVA.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interests were disclosed.
Acknowledgments
Technical help provided by Dr. Soumya Mukherjee in various ways is gratefully acknowledged. We thank Ms. Charu for the assistance in confocal imaging (University of Delhi, south campus) and Mr. S. K. Das for photography in our department (Department of Botany, University of Delhi).
NB and SCB are jointly responsible for the experimental design, data analysis and manuscript writing. NB contributed to the experimental work. All authors have read and approved the final manuscript and have no conflicts of interest.
Funding
This work was supported in the form of Junior Research Fellowship to NB from University Grants Commission, New Delhi and financial assistance from University of Delhi in the form of R & D grant. PURSE grant received from DST, New Delhi is also gratefully acknowledged.
References
- 1.Matusova R, Rani K, Verstappen FWA, Franssen MCR, Beale MH, Bouwmeester HJ. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol 2005; 139:920-34; PMID:16183851; http://dx.doi.org/ 10.1104/pp.105.061382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drummond RSM, Martínez-Sánchez NM, Janssen BJ, Templeton KR, Simons JL, Quinn BD, Karunairetnam S, Snowden KC. Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE7 is involved in the production of negative and positive branching signals in petunia. Plant Physiol 2009; 151:1867-77; PMID:19846541; http://dx.doi.org/ 10.1104/pp.109.146720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beveridge CA, Kyozuka J. New genes in the strigolactone-related shoot branching pathway. Curr Opin Plant Biol 2010; 13:34-9; PMID:19913454; http://dx.doi.org/ 10.1016/j.pbi.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 4.Kloer DP, Ruch S, Al-Babili S, Beyer P, Schulz GE. The structure of a retinal-forming carotenoid oxygenase. Science 2005; 308:267-9; PMID:15821095; http://dx.doi.org/ 10.1126/science.1108965 [DOI] [PubMed] [Google Scholar]
- 5.Sui X, Kiser PD, Von Lintig J, Palczewski K. Structural basis of carotenoid cleavage: From bacteria to mammals. Arch Biochem Biophys 2013; 539:203-13; PMID:23827316; http://dx.doi.org/ 10.1016/j.abb.2013.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kloer DP, Schulz GE. Structural and biological aspects of carotenoid cleavage. Cell Mol Life Sci 2006; 63:2291-303; PMID:16909205; http://dx.doi.org/ 10.1007/s00018-006-6176-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borowski T, Blomberg MRA, Siegbahn PEM. Reaction mechanism of apocarotenoid oxygenase (ACO): A DFT study. Chem Eur J 2008; 14:2264-76; PMID:18181127; http://dx.doi.org/ 10.1002/chem.200701344 [DOI] [PubMed] [Google Scholar]
- 8.Taiz L, Zeiger E. Plant Physiology. Sunderland, USA: Sinauer Associates Inc.; 2014 [Google Scholar]
- 9.Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol 2009; 150:482-93; PMID:19321710; http://dx.doi.org/ 10.1104/pp.108.134783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawford S, Shinohara N, Sieberer T, Williamson L, George G, Hepworth J, Müller D, Domagalska MA, Leyser O. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 2010; 137:2905-13; PMID:20667910; http://dx.doi.org/ 10.1242/dev.051987 [DOI] [PubMed] [Google Scholar]
- 11.Shinohara N, Taylor C, Leyser O. Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol 2013; 11:e1001474; PMID:23382651; http://dx.doi.org/ 10.1371/journal.pbio.1001474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foo E, Reid JB. Strigolactones: New physiological roles for an ancient signal. J Plant Growth Regul 2013; 32:429-42; http://dx.doi.org/ 10.1007/s00344-012-9304-6 [DOI] [Google Scholar]
