Abstract
Successful execution of germination program greatly depends on the seeds’ oxidative homeostasis. We recently identified new roles for the H2O2-reducing enzyme ascorbate peroxidase 6 (APX6) in germination control and seeds’ stress tolerance. APX6 replaces APX1 as the dominant APX in dry seeds, and its loss-of-function results in reduced germination due to over accumulation of ROS and oxidative damage. Metabolic analyses in dry apx6 seeds, revealed altered homeostasis of primary metabolites including accumulation of TCA cycle metabolites, ABA and auxin, supporting a novel role for APX6 in regulating cellular metabolism. Increased sensitivity of apx6 mutants to ABA or IAA in germination assays indicated impaired perception of these signals. Relative suppression of ABI3 and ABI5 expression, and induction of ABI4, suggested the activation of a signaling route inhibiting germination in apx6 seeds that is independent of ABI3. Here we provide additional evidence linking ABI4 with ABA- and auxin-controlled inhibition of germination and suggest a hypothetical model for the role of APX6 in the regulation of the crosstalk between these hormones and ROS.
Keywords: ascorbate peroxidase, APX, Seed, germination, ROS, redox, auxin, ABA, ABI3, ABI4, ABI5
Oxidative imbalances due to stress-induced perturbations during the seed's development can greatly reduce its potential for proper germination. Over-accumulation of reactive oxygen species (ROS) can cause oxidative damage to a wide range of cellular components, including DNA, proteins and lipids, and reduce seeds ability to germinate.1-3 An optimal range of ROS levels, termed the ‘oxidative window of germination’, is required for successful germination; 2 too little ROS will result in suppressed germination (e.g., in dormant seeds) and too much ROS will lead to delaying or inhibiting germination due to accumulation oxidative damage.2 A tight regulation is therefore required to balance ROS production and scavenging and maintain the cellular redox poise.
Cytosolic ascorbate peroxidase 6 (APX6) play an important role in the regulation of the oxidative state in ‘mature drying’ seeds as well as during early stages of germination, and loss of function mutants show reduced germination rate compared to WT seeds.4
In addition to the role of APXs as major H2O2-reducing enzymes, they are important components of the Foyer-Halliwel-Asada pathway, also known as the ascorbic acid-glutathione (AA-GSH) cycle that function in the cytosol and plastids. As such, APX activity also contribute to maintaining cellular redox homeostasis by regulating the ratios of the redox couples AA-dehydroascorbate (DHA), GSH-GSSH and NAD(P)+-NAD(P)H.5 Accumulation of tricarboxylic acid (TCA) cycle metabolites, altered levels of hormones and amino acids in apx6 seeds, suggested that APX6 play important regulatory role in seed metabolism by modulating ROS and redox signals.4
APX6 is Required for Seeds’ Stress Tolerance
Proper execution of the desiccation program, accumulation of storage proteins and transcription of genes that are translated during the imbibition phase will determine the seed's commitment for germination.6-10 Therefore, ‘seed vigor’, i.e., the potential for rapid uniform emergence and development under a wide range of field conditions, greatly depends on proper execution of seed maturation and desiccation related processes.10 Knockout apx6 seeds showed increased sensitivity to hyperosmotic conditions (NaCl and Sorbitol) or heat stress (HS) during germination. In addition, apx6 seeds collected following HS applied during the ‘mature drying’ phase showed sever retardation in germination under favorable conditions.4 These observations suggested that APX6 has an important role in protecting seeds and maintaining their vigor.
APX6-Associated Crosstalk Between ROS, ABA and Auxin May Involve ABI4
Increased ROS level in apx6 is associated with higher expression level of the transcription factor ABI4 in dry and imbibed seeds.4 Accumulated evidence from recent years suggest that ABI4 is activated in response to redox signals involved in retrograde signaling, developmental processes as well as in response to stress.11–13
In addition ABI4-mediated pathways and AA-dependent processes in Arabidopsis were suggested to function interdependently.11
ABI4 was also recently shown to positively regulate dormancy by promoting ABA synthesis.14 Moreover, AA function as a co-factor in enzymatic activities including for nine-cis-epoxycarotenoid dioxygenase (NCED) enzyme that synthesizes the ABA precursor xanthoxin.15 Furthermore, in the AA-deficient mutant vtc1, the level of ABA was 60% higher compared to the WT due to an increase in ABA synthesis transcripts including that of NCED.16 Interestingly, a similar increase in ABA level observed in dry seeds of apx6 was associated with changes in the AA pool.4
Hence, potentially ABI4 pathway could be activated in response to the ROS/redox signal generated in apx6 seeds.
