Abstract
Helicobacter pylori (H. pylori) is a risk factor of gastric carcinoma, and inflammation with H.pylori infection has widely been suggested to trigger gastric carcinogenesis through “inflammation-carcinoma chain” (non-atrophic gastritis (NAG) → chronic atrophic gastritis (CAG) → intestinal metaplasia (IM) → dysplasia (DYS) and gastric carcinoma (GC)). Connexin43 (Cx43) is a major constituent of gap junction in normal gastric mucosa (NGM) and it is continuously down-regulated from normal gastric mucosa to precancerous lesions or ultimate gastric carcinoma, which shows novel target against gastric carcinoma by preventing the Cx43 decline. Our previous studies demonstrated that H. pylori infection in gastric mucosa down-regulates Cx43 expression, but its mechanism remains unknown. The transcriptional factor, GATA binding protein 3 (GATA-3) is the key to regulate adaptive immune response, which possibly relates to inflammation toward malignant transformation. Here the substantial rising of GATA-3 was screened by transcriptional factor microarray along the developmental stages of H. pylori associated gastric carcinoma. Moreover, the increased GATA-3 and inhibited Cx43 were confirmed in clinical specimens, Mongolian gerbils and normal gastric epithelial cell line GES-1 with H. pylori infection. GATA-3 silencing generated the Cx43 restoration both in intermediate differentiation gastric cancer cells BGC-803 and in H. pylori infected GES-1 cells. Dual-luciferase reporter assay further revealed the GATA-3 as one of Cx43 down-regulators by directly binding to its promoters. Together, the incremental GATA-3 is found in H. pylori associated gastric carcinogenesis, which is responsible for Cx43 inhibition as well.
Keywords: carcinogenesis, Connexin43, gastric carcinoma, GATA-3, Helicobacter pylori, inflammation-carcinoma chain, transcription factor
Abbreviations
- GATA-3
GATA binding protein 3
- Cx43
connexin43
- H. pylori
Helicobacter pylori
- GJ
gap junction
- GC
gastric carcinoma
- TF
transcriptional factor
- ILC
innate lymphoid cell
- Th1/Th2 cell
type 1/2 T helper cell
- PBMC
peripheral blood mononuclear cell
- IFN-γ
interferon-gamma
- CagA
cytotoxin-associated gene A
- VacA
vacuolating cytotoxin gene A
- PBS
phosphate buffered saline
- NAG
non-atrophic gastritis
- CAG
chronic atrophic gastritis
- IM
intestinal metaplasia
- DYS
dysplasia
- NGM
normal gastric mucosa
Introduction
Gap junctions (GJs) are plasma membrane domains and channel-like structures allowing cytoplasm of adjacent cells to directly share ions and small molecules <1 kDa.1 Connexin43 (Cx43, also known as gap junction protein α 1, 43kDa, GJA1) is major constituent of GJs in gastric mucosa,2 playing pivotal roles in gastric epithelial cell-cell communication. The sustained Cx43 reduction would exhibit increased susceptibility to gastric carcinoma,3 and our previous studies reveal that Cx43 is downregulated in gastric carcinoma and precancerous lesion.4
Helicobacter pylori (H. pylori) have been classified as type I carcinogen by the WHO/International Agency for Research on Cancer.5 One theory detailing the progression of gastric carcinoma with H. pylori infection is the “inflammation-carcinoma chain” which involves sequential pathological steps: the epithelium undergoes injury in chronic gastritis, followed by atrophy gastritis (in particular loss of parietal cells which is the sign of precancerous lesion), intestinal metaplasia, dysplasia, and eventually carcinoma. Inflammatory status with persistent H. pylori infection contributes to neoplastic transformation,6 and the inhibited Cx43 expression is reported to be associated with H. pylori infection.7 These findings lead to hypothesis that H. pylori may exert its carcinogenic properties by reducing Cx43 in developmental stages of gastric carcinoma. The primary inducer by which the infection of H. pylori causes loss of Cx43 in gastric mucosa, however, remains enigmatic. Mechanisms on how H. pylori infection interplays with gastric mucosa and gradually adds to GCs need to be addressed in addition to the impaired Cx43.
