Abstract
It has been hypothesized that inflammatory response triggered by surgery might induce the release of molecules that could promote proliferation, invasion and metastasis of surviving cancer cells. To test this hypothesis, the levels of multiple inflammation-related circulating factors were analyzed in patients undergoing surgery for colorectal cancer. A Luminex xMAP system was used to simultaneously assess levels of IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, FGF, eotaxin, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α and VEGF in 20 colorectal cancer patients and 10 age-matched non-neoplastic patients. In cancer patients analyses were performed at baseline (before surgery) and at different time points (up to 30 days) following laparoscopic surgery. Significantly higher levels of IL-1β, IL-7, IL-8, G-CSF, IFN-γ and TNF-α were detected in colorectal cancer patients compared to controls at baseline. In colorectal cancer patients, circulating levels decreased progressively following surgery and after day 30 post-surgery were no longer different from controls. These findings suggest that expression levels of several cytokines are higher in colorectal cancer patients compared to control subjects and no significant increase in several inflammation-related circulating factors is observed following laparoscopic surgery for cancer. Confirmation and validation in a different and larger cohort of patients are warranted.
Keywords: colon cancer, cancer biology, surgery, inflammation, cytokines, Luminex xMAP, serum markers
Abbreviations
- EMT
Epithelial Mesenchymal Transition
- CSC
Cancer Stem Cells
- IL
Interleukin
- Ra
Receptor antagonist
- FGF-b
Fibroblast Growth Factor-basic
- G-CSF
Granulocyte Colony Stimulating Factor
- IFN-γ
Interferon γ
- IP-10
IFN-γ Inducible Protein 10
- HuMCP-1
Human Monocyte Chemoattractant Protein 1, MIP-1α
- and 1β
Macrophage Inflammatory Protein 1α and 1β
- PDGF-BB
Platelet Derived Growth Factor-BB
- RANTES
Regulated upon Activation, Normal T-cell Expressed Secreted
- TNF-α
Tumor Necrosis Factor-α
- VEGF
Vascular Endotelial Growth Factor
- CRC
Colorectal Cancer
Introduction
Inflammation plays an important role in various phases of tumor development.1 Chronic inflammation conditions have been associated with an increased risk of cancer and the presence of an inflammatory tumor microenvironment has been proposed as an important hallmark of cancer.2,3 Although the precise mechanisms linking chronic inflammation to cancer development are unknown, several factors have been implicated in this association including the activation of tissue repair and regeneration processes.3-5 In this scenario, cancers have been also described as “wounds that won't heal”6 and several important phenomena occurring during the process of tumor development (i.e., the so-called epithelial mesenchymal transition or EMT) appear to be, at least in part, mediated by cytokines and several other soluble inflammatory molecules and growth factors. Most of these soluble mediators are physiologically released in the sites of wound repair but are also present in tumor microenvironment being able to promote a modification of epithelial cells phenotype.7 Tumor-infiltrating leucocytes as well as cytokines, and especially interleukins (ILs), are critical components of the inflammatory tumor microenvironment and several studies have shown that they can influence many aspects of tumor growth and progression.7-11
In the last years, accumulating evidence has suggested that cancers display a hierarchical organization in which self-renewal potential and tumor initiation ability are restricted to a rare subpopulation of cancer stem cells (CSCs) present in the bulk of more differentiated cancer cells.12 Inflammatory cytokines, growth factors and soluble mediators involved in tissue repair and regeneration processes likely interact with one another in complex ways and act at multiple levels being able to ultimately promote cancer formation by affecting CSCs functions.12-15 Moreover, inflammatory cytokines can play a pivotal role being able to exert pleiotropic effects both directly on tumor cells (i.e., EMT induction and CSCs activation) and indirectly by promoting favorable conditions within the microenvironment (including the suppression of the antitumor activity of immune cells).2,4,7,12,13,16-18
Since surgery, chemotherapy and radiation therapies, by damaging both normal and cancer tissues, trigger a strong inflammatory response, it has been hypothesized that they might induce the release of molecules that could facilitate both EMT induction and stem cell activation and ultimately promote proliferation, invasion and metastasis of surviving cancer cells.19
Inflammatory cytokines are grouped into 9 categories: chemokines, interferons (IFNs), tumor necrosis factors (TNFs), transforming growth factor-β (TGF-β) family members, interleukin-1 (IL-1) family members (such as IL-1α, IL-1β, and IL-18), IL-10 family members, IL-17 family members, haematopoietic growth factors (such as IL-1, G-CSF, GM-CSF), and platelet derived growth factors (PDGFs).20
Cytokines exert their effects by binding to specific receptors on target cells and activating downstream pathways which might merge and diverge at several points. Moreover, a cytokine can bind to different receptors with varying affinities and on different cells leading to multiple, distinct consequences. Activation of oncogenes, such as Ras, can induce inflammatory cytokine expression. Activation of NF-κB, the major pathway that regulates immune inflammatory responses, is a frequent event in cancer cells (tumorigenesis) and can induce pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6.5
In this study we took advantage of a Multi-Analyte Profiling technology to perform a case-control study comparing the levels of several circulating cytokines, chemokines and growth factors in colorectal cancer patients compared to non-neoplastic patients and to evaluate the same parameters during a time course following laparoscopic surgery for colorectal cancer.