Regulation of Germination by ABA and Auxin
In addition to increased level of ABA, apx6 seeds accumulated high level of auxin.4
Auxin action in seeds dormancy requires the ABA signaling pathway (and vice versa), indicating that the roles of auxin and ABA in seed dormancy are interdependent.17
High levels of auxin and activation of IAA signaling enhance ABA-mediated dormancy by supporting the persistence of the expression of ABI3, the major regulator of seed dormancy.17 Increased sensitivity of apx6 seeds to either ABA or IAA, suggested these signals might be involved in the germination inhibition phenotype of the mutants.4 However, since ABI3 and ABI5 expression were relatively suppressed in apx6 seeds, it is likely that the crosstalk between auxin and ABA does not involve activation of the ABI3 signaling route, but rather the ABI4 rout.
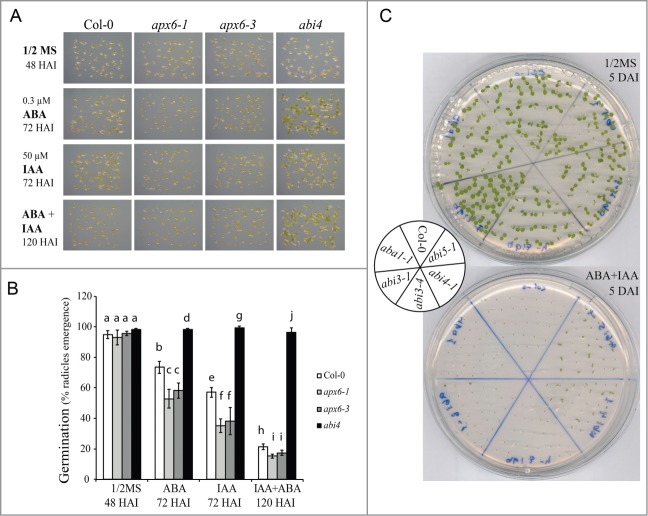
Here we tested whether combination of both ABA and IAA would enhance the inhibition of germination of apx6 and whether abi4 is also insensitive to auxin (Fig. 1). Results show that combination of ABA and IAA enhance the inhibitory effect of germination and that both apx6 lines are more sensitive compared to the WT (Fig. 1). This further suggests that over accumulation of ROS in seeds increase sensitivity to both ABA and auxin. In contrast, reduced ability to generate ROS in Arabidopsis mutant deficient in 2 major respiratory burst oxidase homologs rbohD/F, decreased sensitivity to ABA-mediated auxin response in roots.18
Figure 1.
Germination response to ABA and IAA. Freshly harvested seeds were geminated on 1/2MS supplemented with ABA, IAA or their combination. Sample pictures of seed germination (A) and histograms (B) of germination were recorded at the indicated hours after imbibition (HAI). Standard deviations represent an average of 6 replicates of 50 seeds each. Different lowercase letters indicate values from the seedling lines significantly differ from each other within each treatment by one-way ANOVA and Bonferroni test (P < 0.05). (C) Germination of ABA-related mutants on 1/2MS agar plates without (top) and with 0.3 μM ABA+50 μM IAA (bottom). Photograph was taken 5 d after imbibition (DAI).
In striking contrast to apx6, abi4 maintained close to 100% germination under all conditions, indicating that this mutant is also insensitive to auxin and resistant to its combination with ABA (Fig. 1). Additionally we tested whether other ABA-related mutants behave similarly to abi4 on IAA containing media. Both aba1 and the abi3 lines showed sensitivity to auxin (Fig. 1C and S1), suggesting that nor ABA synthesis or ABI3 are required for auxin-mediated inactivation of germination. Surprisingly, abi5 mutant line showed IAA-insensitivity similar to abi4 (Fig. 1C and S1), supporting a role for abi5 in the response to auxin.