The half-life of Cx43 is 1.5–5 h, and its expression is transcriptionally regulated.8 On the other hand, Cx43 breakdown is temporarily engendered by decreased gap junction channel “Open Time” which could be regulated by a broad range of cancer- and inflammation-associated factors.9 One transcriptional factor (TF) of interest and great importance, GATA binding protein 3 (GATA-3), has varied functions in innate and adaptive immunity in terms of regulating activities of T helper (Th) cells and innate lymphoid cells (ILCs). Moreover, GATA-3 binds to consensus GATA or GATA-like motifs located in a promoter region with high affinity and has been shown to interact with enhanced p38 and extracellular signal-regulated kinase-1/2 (ERK-1/2)-mitogen-activated protein kinases (MAPK).10,11 The p38-MAPK involves signaling pathways partially down-regulating expression of Cx43,12 meanwhile p38-MAPK mediated pathway is selectively regulated by Cx43 in tumor cells, which could thus be one of mediators in secondary malignancies.13 There is no evidence so far to elucidate the direct GATA-3-mediated Cx43 change in gastric carcinogenesis by H. pylori, and it is the topic of our study since multiple GATA-3 binding sites on Cx43 promoters are mentioned by prediction tool (TFSEARCH, version 1.3). To highlight the impact of GATA-3 and Cx43 on gastric neoplasia, we therefore examined the tendency of GATA-3 in combine with Cx43 expression throughout inflammation-carcinoma chain in the presence of H. pylori using clinical specimens. Moreover, we studied potential links between GATA-3 and Cx43 in H. pylori-induced gastric epithelial cells in vitro and Mongolian gerbils models, and bindings of GATA-3 on Cx43 promoters were further investigated.
Results
Global response of transcriptional factors in H. pylori associated gastric carcinogenesis
A promising TF, GATA-3, came to light under the comparisons of H. pylori (−) NGM, H. pylori (+) NAG, CAG, IM, DYS and GC (Table. 1) in view of screening criteria: the consistent trend along the gastric carcinogenesis; the significant fold change in between adjacent stages with clinical significance; the moderate or high endogenic expression for specific and effective regulation. GATA-3 lowly expressed in NGM but, more intriguing, rose rapidly upon H. pylori induced NAG, started its enrichment in H. pylori (+) CAG and kept at high level in H. pylori (+) IM and DYS, peaked in the ultimate H. pylori (+) GC. It was the first time of us to demonstrate that H. pylori infection boosted GATA-3 expression under the comparison between H. pylori (+) NAG and H. pylori (−) NGM. Seeing that H. pylori (+) CAG was known as the initiating phase of gastric precancerous lesion, the further multiplied GATA-3 in this stage could be interpreted as the consequence of gastric epithelial malignant transformation. Moreover, GATA-3 derived its cancerous characterization from the continuous high expression in IM, DYS and GC. These collective data verified the pertinent role of incremental GATA-3 in H. pylori involved gastric epithelial inflammation-carcinoma chain.
Table 1.
Transcriptional Factors DNA array in H. pylori associated gastric carcinogenesis (standardized value)