Results
In this study, circulating levels of 27 soluble mediators: IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, basic FGF, eotaxin, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1 (MCAF), MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α and VEGF were evaluated in 20 colorectal cancer (CRC) patients (cases) and 10 age and sex-matched non-cancer subjects (controls) (Table 2). Blood samples were collected at the day of surgery. In CRC patients analyses were also performed on serum specimens collected at different time intervals, up to 30 days, post laparoscopic surgery.
Table 2.
Values (mean+SD) of several inflammation-related circulating factors in controls and in colorectal cancer patients at baseline (before surgery) and at different time points post-surgery
| Post surgery |
||||||||
|---|---|---|---|---|---|---|---|---|
| Controls | Before surgery | Day 1 | Day 2 | Day 3 | Day 4 | Day 7 | Day 30 | |
| IL-1β | 2,54 ± 0,73 | 584, ± 4,0 | 4,0 ± 1,02 | 3,77 ± 1,15 | 3,31 ± 0.59 | 3,33 ± 0,96 | 3,26 ± 0.72 | 2,99 ± 0,82 |
| IL-1rα | 155,79 ± 111,72 | 416,08 ± 354,34 | 398,45 ± 433,91 | 343,89 ± 299,96 | 268,72 ± 269,71 | 274,97 ± 207,57 | 283,30 ± 189,34 | 215,66 ± 155,06 |
| IL-2 | 3,90 ± 6,77 | 18,57 ± 30,25 | 13,92 ± 32,04 | 12,14 ± 30,89 | 14,01 ± 28,62 | 13,88 ± 27,46 | 9,20 ± 14,58 | 7,77 ± 13,55 |
| IL-4 | 6,33 ± 1,63 | 7,92 ± 2,27 | 7,49 ± 1,94 | 7,59 ± 2,51 | 6,34 ± 1,84 | 6,32 ± 1,30 | 6,20 ± 1,43 | 6,50 ± 1,94 |
| IL-5 | 4,05 ± 1,75 | 6,85 ± 3,41 | 4,85 ± 3,12 | 4,73 ± 2,63 | 3,22 ± 1,63 | 3,23 ± 1,73 | 3,60 ± 1,55 | 3,88 ± 2,02 |
| IL-6 | 11,65 ± 4,89 | 44,88 ± 63,86 | 98,88 ± 67,12 | 56,97 ± 54,08 | 36,78 ± 36,81 | 35,87 ± 37,83 | 28,58 ± 23,71 | 26,41 ± 44,43 |
| IL-7 | 13,18 ± 3,24 | 26,80 ± 9,48 | 22,10 ± 10,45 | 21,51 ± 10,35 | 15,01 ± 7,70 | 15,12 ± 7,29 | 15,78 ± 5,97 | 16,95 ± 8,07 |
| IL-8 | 29,90 ± 2,68 | 57,32 ± 26,27 | 62,82 ± 23,39 | 61,58 ± 23,66 | 46,30 ± 18,23 | 46,77 ± 18,19 | 43,24 ± 12,33 | 41,17 ± 11,90 |
| IL-9 | 21,52 ± 4,92 | 52,02 ± 62,77 | 46,11 ± 57,67 | 36,57 ± 37,15 | 44,89 ± 49,38 | 44,32 ± 49,50 | 40,06 ± 40,14 | 36,30 ± 47,31 |
| IL-10 | 7,83 ± 2,99 | 18,65 ± 13,50 | 20,34 ± 15,43 | 16,80 ± 11,82 | 15,07 ± 16,25 | 14,11 ± 12,16 | 13,19 ± 8,57 | 13,13 ± 10,65 |
| IL-12 | 55,98 ± 14,88 | 111,27 ± 54,66 | 101,06 ± 47,51 | 92,79 ± 54,23 | 96,31 ± 65,77 | 99,25 ± 64,75 | 98,18 ± 57,01 | 90,09 ± 61,64 |
| IL-13 | 4,16 ± 2,05 | 11,39 ± 5,28 | 11,05 ± 6,29 | 13,74 ± 17,22 | 9,57 ± 3,72 | 9,20 ± 3,64 | 9,41 ± 4,28 | 8,79 ± 5,30 |
| IL-15 | 0,08 ± 0,00 | 2,91 ± 6,95 | 2,07 ± 4,12 | 1,06 ± 2,98 | 1,64 ± 2,82 | 1,21 ± 2,43 | 1,67 ± 4,51 | 1,04 ± 3,53 |
| IL-17 | 38,52 ± 15,58 | 99,54 ± 77,39 | 102,98 ± 61,44 | 86,47 ± 74,09 | 53,12 ± 31,20 | 53,09 ± 34,19 | 58,91 ± 44,24 | 74,24 ± 80,49 |