- 13.Cheng X, Ruyter-Spira C, Bouwmeester H. The interaction between strigolactones and other plant hormones in the regulation of plant development. Front Plant Sci 2013; 4:1–16; PMID:23785379; http://dx.doi.org/ 10.3389/fpls.2013.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, et al.. Strigolactone inhibition of shoot branching. Nature 2008; 455:189-94; PMID:18690209; http://dx.doi.org/ 10.1038/nature07271 [DOI] [PubMed] [Google Scholar]
- 15.Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al.. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008; 455:195-200; PMID:18690207; http://dx.doi.org/ 10.1038/nature07272 [DOI] [PubMed] [Google Scholar]
- 16.Foo E, Bullier E, Goussot M, Foucher F, Rameau C, Beveridge CA. The branching gene RAMOSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell 2005; 17:464-74; PMID:15659639; http://dx.doi.org/ 10.1105/tpc.104.026716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. Cellular organisation of the Arabidopsis thaliana root. Development 1993; 119:71-84; PMID:8275865 [DOI] [PubMed] [Google Scholar]
- 18.Torrey JG. The induction of lateral roots by indoleacetic acid and root decapitation. Am J Bot 1950; 37:257-64; http://dx.doi.org/ 10.2307/2437843 [DOI] [Google Scholar]
- 19.Blakely LM, Durham M, Evans TA, Blakely RM. Experimental studies on lateral root formation in radish seedling roots. I. General methods, developmental stages, and spontaneous formation of laterals. Bot Gaz 1982; 143:341-52; http://dx.doi.org/ 10.1086/337308 [DOI] [Google Scholar]
- 20.Klee HJ, Horsch RB, Hinchee MA, Hein MB, Hoffmann NL. The effects of overproduction of two Agrobacterium tumefaciens T-DNA auxin biosynthetic gene products in transgenic petunia plants. Gene Dev 1987; 1:86-96; http://dx.doi.org/ 10.1101/gad.1.1.86 [DOI] [Google Scholar]
- 21.Kares C, Prinsen E, Van Onckelen H, Otten L. IAA synthesis and root induction with iaa genes under heat shock promoter control. Plant Mol Biol 1990; 15:225-36; PMID:2129423; http://dx.doi.org/ 10.1007/BF00036909 [DOI] [PubMed] [Google Scholar]
- 22.De Smet I. Lateral root initiation: one step at a time. New Phytol 2012; 193:867-73; PMID:22403823; http://dx.doi.org/ 10.1111/j.1469-8137.2011.03996.x [DOI] [PubMed] [Google Scholar]
- 23.Celenza JL Jr, Grisafi PL, Fink GR. A pathway for lateral root formation in Arabidopsis thaliana. Gene Dev 2015; 9:2131-42; http://dx.doi.org/ 10.1101/gad.9.17.2131 [DOI] [PubMed] [Google Scholar]
- 24.Negi S, Ivanchenko MG, Muday GK. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J 2008; 55:175-87; PMID:18363780; http://dx.doi.org/ 10.1111/j.1365-313X.2008.03495.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Negi S, Sukumar P, Liu X, Cohen JD, Muday GK. Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant J 2010; 61:3-15; PMID:19793078; http://dx.doi.org/ 10.1111/j.1365-313X.2009.04027.x [DOI] [PubMed] [Google Scholar]
- 26.Lewis DR, Negi S, Sukumar P, Muday GK. Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development 2011; 138:3485-95; PMID:21771812; http://dx.doi.org/ 10.1242/dev.065102 [DOI] [PubMed] [Google Scholar]
- 27.Muday GK, Rahman A, Binder BM. Auxin and ethylene: collaborators or competitors? Trends Plant Sci 2012; 17:181-95; PMID:22406007; http://dx.doi.org/ 10.1016/j.tplants.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 28.Guo D, Liang J, Qiao Y, Yan Y, Li L, Dai Y. Involvement of G1-to-S transition and AhAUX-dependent auxin transport in abscisic acid-induced inhibition of lateral root primodia initiation in Arachis hypogaea L. J Plant Physiol 2012; 169:1102-11; PMID:22633819; http://dx.doi.org/ 10.1016/j.jplph.2012.03.014 [DOI] [PubMed] [Google Scholar]