|
H. pylori (-) |
H. pylori (+) |
|||||
|---|---|---|---|---|---|---|
| Gene Name | NGM | NAG | CAG | IM | DYS | GC |
| AP-1 | 0.364475 | 0.236852 | 0.437483 | 0.109007 | 0.33254 | 0.557029 |
| CdxA/NKX2 | 0.025694 | 0.162097 | 0.303745 | 0.077073 | 0.23202 | 0.373394 |
| MyoD | 0.755692 | 0.708577 | 0.575592 | 0.815667 | 0.69901 | 0.896107 |
| c-Myc-responsive region | 0.021542 | 0.151038 | 0.17115 | 0.155812 | 0.45871 | 0.531179 |
| MZF1 | 0.130042 | 0.212263 | 0.390068 | 0.063666 | 0.70764 | 0.640325 |
| myc-CF1 | 0.042735 | 0.120381 | 0.08333 | 0.065778 | 0.08691 | 0.220635 |
| USF-1 | 0.59681 | 0.586308 | 0.504982 | 0.274977 | 0.68181 | 0.724868 |
| Nkx-2.5 | 0.07258 | 0.189742 | 0.212145 | 0.06976 | 0.26695 | 0.160128 |
| SRY | 0.123837 | 0.22467 | 0.054424 | 0.18344 | 0.02147 | 0.006878 |
| NF-1(2) | 0.193751 | 0.20134 | 0.0334 | 0.390525 | 0.23373 | 0.499282 |
| ADR1 | 0.839109 | 0.792412 | 0.768519 | 0.863365 | 0.74375 | 0.850454 |
| p300 | 0.812017 | 0.802346 | 0.77387 | 0.901597 | 0.65291 | 0.594067 |
| E12/E47 | 0.177671 | 0.206015 | 0.147324 | 0.25466 | 0.05314 | 0.457672 |
| E2F-1 | 0.973607 | 0.909062 | 0.913756 | 1.02194 | 0.98074 | 1.004649 |
| NF-E1 (YY1) | 0.828665 | 0.737391 | 0.773008 | 0.838459 | 0.75935 | 0.906803 |
| GATA-3 | 0.181778 | 0.28055 | 0.48277 | 0.59822 | 0.4974 | 0.64002 |
| NF-Y | 0.10094 | 0.151803 | 0.026937 | 0.045057 | 0.02396 | 0.073318 |
| HLF | 0.11418 | 0.1398 | 0.04269 | 0.090441 | 0.04606 | 0.065457 |
| EVI-1 | 0.725453 | 0.385328 | 0.855196 | 0.916467 | 0.88423 | 0.898413 |
| LyF-1 | 0.744593 | 0.750652 | 0.73725 | 0.894202 | 0.87372 | 0.895843 |
| 1-Oct | 0.13087 | 0.12645 | 0.172252 | 0.116971 | 0.0952 | 0.181557 |
| c-Ets-1 | 0.116976 | 0.09053 | 0.079786 | 0.307845 | 0.26178 | 0.323432 |
| HFH-2 | 0.951846 | 0.972714 | 1.017406 | 1.064722 | 1.05287 | 1.053023 |
GATA-3 enrichment and Cx43 down-regulation are confirmed in H. pylori dependent gastric lesion and carcinogenesis in clinical specimens
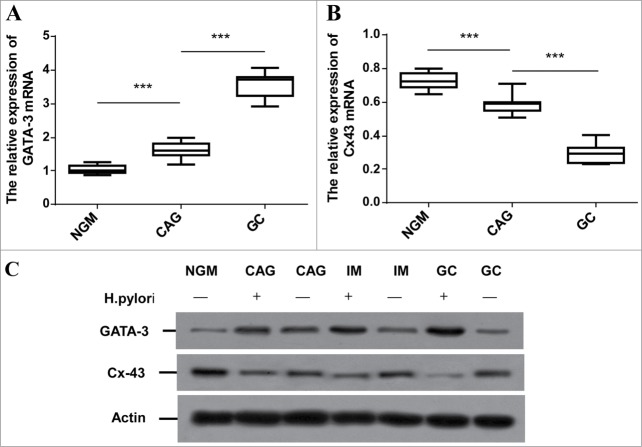
In consistent with TFs microarray, GATA-3 mRNA was time-course enriched in distinct H. pylori involved cancerous checkpoints compared to normal mucosa (Fig.1A, p < 0.001). The down-regulated Cx43 mRNA levels were detected in the same samples (Fig.1B, p < 0.001). We further investigated the GATA-3 and Cx43 protein level in H. pylori-mediated GCs progression. Western blot analysis on representative specimens from H. pylori (−) NGM, H. pylori (−/+) CAG, IM and GC displayed that H. pylori infection did associate with GATA-3 up-regulation and Cx43 inhibition along the GCs progression (Fig.1C). These results suggested that H. pylori harbors a common GATA-3 activation and inhibited Cx43, which were favorable for accelerating gastric carcinogenesis.
Figure 1.
Comparison of GATA-3 and Cx43 expression among developmental stages of H. pylori associated gastric carcinoma in clinical specimens. (A, B) Quantitative RT-PCR analysis of GATA-3 (A) and Cx43 (B) in H. pylori (-) NGM, H. pylori (+) CAG and GC patients (n=15). *** p < 0.001. (C) Representative images of GATA-3 and Cx43 protein levels in H. pylori (-) NGM, H. pylori (-/+) CAG, IM and GC.