| Eotaxin | 298,86 ± 98,87 | 387,86 ± 199,42 | 243,66 ± 184,13 | 251,30 ± 151,34 | 261,54 ± 178,71 | 267,50 ± 208,99 | 278,54 ± 192,13 | 348,17 ± 203,77 |
| FGF basic | 77,33 ± 23,24 | 153,66 ± 75,67 | 161,57 ± 77,02 | 150,31 ± 84,28 | 100,49 ± 42,02 | 102,17 ± 45,31 | 121,69 ± 69,86 | 131,00 ± 77,15 |
| G-CSF | 43,55 ± 7,33 | 98,51 ± 35,79 | 104,44 ± 29,86 | 99,37 ± 38,37 | 99,85 ± 38,30 | 97,71 ± 33,92 | 96,30 ± 35,23 | 80,39 ± 41,27 |
| GM-CSF | 3,63 ± 5,83 | 31,29 ± 46,59 | 28,97 ± 27,01 | 21,05 ± 19,03 | 26,81 ± 22,46 | 25,89 ± 20,91 | 25,59 ± 19,57 | 21,16 ± 41,75 |
| IFN-γ | 174,54 ± 36,43 | 334,58 ± 148,27 | 321,57 ± 129,63 | 318,79 ± 134,61 | 289,99 ± 145,36 | 291,54 ± 136,62 | 301,83 ± 139,41 | 267,76 ± 124,80 |
| IP-10 | 2288,21 ± 493,54 | 3304,68 ± 1560,38 | 1698,88 ± 885,91 | 2032,41 ± 1055,54 | 1884,19 ± 854,86 | 2189,06 ± 990,88 | 2138,98 ± 981,23 | 3476,95 ± 1897,59 |
| MCP-1 | 45,90 ± 12,95 | 58,51 ± 54,13 | 65,73 ± 62,18 | 62,96 ± 63,30 | 59,84 ± 44,63 | 53,39 ± 38,35 | 44,24 ± 27,62 | 49,27 ± 66,88 |
| MIP-1α | 6,21 ± 0,92 | 11,63 ± 3,68 | 10,63 ± 4,71 | 9,61 ± 3,76 | 9,35 ± 5,66 | 9,43 ± 5,70 | 8,42 ± 4,39 | 9,03 ± 4,67 |
| PDGF-β | 9456,94 ± 4340,63 | 9235,20 ± 5753,41 | 7656,33 ± 3929,09 | 6506,11 ± 3286,27 | 5150,96 ± 2590,16 | 5240,80 ± 2472,11 | 5655,20 ± 3106,92 | 6642,00 ± 3580,99 |
| MIP-1β | 169,22 ± 51,55 | 177,42 ± 57,74 | 183,36 ± 70,93 | 161,94 ± 63,84 | 165,64 ± 68,05 | 163,95 ± 64,02 | 143,08 ± 41,21 | 140,90 ± 41,71 |
| RANTES | 53108 ± 32242 | 63387 ± 55281 | 83117 ± 98018 | 77142 ± 71661 | 48071 ± 38743 | 48984 ± 63302 | 46899 ± 31289 | 36285 ± 18503 |
| TNF-α | 37,90 ± 2,91 | 69,02 ± 16,30 | 66,71 ± 17,83 | 66,41 ± 20,55 | 63,19 ± 17,70 | 61,77 ± 17,46 | 63,27 ± 22,95 | 59,49 ± 21,03 |
| VEGF | 131,93 ± 76,26 | 348,22 ± 411,64 | 297,07 ± 225,34 | 249,82 ± 226,90 | 185,82 ± 132,60 | 184,30 ± 116,90 | 171,48 ± 120,53 | 193,11 ± 142,20 |
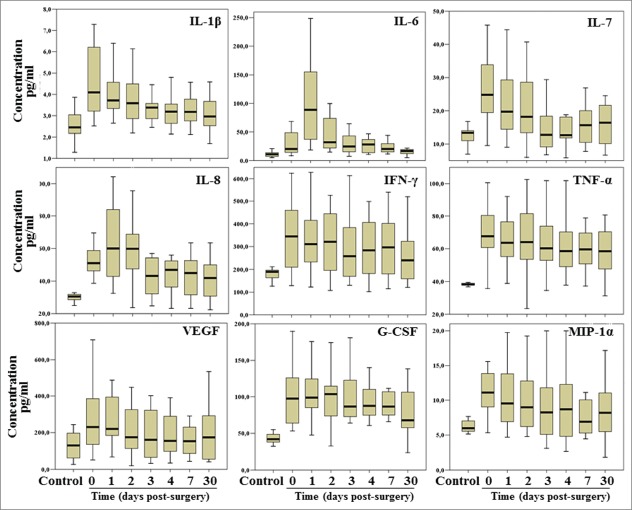
As shown in Table 3, levels of IL-1β, IL-7, IL-8, G-CSF, IFN-γ and TNF-α appeared to be significantly higher in CRC patients compared to control subjects. These increased circulating levels decreased progressively following surgery and after 30 d were no longer different from what observed in controls. Different cytokines displayed a different kinetic: thus, while IL-1β, IL-7 and IFN-γ decreased at a level comparable to healthy control already 1 day post-surgery, IL-8 decreased at a level compared to controls only at day 3 post-surgery, while G-CSF and TNF-α showed a significant decrease only at longer time points, after 30 days post-surgery. Moreover, the levels of IL-1β and IL-7 at day 30 post-surgery were significantly lower than those observed before surgery (Fig. 1). Only IL-6 displayed a transient increase of the circulating levels at day 1 post-surgery but it rapidly decreased thereafter while for IP-10 we observed