- 29.Bielach A, Podlesáková K, Marhavy P, Duclercq J, Cuesta C, Müller B, Grunewald W, Tarkowski P, Benková E. Spatio temporal regulation of lateral root organogenesis in Arabidopsis by cytokinin. Plant Cell 2012; 24:3967-81; PMID:23054471; http://dx.doi.org/ 10.1105/tpc.112.103044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao F, Shen J, Brady SR, Muday GK, Asami T, Yang Z. Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiol 2004; 134:1624-31; PMID:15047895; http://dx.doi.org/ 10.1104/pp.103.036897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koltai H, Dor E, Hershenhorn J, Joel DM, Weininger S, Lekalla S, Shealtiel H, Bhattacharya C, Eliahu E, Resnick N, et al.. Strigolactones'effect on root growth and root hair elongation may be mediated by auxin efflux carriers. J Plant Growth Regul 2010; 29:129-36; http://dx.doi.org/ 10.1007/s00344-009-9122-7 [DOI] [Google Scholar]
- 32.Kapulnik Y, Resnick N, Mayzlish-Gati E, Kaplan Y, Wininger S, Hershenhorn J, Koltai H. Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. J Exp Bot 2011; 62:2915-24; PMID:21307387; http://dx.doi.org/ 10.1093/jxb/erq464 [DOI] [PubMed] [Google Scholar]
- 33.Ruyter-Spira C, Kohlen W, Charnikhova T, Van Zeijl A, Van Bezouwen L, De Ruijter N, Cardoso C, López-Ráez JA, Matusova R, Bours R, et al.. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiol 2011; 155:721-34; PMID:21119044; http://dx.doi.org/ 10.1104/pp.110.166645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li SW, Xue L, Xu S, Feng H, An L. Mediators, genes and signaling in adventitious rooting. Bot Rev 2009; 75: 230-47; http://dx.doi.org/ 10.1007/s12229-009-9029-9 [DOI] [Google Scholar]
- 35.Yadav S, David A, Bhatla SC. Nitric oxide modulates specific steps of auxin-induced adventitious rooting in sunflower. Plant Signal Behav 2010; 5:1163-66; PMID:20948300; http://dx.doi.org/ 10.4161/psb.5.10.12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellamine J, Penel C, Greppin H, Gaspar T. Confirmation of the role of auxin and calcium in the late phases of adventitious root formation. Plant Growth Regul 1998; 26:191-94; http://dx.doi.org/ 10.1023/A:1006182801823 [DOI] [Google Scholar]
- 37.Li YH, Zou MH, Feng BH, Huang X, Zhang Z, Sun GM. Molecular cloning and characterization of the genes encoding an auxin efflux carrier and the auxin influx carriers associated with the adventitious root formation in mango (Mangifera indica L.) cotyledon segments. Plant Physiol Biochem 2012; 55:33-42; PMID:22522578; http://dx.doi.org/ 10.1016/j.plaphy.2012.03.012 [DOI] [PubMed] [Google Scholar]
- 38.Sukumar P, Maloney GS, Muday GK. Localized induction of the ATP-binding cassette B19 auxin transporter enhances adventitious root formation in Arabidopsis. Plant Physiol 2013; 162:1392-405; PMID:23677937; http://dx.doi.org/ 10.1104/pp.113.217174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Péret B, De Rybel B, Casimiro I, Benková E, Swarup R, Laplaze L, Beeckman T, Bennett MJ. Arabidopsis lateral root development: an emerging story. Trends Plant Sci 2009; 14:399-408; PMID:19559642; http://dx.doi.org/ 10.1016/j.tplants.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 40.Péret B, Middleton AM, French AP, Larrieu A, Bishopp A, Njo M, Wells DM, Porco S, Mellor N, Band LR, et al.. Sequential induction of auxin efflux and influx carriers regulates lateral root emergence. Mol Syst Biol 2013; 9:699; PMID:24150423; http://dx.doi.org/ 10.1038/msb.2013.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dastidar MG, Jouannet V, Maizel A. Root branching: mechanisms, robustness and plasticity. WIREs Dev Biol 2012; 1:329-343; PMID:23801487; http://dx.doi.org/ 10.1002/wdev.17 [DOI] [PubMed] [Google Scholar]