H. pylori triggers Cx43 inhibition and GATA-3 augmentation in vitro
The mRNA and protein levels were measured after the co-culture of H. pylori and GES-1 cells monolayer. Cells only changing media served as controls. GATA-3 mRNA boosted in 24h H. pylori infection and kept in higher expression in 48h (Fig. 2A, p < 0.001). The Cx43 mRNA decreased with prolonged time (Fig. 2B, p < 0.01). The shrinking profile of H. pylori infected every individual was observed compared with non-H. pylori infected cells monolayer (Fig. 2C).The increasingly time-dependent GATA-3 protein levels were detected as well as decreased Cx43 protein levels (Fig. 2D) that were in agreement with the report that H. pylori correlated to GJs inhibition.14 These findings demonstrated the strong effects of H. pylori infection on gastric mucosa within a short time in vitro.
Figure 2.
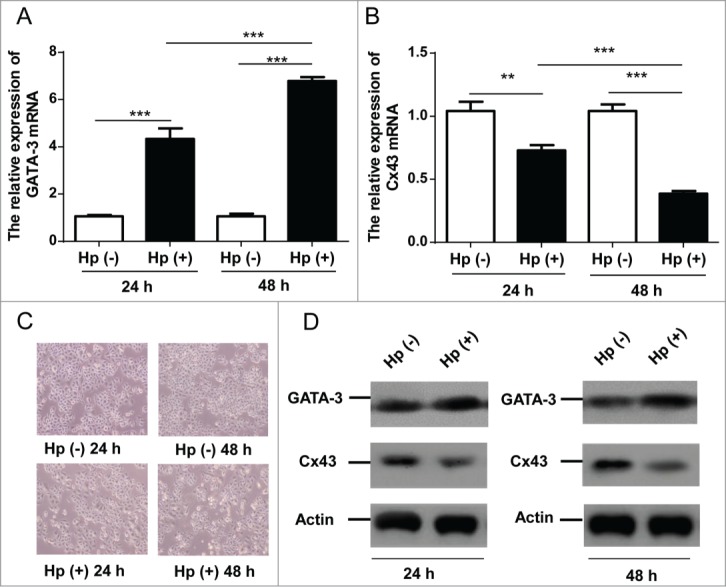
The effects of H. pylori on GATA-3 and Cx43 expressions in GES-1 cells. (A, B) Quantitative RT-PCR analysis of GATA-3 (A) and Cx43 (B) in GES-1 cells with H. pylori 24 and 48 hours infections. ** p < 0.01; *** p < 0.001. (C) Morphology of normal GES-1 cells and 24/48 hours H. pylori infected GES-1 cells. (D) Western blot analysis of GATA-3 and Cx43 protein levels in GES-1 cells under 24 and 48 hours H. pylori infections.
GATA-3 negatively regulates Cx43 by directly binding its promoters
Notably, the ascendant GATA-3 was accompanied with Cx43 disruption in response to H. pylori infection, which brought an idea whether GATA-3 regulates Cx43 expression independent of p38/MAPK/ERK pathway. As GATA family proteins were similar in structure, it might not be the best way to measure specific functions in GATA-3 over-expression studies. We therefore in particular attenuated GATA-3 expression. H. pylori and GES-1 monolayers were co-cultured at the ratio of 35:1 for 48 h, and GATA-3 mRNA activation was in line with previous H. pylori infection in vitro experiments. The successful GATA-3 silencing was then conducted using mixed strands of small interfering RNA (siRNA-1, siRNA-2) in light of the downregulated GATA-3 mRNA (Fig. 3A, p < 0.001) as well as ∼90% transfection efficiency (Fig. S1). The elevation of Cx43 mRNA (Fig. 3B, p < 0.001) and protein levels (Fig.3C) indicated that H. pylori mediated Cx43 disruption could be alleviated by restraining the GATA-3 activation.
Figure 3.