a statistically significant increase during the first 3 days-post surgery compared to baseline levels. An increase after surgery was also observed for IL-8 and G-CSF whose levels were already higher in patients than in controls and further increased initially in the first 2-days after surgery to decrease thereafter. No significant differences were observed between cases and controls and among the analyzed time points for the other analytes included in this study.
Table 3.
Results of the comparisons between colorectal cancer patients and controls and among different time points after surgery in patients
| C* vs 0** | 0 vs 1 | 0 vs 2 | 0 vs 3 | 0 vs 7 | 0 vs 30 | C vs 30 | |
|---|---|---|---|---|---|---|---|
| IL-1β | 0.001§ | 0.047 | 0.012 | 0.006 | 0.005 | 0.001 | n.s. |
| IL-1rα | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| IL-2 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| IL-4 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| IL-5 | n.s. | n.s. | n.s. | 0.003 | 0.014 | 0.007 | n.s. |
| IL-6 | n.s. | 0.024 | n.s. | n.s. | n.s. | n.s. | n.s. |
| IL-7 | 0.002 | n.s. | n.s. | 0.008 | 0.018 | 0.012 | n.s. |
| IL-8 | 0.014 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| IL-9 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| IL-10 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| IL-12 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| IL-13 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| IL-15 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| IL-17 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Eotaxin | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| FGF basic | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| G-CSF | 0.003 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| GM-CSF | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| IFN-γ | 0.049 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| IP-10 | n.s. | 0.002 | 0.044 | 0.044 | n.s. | n.s. | n.s. |
| MCP-1 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| MIP-1α | 0.05 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| PDGF-β | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| MIP-1β | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| RANTES | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| TNF-α | 0.001 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| VEGF | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
C = control subjects; ** = numbers indicate the time-point of blood collection in colorectal cancer patients (0 = baseline before surgery; 1–30 = days post-surgery). § = levels were compared by 2-way ANOVA with Bonferroni correction.
Figure 1.
Box-plot showing results of the soluble mediators which displayed the most striking variations between colorectal cancer patients and controls. Outliers were not reported.