These results suggest that ABI4 may be a convergence target point for ABA- and auxin-controlled inhibition of germination and that ABI5 is also involved in this response.
Crosstalk between ABA and auxin involving ABI4 has been shown in Arabidopsis lateral root development,19 primary root growth and seeds germination,20 and in time of flowering.21 Furthermore, cooperation or interaction between ABI4 and ABI5 in gene regulation was previously reported in germination control and response to stress.22-25 However, since ABI5 expression is relatively suppressed in apx6 seeds,4 it is likely that the mutant's sensitivity to auxin is not mediated by this transcription factor.
Therefore we suggest a hypothetical model in which APX6 is the modulator of the cytosolic ROS and redox signals that regulates germination in seeds. This regulation involves ABA and auxin metabolism and signaling and is mediated by ABI4 (Fig. 2).
Figure 2.
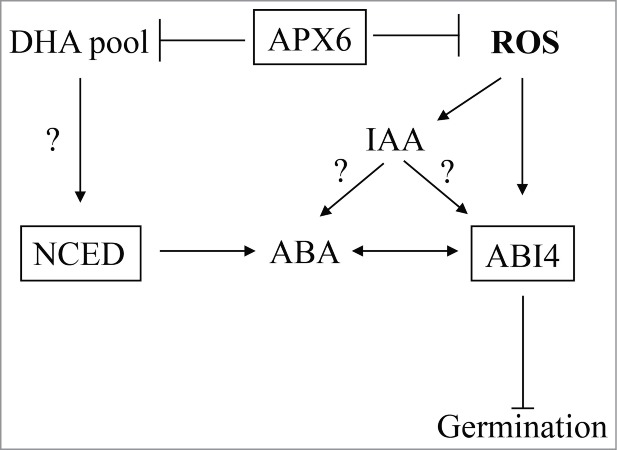
Hypothetical model for APX6-regulated ROS/redox signaling in seeds. APX6 function in seeds to reduce the levels of ROS and redox imbalances during stress, protecting seeds and maintain their vigor. ROS and redox signals generated in the absence of functional APX6 or during sever stress conditions lead to increased levels of ABA and auxin and promote their inhibitory effect on germination. APX6 effect the balance between the AA pool and DHA pool.5 Decreased availability of AA result in activation of ABA biosynthesis genes including NECD leading accumulation of ABA.15 ABI4 expression and pathway are activated in response to the increase in oxidative load and the level of ABA. It is not clear yet if ABI4 responds directly to auxin or indirectly by enhancing the ABA-mediate response.
Funding Statement
This work was supported by the Marie Curie Actions-International Career. Integration Grant (grant no. 293999), the Israel Science Foundation (grant no. 938/11) and the Binational Agricultural Research & Development Fund (project IS-4652-13 R).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Parkhey S, Naithani SC, Keshavkant S. ROS production and lipid catabolism in desiccating Shorea robusta seeds during aging. Plant Physiol Biochem 2012; 57:261-7; http:www.ncbi.nlm.nih.govpubmed22766395; PMID:22766395; http://dx.doi.org/ 10.1016/j.plaphy.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 2. Bailly C, El-Maarouf-Bouteau H, Corbineau F. From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C R Biol 2008; 331:806-14; http:www.ncbi.nlm.nih.govpubmed18926495; PMID:18926495; http://dx.doi.org/ 10.1016/j.crvi.2008.07.022 [DOI] [PubMed] [Google Scholar]
- 3. Chen Q, Yang L, Ahmad P, Wan X, Hu X. Proteomic profiling and redox status alteration of recalcitrant tea (Camellia sinensis) seed in response to desiccation. Planta 2011; 233:583-92; http:www.ncbi.nlm.nih.govpubmed21120520; PMID:21120520; http://dx.doi.org/ 10.1007/s00425-010-1322-7 [DOI] [PubMed] [Google Scholar]