- 42.Lavenus J, Goh T, Roberts I, Guyomarc'h S, Lucas M, De Smet I, Fukaki H, Beeckman T, Bennett M, Laplaze L. Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci 2013; 18:450-58; PMID:23701908; http://dx.doi.org/ 10.1016/j.tplants.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 43.De Klerk GJ, Keppel M, Ter Brugge J, Meekes H. Timing of the phases in adventitious root formation in apple microcuttings. J Exp Bot 1995; 46:965-72; http://dx.doi.org/ 10.1093/jxb/46.8.965 [DOI] [Google Scholar]
- 44.De Klerk GJ, Van Der Krieken W, De Jong JC. The formation of adventitious roots: new concepts, new possibilities. In Vitro Cell Dev Biol Plant 1999; 35:189-199; http://dx.doi.org/ 10.1007/s11627-999-0076-z [DOI] [Google Scholar]
- 45.Bellamine J, Penel C, Greppin H, Gaspar T. Confirmation of the role of auxin and calcium in the late phases of adventitious root formation. Plant Growth Regul 1998; 26:191-94; http://dx.doi.org/ 10.1023/A:1006182801823 [DOI] [Google Scholar]
- 46.Rasmussen A, Mason MG, De Cuyper C, Brewer PB, Herold S, Agusti J, Geelen D, Greb T, Goormachtig S, Beeckman T, et al.. Strigolactones suppress adventitious rooting in Arabidopsis and pea. Plant Physiol 2012; 158:1976-87; PMID:22323776; http://dx.doi.org/ 10.1104/pp.111.187104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pacurar DI, Perrone I, Bellini C. Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol Plant 2014; 151:83-96; PMID:24547793; http://dx.doi.org/ 10.1111/ppl.12171 [DOI] [PubMed] [Google Scholar]
- 48.Shen H, Luong P, Huq E. The F-box protein MAX2 functions as a positive regulator of photomorphogenesis in Arabidopsis. Plant Physiol 2007; 145:1471-83; PMID:17951458; http://dx.doi.org/ 10.1104/pp.107.107227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen H, Zhu L, Bu QY, Huq E. MAX2 affects multiple hormones to promote photomorphogenesis. Mol Plant 2012; 5:750-62; PMID:22466576; http://dx.doi.org/ 10.1093/mp/sss029 [DOI] [PubMed] [Google Scholar]
- 50.Tsuchiya Y, Vidaurre D, Toh S, Hanada A, Nambara E, Kamiya Y, Yamaguchi S, McCourt P. A small molecule screen identifies new functions for the plant hormone strigolactone. Nat Chem Biol 2010; 6:741-49; PMID:20818397; http://dx.doi.org/ 10.1038/nchembio.435 [DOI] [PubMed] [Google Scholar]
- 51.Hu Z, Yan H, Yang J, Yamaguchi S, Maekawa M, Takamure I, Tsutsumi N, Kyozuka J, Nakazono M. Strigolactones negatively regulate mesocotyl elongation in rice during germination and growth in darkness. Plant Cell Physiol 2010; 51:1136-42; PMID:20498118; http://dx.doi.org/ 10.1093/pcp/pcq075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urquhart S, Foo E, Reid JB. The role of strigolactones in photomorphogenesis of pea is limited to adventitious rooting. Physiol Plant 2015; 153:392-402; PMID:24962787; http://dx.doi.org/ 10.1111/ppl.12246 [DOI] [PubMed] [Google Scholar]
- 53.Ivanchenko MG, Muday GK, Dubrovsky JG. Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J 2008; 55:335-47 [DOI] [PubMed] [Google Scholar]
- 54.Jusaitis M. Rooting response of mung bean cuttings to 1- aminocyclopropane-1-carboxylic acid and inhibitors of ethylene biosynthesis. Sci Hortic 1986; 29:77-85; http://dx.doi.org/ 10.1016/0304-4238(86)90033-6 [DOI] [Google Scholar]
- 55.Liu J, Mukherjee I, Reid DM. Adventitious rooting in hypocotyls of sunflower (Helianthus annuus) seedlings. III. The role of ethylene. Physiol Plant 1990; 78:268-76; http://dx.doi.org/ 10.1111/j.1399-3054.1990.tb02091.x [DOI] [Google Scholar]
- 56.Pan R, Wang J, Tian X. Influence of ethylene on adventitious root formation in mung bean hypocotyl cuttings. Plant Growth Regul 2002; 36:135-39; http://dx.doi.org/ 10.1023/A:1015051725089 [DOI] [Google Scholar]