Discussion
It has been postulated that cancer treatments, and mainly surgery, might affect proliferation and survival of residual CSCs, ultimately influencing disease evolution and outcome, due to the release of soluble mediators during the wound healing process following treatment-induced tissue injury.19
Our result are in agreement with previous studies reporting an increased level of several circulating cytokines in cancer patients and suggest an important role of cancer cells and/or tumor-infiltrating leucocytes in the production of such soluble mediators10,11,21 (Fig. 1 and Table 3).
These findings do not confirm our initial hypothesis that surgery may affect the survival of residual cancer cells by increasing the levels of inflammatory cytokines. Indeed, laparoscopic surgery for CRC induced only a slight increase in a limited subset of cytokines, namely IL-6, IL-8, IP-10 and G-CSF, whereas its effect on the majority of the analyzed soluble mediators appeared irrelevant compared to the effects induced by the presence of the tumor bulk.
It is of interest that most of the circulating factors found to be increased in CRC patients are considered pro-inflammatory mediators thus suggesting that cancer patients display an inflammatory state which is due to and sustained by tumor bulk and likely involving both neoplastic as well as nonmalignant stromal and inflammatory cells.5-8,15,22 Several potent soluble mediators are released during inflammation and they are finely regulated in a temporally and spatially regulated fashion to prevent them from acting outside the process itself. If this occurs, the same factors playing important roles during the inflammation process can promote unintended results, including tumor development3,4 and the findings of the present study confirm that in cancer patients they are mainly produced within tumor microenvironment.
Previous studies have analyzed the expression of serum cytokines in human cancers reporting an increased level of inflammation-related factors in several human malignancies.5,9-11,21-25 To our knowledge, our study if the first using a xMAP system to simultaneously assess expression levels of several circulating factors in cancer patients at the baseline and in the post-operatory while carefully controlling for cigarette smoking and other potential confounders.
We demonstrated that several cytokines are increased in cancer patients, thus confirming similar results recently reported by multiplexed analysis in CRC patients.26 They include IL-1β, IL-8 and TNF-α which are considered the main pro-inflammatory mediators. IL-1β and TNF-α are known to promote tumor development under chronic inflammation. IL-1β can promote colon cells proliferation through activation of COX-2 and Wnt-dependent pathways.27 IL-8 is induced by TNFα and IL-1. It is produced by different tumor cells, including colon cancer cells and its increased levels have been associated with tumor size, depth of infiltration, or increasing stage of disease in different cancers, including colon cancer.25
CRC patients also displayed higher levels of G-CSF which has been reported to stimulate angiogenesis and promotes tumor growth.28
More surprising were the observed higher levels IFN-γ which has been reported to exert tumor-suppressive functions protecting the host against tumor formation.5,10,29,30 However its activity is probably counteracted in the presence of several opposing signals such as those exerted by the other cytokines found to be increased in patients.
The observed transient increase of IL-6 following surgery is also of interest and might affect disease evolution. IL-6 is a major player in acute inflammation in combination with IL-1 and TNF-α and can be produced by a variety of cells tumor cells contributing to create a microenvironment advantageous to cancer growth.4,5,15,22 Thus, an increase in IL-6, as well as of G-CSF, was expected following surgery given their role in the recruitment and migration of neutrophils into the damaged tissue. However, given their ability to stimulate tumorigenesis as well as cancer cells it cannot be completely excluded that, in association with the observed increase in IL-8 and IP-10 levels, they might have a stimulatory effect on the growth of residual cancer cells both at primary and metastatic sites.
In conclusion, contrary to the expectations, we did not observe significant increases in the expression levels of several inflammation-related circulating factors following surgery for CRC. Our series only included laparoscopic patients and, although we did not compare them with patients undergoing open surgery, our findings are in agreement with several previous evidence suggesting a reduced inflammatory response in laparoscopic surgical procedures compared to conventional surgery.31
Moreover, our findings confirm previous single-marker association studies demonstrating that expression levels of several cytokines are higher in CRC patients compared to control subjects and that they progressively decrease after surgery. These findings suggest that the presence of tumor bulk is per se able to induce a chronic, low-grade inflammatory state and it will be of interest to analyze how and whether it might correlate with disease outcome and with the development of complications (i.e., the development of cachessia and/or hyperalgesic states). Moreover, although our observations relate to a limited number of patients and warrant further replication and confirmation in a larger cohort, it will be important to determine whether profiles of circulating inflammatory cytokines might be helpful for the identification of CRC patients and/or might provide the potential to identify new therapeutic options for the management of CRC patients.