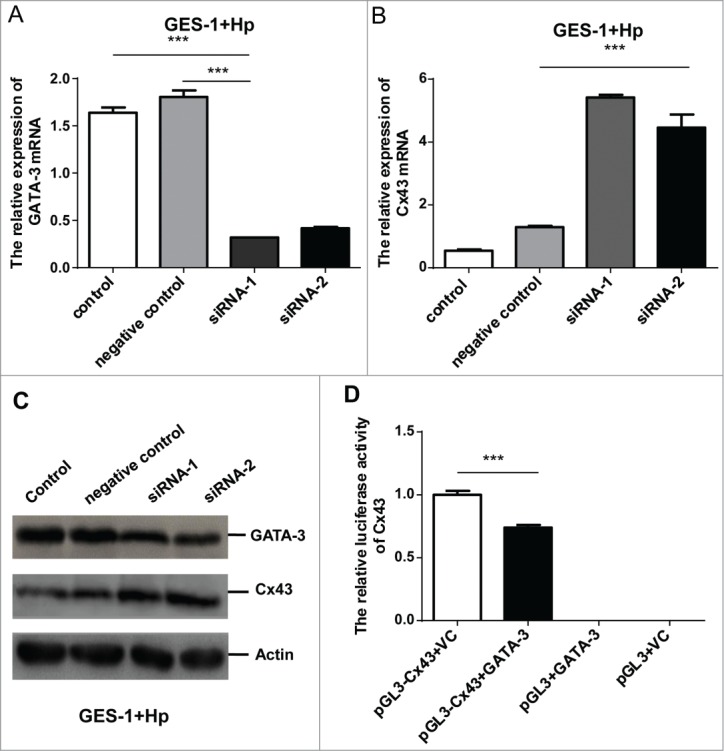
GATA-3 negatively regulates Cx43 by directly binding to its promoters. (A, B) Quantitative RT-PCR analysis of GATA-3 (A) and Cx43 (B) levels in H. pylori infected GES-1 cells treated with a scramble control, GATA-3 siRNA-1, GATA-3 siRNA-2. (C) Western blot analysis of GATA-3 and Cx43 protein levels in H. pylori infected GES-1 cells treated with a scramble control, GATA-3 siRNA-1, and GATA-3 siRNA-2. (D) GATA-3 direct binds to Cx43 promoters. HEK-293T cells co-transfected with GATA-3 and Cx43-pGL3 plasmids. The results are displayed as the ratio of firefly luciferase activity in the pGL3-Cx43-GATA-3-transfected cells to the activity in the pGL3-Cx43 controls. *** p < 0.001.
Furthermore, a higher GATA-3 expression was found in moderate differentiated gastric cancer cell line BGC-803 in comparison of normal gastric cancer cell line GES-1, partly demonstrating the GATA-3 elevation as a phenomenon of gastric carcinoma besides H. pylori inflammation. The BGC-803 cells were thereafter subjected to GATA-3 silencing (Fig. S2A, p < 0.01). The increasing Cx43 mRNA in BGC-803 cells treated by GATA-3 knock-down could be seen (Fig. S2B, p < 0.001), suggesting that silencing of GATA-3 would result in up-regulated Cx43 expression in GCs cells.
Assuming a major contribution of increased GATA-3 expression to the Cx43 inhibition in the H. pylori infected gastric mucosa, we next clarified whether GATA-3 directly transcriptionally regulates Cx43 gene. We performed a luciferase reporter assay on the basis of facts that conserved GATA-3 binding sites were predicted in the 2.2-kb and 0.1-kb promoter region of the Cx43 gene. HEK293T cells were successfully subjected to the 2.3-kb Cx43 promoters with and without GATA site substitution (Fig. S3) based on sequence alignment (Fig. S4). The activity of the wild-type Cx43 promoter reporter (Cx43-WT-LUC) dropped by 30% upon co-transfection of GATA-3 over-expressing plasmid (pEF-GATA-3) compared to GATA-3 empty plasmid (Fig. 3D, p < 0.001). These results clearly elucidated that GATA-3 reduced the activity of Cx43 gene expression through the conserved GATA binding sites.