- 57.Drew MC, Jackson MB, Giffard S. Ethylene-promoted adventitious rooting and development of cortical air spaces (aerenchyma) in roots may be adaptive responses to flooding in Zea mays L. Planta 1979; 147:83-88; PMID:24310899; http://dx.doi.org/ 10.1007/BF00384595 [DOI] [PubMed] [Google Scholar]
- 58.Drew MC, He CJ, Morgan PW. Decreased ethylene biosynthesis, and induction of aerenchyma, by nitrogen- or phosphate- starvation in adventitious roots of Zea mays L. Plant Physiol 1989; 91:266-71; PMID:16667008; http://dx.doi.org/ 10.1104/pp.91.1.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stöhr C, Stremlau S. Formation and possible roles of nitric oxide in plant roots. J Exp Bot 2006; 57:463-70; PMID:16356940; http://dx.doi.org/ 10.1093/jxb/erj058 [DOI] [PubMed] [Google Scholar]
- 60.Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L. Nitric oxide is required for root organogenesis. Plant Physiol 2002; 129:954-56; PMID:12114551; http://dx.doi.org/ 10.1104/pp.004036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pagnussat GC, Lanteri ML, Lamattina L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol 2003; 132:1241-48; PMID:12857806; http://dx.doi.org/ 10.1104/pp.103.022228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L. Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol 2004; 135:279-86; PMID:15122018; http://dx.doi.org/ 10.1104/pp.103.038554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lanteri ML, Pagnussat GC, Lamattina L. Calcium and calcium dependent protein kinases are involved in nitric oxide-and auxin induced adventitious root formation in cucumber. J Exp Bot 2006; 57:1341-51; PMID:16531462; http://dx.doi.org/ 10.1093/jxb/erj109 [DOI] [PubMed] [Google Scholar]
- 64.Mendez-Bravo A, Raya-Gonzalez J, Herrera-Estrella L, López-Bucio J. Nitric oxide is involved in alkamide-induced lateral root development in Arabidopsis. Plant Cell Physiol 2010; 51:1612-26; PMID:20685967; http://dx.doi.org/ 10.1093/pcp/pcq117 [DOI] [PubMed] [Google Scholar]
- 65.De Rybel B, Audenaert D, Xuan W, Overvoorde P, Strader LC, Kepinski S, Hoye R, Brisbois R, Parizot B, Vanneste S, et al.. A role for the root cap in root branching revealed by the non-auxin probe naxillin. Nat Chem Biol 2012; 8:798-805; PMID:22885787; http://dx.doi.org/ 10.1038/nchembio.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schlicht M, Ludwig-Müller J, Burbach C, Volkmann D, Baluska F. Indole-3-butyric acid induces lateral root formation via peroxisome-derived indole-3-acetic acid and nitric oxide. New Phytol 2013; 200:473-82; PMID:23795714; http://dx.doi.org/ 10.1111/nph.12377 [DOI] [PubMed] [Google Scholar]
- 67.Proust H, Hoffmann B, Xie X, Yoneyama K, Schaefer DG, Yoneyama K, Nogué F, Rameau C. Strigolactones regulate protonema branching and act as a quorum sensing-like signal in the moss Physcomitrella patens. Development 2011; 138:1531-39; PMID:21367820; http://dx.doi.org/ 10.1242/dev.058495 [DOI] [PubMed] [Google Scholar]
- 68.Porcel R, Aroca R, Ruiz-Lozano JM. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron Sustainable Dev 2012; 32:181-200; http://dx.doi.org/ 10.1007/s13593-011-0029-x [DOI] [Google Scholar]
- 69.Rasmussen A, Depuydt S, Goormachtig S, Geelen D. Strigolactones fine-tune the root system. Planta 2013. 238:615-26; PMID:23801297; http://dx.doi.org/ 10.1007/s00425-013-1911-3 [DOI] [PubMed] [Google Scholar]
- 70.Kohlen W, Charnikhova T, Liu Q, Bours R, Domagalska MA, Beguerie S, Verstappen F, Leyser O, Bouwmeester H, Ruyter-Spira C. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol 2011; 155:974-87; PMID:21119045; http://dx.doi.org/ 10.1104/pp.110.164640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kretzschmar T, Kohlen W, Sasse J, Borghi L, Schlegel M, Bachelier JB, Reinhardt D, Bours R, Bouwmeester HJ, Martinoia E. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 2012; 483: 341-44; PMID:22398443; http://dx.doi.org/ 10.1038/nature10873 [DOI] [PubMed] [Google Scholar]