In fact, not only inflammatory conditions can precede and, likely, promote tumor initiation and development, but a “smoldering” inflammation can sustain survival of malignant cells at all phases of tumor development, stimulating angiogenesis and metastasis and suppressing antitumor immune responses. Thus, targeting of this inflammatory state may offer new therapeutic opportunities in cancer therapy and further studies to identify the underlying mechanisms and whether it can be affected by antineoplastic therapy are warranted.
Materials and Methods
Study subjects
From June 2012 to December 2013, we enrolled 30 potential subjects, affected by primary, previously untreated, colorectal cancer (CRC) admitted for surgery at the Division of Surgery of the Policlinico Agostino Gemelli, Università Cattolica del Sacro Cuore, Rome, Italy. Inclusion criteria were: patients with diagnosis of CRC candidate to surgery and not receiving neoadjuvant therapy. Exclusion criteria were: inflammatory bowel diseases, atherosclerosis, diabetes, autoimmune diseases, infections, smoking and immunosuppressive or immunomodulatory therapy. After receiving the confirmation of the diagnosis, with staging according to the TNM classification, and the treatment planning, the patients signed an informed consent for the participation to the present study, previously approved by our local ethical committee. After excluding stage 0 and stage IV cases, 20 CRC patients who underwent cancer resection by laparoscopy and displayed a regular postoperative course (no fever, infection and/or other complications) were included in the study. Clinical data of the patients are summarized in Table 1.
Table 1.
Characteristics of colorectal-cancer patients included in this study
| N = 20 (10F-10M; mean age 60y; range 32–75) | |
|---|---|
| Site of the neoplasia | |
| Right colon | 10 |
| Left colon | 3 |
| Sigmoid | 5 |
| Rectum | 2 |
| Surgery | |
| Right hemicolectomy | 10 |
| Left hemicolectomy | 3 |
| Sigmoid resection | 5 |
| Rectal resection | 2 |
| Histology | |
| Adenocarcinoma (3 mucinous type ) | |
| Staging | |
| Stage I | 5 |
| Stage II | 9 |
| Stage III | 6 |
| Grading | |
| G2 | 17 |
| G3 | 3 |
| Follow Up | |
| Non evidence of disease | 19 |
| Alive with disease | 1 |
For controls, sera were collected from 10 sex, age and BMI-matched subjects admitted in the same Surgery Unit and exposed to the same environmental conditions but candidate to surgery for non-neoplastic diseases (5 women and 5 men; age range 34–82, mean 59) with a personal history negative for malignancy, without recent trauma or surgery, and who were not pregnant. Disease affecting control subjects included: perianal benign lesions (n = 5), inguinal hernia (n = 3), varicose vein (n = 1) and breast fibroma (n = 1). Exclusion criteria were the same than for CRC patients. Controls were also informed about the objectives of the present study and signed an informed consent for their enrollment.
Blood samples were always collected early in the morning to control for possible circadian variability in the analyzed parameters and sera were processed at 3000 rpm for 15 minutes, frozen within 2 hours from collection and stored at −80°C until analysis.
Luminex assay
A Luminex xMAP system (Bio-Plex 200 System, Bio-Rad Laboratories, Hercules, CA) which is a multiplex biometric enzyme-linked immunosorbent assay (ELISA)-based immunoassay, containing dyed microspheres conjugated with a monoclonal antibody specific for a target protein, was used according to the manufacturer's instructions. We used the BioPlex Human Cytokine Assay (BioRad) which allows the simultaneous detection of the following circulating analytes: IL-1β (Interleukin-1β), IL-1Ra (Receptor antagonist), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-17, eotaxin, FGF-b (Fibroblast Growth Factor-basic), G-CSF (Granulocyte Colony Stimulating Factor), IFN-γ (Interferon γ), IP-10 (IFN-γ Inducible Protein 10), HuMCP-1 (Human Monocyte Chemoattractant Protein 1), MIP-1α and 1β (Macrophage Inflammatory Protein 1α and 1β), PDGF-BB (Platelet Derived Growth Factor-BB), RANTES (Regulated upon Activation, Normal T-cell Expressed Secreted), TNF-α (Tumor Necrosis Factor-α) and VEGF (Vascular Endotelial Growth Factor). Concentrated human recombinant analytes were provided by the vendor (BioRad) and a broad range of standards was used to establish standard curves to maximize the sensitivity and dynamic range of the assay. The concentrations were calculated by a software provided by the manufacturer using the standard curve.32
Serum samples were assayed in duplicate and averaged to calculate concentrations. Both sera from patients and control subjects were included in each analytical batch. Evaluation of pairs of duplicates within the same batch as well as across different batches confirmed the good reproducibility of the measurement (data not shown).