- 4. Chen C, Letnik I, Hacham Y, Dobrev P, Ben-Daniel BH, Vankova R, Amir R, Miller G. Ascorbate peroxidase 6 protects Arabidopsis thaliana desiccating and germinating seeds from stress and mediates crosstalk between ROS, ABA and auxin. Plant Physiol 2014; 166:370-83; http:www.ncbi.nlm.nih.govpubmed25049361; PMID:25049361; http://dx.doi.org/ 10.1104/pp.114.245324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 2011; 155:2-18; 3075780 http:www.ncbi.nlm.nih.govpubmed21205630; PMID:21205630; http://dx.doi.org/ 10.1104/pp.110.167569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rajjou L, Duval M, Gallardo K, Catusse J, Bally J, Job C, Job D. Seed germination and vigor. Annu Rev Plant Biol 2011; 63:507-33; http:www.ncbi.nlm.nih.govpubmed22136565; PMID:22136565; http://dx.doi.org/ 10.1146/annurev-arplant-042811-105550 [DOI] [PubMed] [Google Scholar]
- 7. Rajjou L, Gallardo K, Debeaujon I, Vandekerckhove J, Job C, Job D. The effect of alpha-amanitin on the Arabidopsis seed proteome highlights the distinct roles of stored and neosynthesized mRNAs during germination. Plant Physiol 2004; 134:1598-613; 419834 http:www.ncbi.nlm.nih.govpubmed15047896; PMID:15047896; http://dx.doi.org/ 10.1104/pp.103.036293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holdsworth MJ, Bentsink L, Soppe WJ. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol 2008; 179:33-54; http:www.ncbi.nlm.nih.govpubmed18422904; PMID:18422904; http://dx.doi.org/ 10.1111/j.1469-8137.2008.02437.x [DOI] [PubMed] [Google Scholar]
- 9. Finkelstein R, Reeves W, Ariizumi T, Steber C. Molecular aspects of seed dormancy. Annu Rev Plant Biol 2008; 59:387-415; http:www.ncbi.nlm.nih.govpubmed18257711; PMID:18257711; http://dx.doi.org/ 10.1146/annurev.arplant.59.032607.092740 [DOI] [PubMed] [Google Scholar]
- 10. Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytol 2006; 171:501-23; http:www.ncbi.nlm.nih.govpubmed16866955; PMID:16866955; http://dx.doi.org/ 10.1111/j.1469-8137.2006.01787.x [DOI] [PubMed] [Google Scholar]
- 11. Foyer CH, Kerchev PI, Hancock RD. The ABA-INSENSITIVE-4 (ABI4) transcription factor links redox, hormone and sugar signaling pathways. Plant Signal Behav 2012; 7:276-81; 3404864 http:www.ncbi.nlm.nih.govpubmed22415048; PMID:22415048; http://dx.doi.org/ 10.4161/psb.18770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giraud E, Van Aken O, Ho LH, Whelan J. The transcription factor ABI4 is a regulator of mitochondrial retrograde expression of ALTERNATIVE OXIDASE1a. Plant Physiol 2009; 150:1286-96; 2705018 http:www.ncbi.nlm.nih.govpubmed19482916; PMID:19482916; http://dx.doi.org/ 10.1104/pp.109.139782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M, Lim J, Mittler R, Chory J. Signals from chloroplasts converge to regulate nuclear gene expression. Science 2007; 316:715-9; http:www.ncbi.nlm.nih.govpubmed17395793; PMID:17395793; http://dx.doi.org/ 10.1126/science [DOI] [PubMed] [Google Scholar]
- 14. Shu K, Zhang H, Wang S, Chen M, Wu Y, Tang S, Liu C, Feng Y, Cao X, Xie Q. ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in arabidopsis. PLoS Genet 2013; 9:e1003577; 3688486 http:www.ncbi.nlm.nih.govpubmed23818868; PMID:23818868; http://dx.doi.org/ 10.1371/journal.pgen.1003577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arrigoni O, De Tullio MC. The role of ascorbic acid in cell metabolism: between gene-directed functions and unpredictable chemical reactions. J Plant Physiol 2000; 157:481-8; http://dx.doi.org/ 10.1016/S0176-1617(00)80102-9 [DOI] [Google Scholar]