- 72.Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, et al.. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 2001; 13:843-52; PMID:11283340; http://dx.doi.org/ 10.1105/tpc.13.4.843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sugimoto Y, Ali AM, Yabuta S, Kinoshita H, Inanaga S, Itai A. Germination strategy of Striga hermonthica involves regulation of ethylene biosynthesis. Physiol Plant 2003; 119:137-45; http://dx.doi.org/ 10.1034/j.1399-3054.2003.00162.x [DOI] [Google Scholar]
- 74.Koltai H. Strigolactones activate different hormonal pathways for regulation of root development in response to phosphate growth conditions. Ann Bot 2013; 112:409-15; PMID:23059852; http://dx.doi.org/ 10.1093/aob/mcs216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayward A, Stirnberg P, Beveridge C, Leyser O. Interactions between auxin and strigolactone in shoot branching control. Plant Physiol 2009; 151:400-12; PMID:19641034; http://dx.doi.org/ 10.1104/pp.109.137646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frusciante S, Diretto G, Bruno M, Ferrante P, Pietrella M, Prado-Cabrero A, Rubio-Moraga A, Beyer P, Gomez-Gomez L, Al-Babili S, et al.. Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. PNAS 2014; 111:12246-51; PMID:25097262; http://dx.doi.org/ 10.1073/pnas.1404629111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Torres J, Cooper CE, Sharpe M, Wilson MT. Reactivity of nitric oxide with cytochrome c oxidase: interactions with the binuclear centre and mechanism of inhibition. J Bioenergy Biomemb 1998; 30:63-69; PMID:9623807; http://dx.doi.org/ 10.1023/A:1020559528124 [DOI] [PubMed] [Google Scholar]
- 78.Brown GC. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett 1995; 369:136-39; PMID:7649245; http://dx.doi.org/ 10.1016/0014-5793(95)00763-Y [DOI] [PubMed] [Google Scholar]
- 79.Giulivi C. Functional implications of nitric oxide produced by mitochondria in mitochondrial metabolism. Biochem J 1998; 332:673-79; PMID:9620869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Drapier JC. Interplay between NO and (Fe-S) clusters: relevance to biological systems. Methods 1997; 11:319-29; PMID:9073575; http://dx.doi.org/ 10.1006/meth.1996.0426 [DOI] [PubMed] [Google Scholar]
- 81.Boller T, Herner RC, Kende H. Assay for and enzymatic formation of an ethylene precursor, 1-Aminocyclopropane-1-carboxylic acid. Planta 1979; 145:293-03; PMID:24317737; http://dx.doi.org/ 10.1007/BF00454455 [DOI] [PubMed] [Google Scholar]
- 82.Lizada MCC, Yang SF. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem 1979; 100:140-45; PMID:543532; http://dx.doi.org/ 10.1016/0003-2697(79)90123-4 [DOI] [PubMed] [Google Scholar]
- 83.Truernit E, Bauby H, Belcram K, Barthélémy J, Palauqui JC. OCTOPUS, a polarly localised membrane-associated protein, regulates phloem differentiation entry in Arabidopsis thaliana. Development 2012; 139:1306-15; PMID:22395740; http://dx.doi.org/ 10.1242/dev.072629 [DOI] [PubMed] [Google Scholar]
- 84.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248-54; PMID:942051; http://dx.doi.org/ 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 85.Mathieu S, Bigey F, Procureur J, Terrier N, Günata Z. Production of a recombinant carotenoid cleavage dioxygenase from grape and enzyme assay in water-miscible organic solvents. Biotechnol Lett 2007; 29:837-41; PMID:17295086; http://dx.doi.org/ 10.1007/s10529-007-9315-8 [DOI] [PubMed] [Google Scholar]