Statistical analysis
Statistical analysis was performed using SPSS software (version 11.5.0; SPSS, Chicago, IL, USA). Two-way Analysis of Variance (ANOVA) tests were used to compare cytokine levels between cases and controls and across time. Bonferroni correction for multiple testing was employed for post-hoc comparisons and statistical significance was considered at the 5% level (P < 0.05).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Financial support from the Università Cattolica del Sacro Cuore (Linea D1) is gratefully acknowledged.
References
- 1. Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005; 7:211-7; PMID:15766659; http://dx.doi.org/ 10.1016/j.ccr.2005.02.013 [DOI] [PubMed] [Google Scholar]
- 2. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357:539-45; PMID:11229684; http://dx.doi.org/ 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 3. Sgambato A, Cittadini A. Inflammation and cancer: a multifaceted link. Eur Rev Med Pharmacol Sci 2010; 14:263-8; PMID:20496533 [PubMed] [Google Scholar]
- 4. Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev 2008; 18:3-10; PMID:18325755; http://dx.doi.org/ 10.1016/j.gde.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 5. Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol 2013; 33 Suppl 1:S79-84; PMID:23225204; http://dx.doi.org/ 10.1007/s10875-012-9847-0 [DOI] [PubMed] [Google Scholar]
- 6. Dvorak H. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315:1650-9; PMID:3537791; http://dx.doi.org/ 10.1056/NEJM198612253152606 [DOI] [PubMed] [Google Scholar]
- 7. Gao F, Liang B, Reddy ST, Farias-Eisner R, Su X. Role of inflammation-associated microenvironment in tumorigenesis and metastasis. Curr Cancer Drug Targets 2014; 14:30-45; PMID:24200082; http://dx.doi.org/ 10.2174/15680096113136660107 [DOI] [PubMed] [Google Scholar]
- 8. Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol 2012; 227(6):2798-803; PMID:21938724 [DOI] [PubMed] [Google Scholar]
- 9. Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med 2013; 64:45-57; PMID:23121183; http://dx.doi.org/ 10.1146/annurev-med-121211-091527 [DOI] [PubMed] [Google Scholar]
- 10. Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol 2013; 14:e218-28; PMID:23639322; http://dx.doi.org/ 10.1016/S1470-2045(12)70582-X [DOI] [PubMed] [Google Scholar]
- 11. Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, Katki HA, Koshiol J, Shelton G, Caporaso NE, et al. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst 2013; 105:1871-80; PMID:24249745; http://dx.doi.org/ 10.1093/jnci/djt309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bomken S, Fiser K, Heidenreich O, Vormoor J. Understanding the cancer stem cell. Br J Cancer 2010; 103:439-45; PMID:20664590; http://dx.doi.org/ 10.1038/sj.bjc.6605821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tanno T, Matsui W. Development and maintenance of cancer stem cells under chronic inflammation. J Nippon Med Sch 2011; 78:138-45; PMID:21720087; http://dx.doi.org/ 10.1272/jnms.78.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu X, Li H, Ren X. Interaction between regulatory T cells and cancer stem cells. Int J Cancer 2012; 131:1491-8; PMID:22592629; http://dx.doi.org/ 10.1002/ijc.27634 [DOI] [PubMed] [Google Scholar]
- 15. Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J Immunol Res 2014; 2014:149185; PMID:24901008; http://dx.doi.org/ 10.1155/2014/149185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bonafe M, Storci G, Franceschi C. Inflamm-aging of the stem cell niche: breast cancer as a paradigmatic example: breakdown of the multi-shell cytokine network fuels cancer in aged people. Bioessays 2011; 34:40-9; PMID:22086861; http://dx.doi.org/ 10.1002/bies.201100104 [DOI] [PubMed] [Google Scholar]
- 17. Jinushi M, Baghdadi M, Chiba S, Yoshiyama H. Regulation of cancer stem cell activities by tumor-associated macrophages. Am J Cancer Res 2012; 2:529-39; PMID:22957305 [PMC free article] [PubMed] [Google Scholar]