GATA-3 augmentation and Cx43 inhibition are found in H. pylori infected Mongolian gerbils
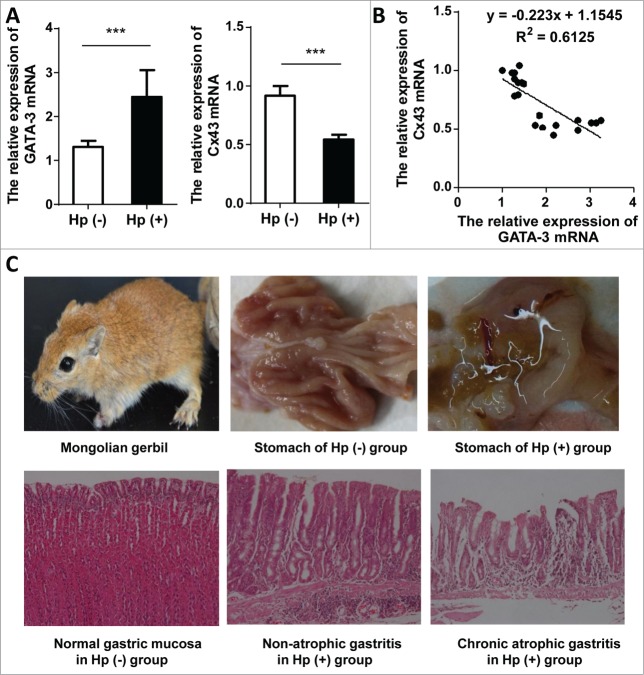
The increased GATA-3 and decreased Cx43 mRNA in H. pylori-infected antrum tissues were observed (Fig. 4A) in comparison with control groups. All data of experimental and control group generated the inverse correlation between GATA-3 and Cx43 (Fig. 4B). Among 10 gerbils under H. pylori infection for 48 weeks, 9 were macroscopically in hyperemia, edema, erosion, hemorrhages and/or food retention, but no ulcer or mass were found. Pathological sections manifested varied morbidity degree with 1 mild inflammation, 5 moderate inflammations, 2 severe inflammations, 1 atrophic gastritis and 1 unchanged. No excisional biopsies were diagnosed with gastric carcinoma. Additionally, no features of inflammatory reaction were found in control group (Fig. 4C). Western blot analysis of GATA-3 and Cx43 were not conducted due to the lack of specific antibodies for Mongolian gerbils, but overall, the tendency of GATA-3 and Cx43 coincided exactly with the previous H. pylori model, and these data supplemented their negative correlation in gastric mucosa.
Figure 4.
The effects of H. pylori on GATA-3 and Cx43 expression in Mongolian gerbils. (A) The alterative GATA-3 and Cx43 mRNA in Mongolian gerbils with H. pylori 48-week infection (n=10) compared to control group (n=10). *** p < 0.001. (B) The correlation of GATA-3 and Cx43 mRNA levels from Mongolian gerbils (10 from experimental group and 10 from control group). (C) Profile of Mongolian gerbil (upper left); typical macroscopic manifestation of normal stomach in control group (top center) and inflammatory edema in experimental group (upper right); histologic sections of normal gastric mucosa (left bottom), H. pylori induced severe non-atrophic gastritis (bottom center), and H. pylori induced atrophic gastritis (right bottom).
Discussions
A critical role of Cx43 in assembly and maintenance of gastric mucosa has been previously reported. Cx43 overexpressions differentially suppress tumor growth via bystander effect (death of a greater number of tumor cells).15 Mucosae are frequently injured, a transient Cx43 reduction is found in wound edges which directly accelerates wound repair by triggering proliferation and migration, then Cx43 returns to homeostatic levels upon wound closure.16 However, Cx43 inhibition would appear under non-termed mucosae restitutions caused by chronic inflammation, whose side effect is disruption of cell-cell communication. H. pylori-associated gastric carcinoma is generally conceptualized as a multi-step process named inflammation-carcinoma chain, our studies strikingly reason that loss of Cx43 in gastric mucosa runs throughout the H. pylori related cancerization, which is similar to the mechanism describing gap junction disruption in HPV-16-associated cervical cancer progression.17
The transcriptional factor GATA-3 has emerged as a critical regulator of both innate and adaptive immunity. Compared to other GATA family member, GATA-3 highly expresses only in the haematopoietic compartment, and specially localizes to cytoplasm in naive T lymphocytes.18 GATA-3 is a marker that has been widely highlighted in urothelial neoplasms19 and breast epithelial tumors.20 It is reported that GATA-3 positively regulates cyclin-D1 which maintains cells in an undifferentiated state, suggesting an oncogenic potentiality.21 Meanwhile several studies assessed that GATA-3 maintains cell differentiation and immunosuppresses tumor dissemination in breast cancer,22,23 indicating the distinct GATA-3 immune functions in various tumors.