- 16. Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH. Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 2003; 15:939-51; http://www.ncbi.nlm.nih.gov/pubmed/12671089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu X, Zhang H, Zhao Y, Feng Z, Li Q, Yang HQ, Luan S, Li J, He ZH. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc Natl Acad Sci U S A 2013; 110:15485-90; 3780901 http:www.ncbi.nlm.nih.govpubmed23986496; PMID:23986496; http://dx.doi.org/ 10.1073/pnas.1304651110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiao Y, Sun L, Song Y, Wang L, Liu L, Zhang L, Liu B, Li N, Miao C, Hao F. AtrbohD and AtrbohF positively regulate abscisic acid-inhibited primary root growth by affecting Ca2+ signalling and auxin response of roots in Arabidopsis. J Exp Bot 2013; 64:4183-92; http:www.ncbi.nlm.nih.govpubmed23963673; PMID:23963673; http://dx.doi.org/ 10.1093/jxb/ert228 [DOI] [PubMed] [Google Scholar]
- 19. Shkolnik-Inbar D, Bar-Zvi D. ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 2010; 22:3560-73; 3015119 http:www.ncbi.nlm.nih.govpubmed21097710; PMID:21097710; http://dx.doi.org/ 10.1105/tpc.110.074641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He J, Duan Y, Hua D, Fan G, Wang L, Liu Y, Chen Z, Han L, Qu LJ, Gong Z. DEXH box RNA helicase-mediated mitochondrial reactive oxygen species production in Arabidopsis mediates crosstalk between abscisic acid and auxin signaling. Plant Cell 2012; 24:1815-33; 3442571 http:www.ncbi.nlm.nih.govpubmed22652060; PMID:22652060; http://dx.doi.org/ 10.1105/tpc.112.098707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu S, Ligang C, Liping Z, Diqiu Y. Overexpression of OsWRKY72 gene interferes in the abscisic acid signal and auxin transport pathway of Arabidopsis. J Biosci 2010; 35:459-71; http:www.ncbi.nlm.nih.govpubmed20826955; PMID:20826955; http://dx.doi.org/ 10.1007/s12038-010-0051-1 [DOI] [PubMed] [Google Scholar]
- 22. Cantoro R, Crocco CD, Benech-Arnold RL, Rodriguez MV. In vitro binding of Sorghum bicolor transcription factors ABI4 and ABI5 to a conserved region of a GA 2-OXIDASE promoter: possible role of this interaction in the expression of seed dormancy. J Exp Bot 2013; 64:5721-35; 3871824 http:www.ncbi.nlm.nih.govpubmed24151305; PMID:24151305; http://dx.doi.org/ 10.1093/jxb/ert347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kong Y, Chen S, Yang Y, An C. ABA-insensitive (ABI) 4 and ABI5 synergistically regulate DGAT1 expression in Arabidopsis seedlings under stress. FEBS Lett 2013; 5873076-82; http:www.ncbi.nlm.nih.govpubmed23942253; PMID:23942253; http://dx.doi.org/ 10.1016/j.febslet.2013.07.045 [DOI] [PubMed] [Google Scholar]
- 24. Reeves WM, Lynch TJ, Mobin R, Finkelstein RR. Direct targets of the transcription factors ABA-Insensitive(ABI)4 and ABI5 reveal synergistic action by ABI4 and several bZIP ABA response factors. Plant Mol Biol 2011; 75:347-63; 3044226 http:www.ncbi.nlm.nih.govpubmed21243515; PMID:21243515; http://dx.doi.org/ 10.1007/s11103-011-9733-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bossi F, Cordoba E, Dupre P, Mendoza MS, Roman CS, Leon P. The Arabidopsis ABA-INSENSITIVE (ABI) 4 factor acts as a central transcription activator of the expression of its own gene, and for the induction of ABI5 and SBE2.2 genes during sugar signaling. Plant J 2009; 59:359-74; http:www.ncbi.nlm.nih.govpubmed19392689; PMID:19392689; http://dx.doi.org/ 10.1111/j.1365-313X.2009.03877.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.