- 18. Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest 2011; 121:3804-9; PMID:21965337; http://dx.doi.org/ 10.1172/JCI57099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harless WW. Cancer treatments transform residual cancer cell phenotype. Cancer Cell Int 2011; 11:1; PMID:21214935; http://dx.doi.org/ 10.1186/1475-2867-11-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guven Maiorov E, Keskin O, Gursoy A, Nussinov R. The structural network of inflammation and cancer: merits and challenges. Semin Cancer Biol 2013; 23:243-51; PMID:23712403; http://dx.doi.org/ 10.1016/j.semcancer.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 21. Kemik O, Kemik AS, Begenik H, Erdur FM, Emre H, Sumer A, Purisa S, Tuzun S, Kotan C. The relationship among acute-phase responce proteins, cytokines, and hormones in various gastrointestinal cancer types patients with cachectic. Hum Exp Toxicol 2011; 31:117-25; PMID:21803781; http://dx.doi.org/ 10.1177/0960327111417271 [DOI] [PubMed] [Google Scholar]
- 22. De Luca A, Lamura L, Gallo M, Maffia V, Normanno N. Mesenchymal stem cell-derived interleukin-6 and vascular endothelial growth factor promote breast cancer cell migration. J Cell Biochem 2012; 113:3363-70; PMID:22644871; http://dx.doi.org/ 10.1002/jcb.24212 [DOI] [PubMed] [Google Scholar]
- 23. Guven-Maiorov E, Acuner-Ozbabacan SE, Keskin O, Gursoy A, Nussinov R. Structural pathways of cytokines may illuminate their roles in regulation of cancer development and immunotherapy. Cancers (Basel) 2014; 6:663-83; PMID:24670367; http://dx.doi.org/ 10.3390/cancers6020663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu VF, Yang J, Lebrun DG, Li M. Understanding the role of cytokines in Glioblastoma Multiforme pathogenesis. Cancer Lett 2011; 316:139-50; PMID:22075379; http://dx.doi.org/ 10.1016/j.canlet.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 25. Sharma J, Gray KP, Harshman LC, Evan C, Nakabayashi M, Fichorova R, Rider J, Mucci L, Kantoff PW, Sweeney CJ. Elevated IL-8, TNF-alpha, and MCP-1 in men with metastatic prostate cancer starting androgen-deprivation therapy (ADT) are associated with shorter time to castration-resistance and overall survival. Prostate 2014; 74:820-8; PMID:24668612; http://dx.doi.org/ 10.1002/pros.22788 [DOI] [PubMed] [Google Scholar]
- 26. Krzystek-Korpacka M, Diakowska D, Kapturkiewicz B, Bebenek M, Gamian A. Profiles of circulating inflammatory cytokines in colorectal cancer (CRC), high cancer risk conditions, and health are distinct. Possible implications for CRC screening and surveillance. Cancer Lett 2013; 337:107-14; PMID:23726839; http://dx.doi.org/ 10.1016/j.canlet.2013.05.033 [DOI] [PubMed] [Google Scholar]
- 27. Kaler P, Godasi BN, Augenlicht L, Klampfer L. The NF-kappaB/AKT-dependent Induction of Wnt Signaling in Colon Cancer Cells by Macrophages and IL-1beta. Cancer Microenviron 2009; PMID:19779850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M. G-CSF stimulates angiogenesis and promotes tumor growth: potential contribution of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun 2002; 297:1058-61; PMID:12359263; http://dx.doi.org/ 10.1016/S0006-291X(02)02335-5 [DOI] [PubMed] [Google Scholar]
- 29. Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer 2004; 4:11-22; PMID:14708024; http://dx.doi.org/ 10.1038/nrc1252 [DOI] [PubMed] [Google Scholar]
- 30. Monteleone G, Pallone F, Stolfi C. The dual role of inflammation in colon carcinogenesis. Int J Mol Sci 2012; 13:11071-84; PMID:23109839; http://dx.doi.org/ 10.3390/ijms130911071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Novitsky YW, Litwin DE, Callery MP. The net immunologic advantage of laparoscopic surgery. Surg Endosc. 2004; 18:1411-9; PMID:15791361; http://dx.doi.org/ 10.1007/s00464-003-8275-x [DOI] [PubMed] [Google Scholar]
- 32. Fabbiani M, Sidella L, Corbi M, Martucci R, Sali M, Colafigli M, Cauda R, Delogu G, Sgambato A, Di Giambenedetto S. HIV-infected patients show impaired cellular immune response to influenza vaccination compared to healthy subjects. Vaccine 2013; 31:2914-8; PMID:23623859; http://dx.doi.org/ 10.1016/j.vaccine.2013.04.033 [DOI] [PubMed] [Google Scholar]