Gastric epithelial mutagenesis initiation or malignancy transformation is in a large part from H. pylori infection followed by inflammation-carcinoma chain. In our studies, H. pylori brings about active GATA-3, explaining this immune response to H. pylori by GATA-3 upregulation solely in gastric mucosa. Additionally, content of GATA-3 positively correlates to H. pylori associated gastric neoplasia. One of its mechanisms is suppressive immune surveillance. Since H. pylori persistent colonization in gastric mucosa is widely reported, it is also perceived as antigen which is responsible for systemic immune tolerance. Once neoplasia appears, the declining immune surveillance to inchoate tumor antigens allows further cancerization in the presence of pathogen.24
GATA-3 is recently identified one of crucial immune tolerance genes by promoting the secretion of IL-4, IL-5, and IL-13 from T helper 2 (Th2) predominant immune response.25 GATA-3 induces the differentiation of Th0 cells toward Th2 cell subtype26 which is responsible for relieved inflammation.27 GATA-3 elevation partly counteracts Th1 cytokines and protects mucosa from serious injury at the early stage of H. pylori infection.28 However, the predominant Th2 response caused by GATA-3 enrichment is known to facilitate H. pylori persistence on mucosa29,30 and immune escape.31 Accordantly, pathogen-dependent GATA-3 activation theoretically restrains Th1 production like IL-2, IFN-γ, thereby breaks the acknowledged T cell-mediated tumor surveillance characterized by Th1 cytokines.32 The markedly higher levels of GATA-3 in T cells have been shown in H. pylori associated gastric carcinoma,33 confirming a shift from Th1 to Th2 response during the progression of H. pylori associated gastric pathologies. Studies on peripheral blood mononuclear cells (PBMCs) in gastric cancer patients report the increasing mRNA levels of GATA-3 dominant Th2 phenotype,34 so GATA-3 abundance supposes to be detected in H. pylori-induced initial gastritis and advanced carcinoma. In combine with the augmented mRNA and protein levels of GATA-3 in our study, we hypothesized that signaling via the T cell antigen receptor (TCR) has mutual promotions of Th2 cytokine stimulation and GATA-3 in response to H. pylori infection, and GATA-3 enrichment cause by H. pylori may foster the gastric carcinogenesis throughout the inflammation-carcinoma chain by creating a tumor microenvironment with the imbalance of Th1/Th2 pathologies.
Since H. pylori adheres to gastric mucosa, it presumably exerts salient impact on gastric mucosa in addition to activating T cell-mediated immunization. Our results provides direct insights of GATA-3 potentiation on gastric epithelial which is in accordant with the trend found in immune cells in H. pylori gastric malignancies, and more attracting studies explaining this consistent tendency in epithelial cells and immune cells are needed to decipher the potential role of gastric epithelial in adaptive immune response establishment.
Major findings focus H. pylori carcinogenicity on loss of tumor suppressive gene on the development of GCs, including Cx433. H. pylori is involved in Src/MEK/ERK and p38 MAPK pathways35 which is capable of Cx43 disruption.36 The expression tendencies in H. pylori-dependent inflammation-carcinoma chain hint that once GCs occur, GATA-3 up-regulation and Cx43 inhibition is frequent as a consequence of GCs, and may be influenced by, but is not all attributed to H. pylori infection. It is confirmed by our results that GATA-3 high expression and Cx43 low expression are tested in GCs cells irrelevant to H. pylori. This character in GCs lets the GATA-3 stimulation be part of immediate events of Cx43 inhibition. GATA-3 specific siRNA in BGC-803 cells and H. pylori infected GES-1 cells reveals significant Cx43 rehabilitation, and this raises intriguing possibilities that GATA-3 directly down-regulates gap junction. Moreover, the luciferase reporter assay shows direct GATA-3 binding sites in Cx43 promoter. These data particularly corroborate the carcinogenesis action of GATA-3 through Cx43 breakdown in H. pylori infected mucosa.
Taken together, H. pylori triggers breakdown of Cx43 expression, which gives rise to stunting of cell-cell communication and partly contributes to gastric cancerous propensity. GATA-3 upregulation is newly screened as an event of H. pylori associated gastric carcinogenesis by TFs microarray. A global view of increasing GATA-3 in the process of H. pylori related GCs progression is presented in clinical specimens, Mongolian gerbils, and normal gastric epithelial cells in vitro. The selective inhibition of GATA-3 considerably leads to Cx43 up-regulation, presumably paving the way to the restoration of Cx43. The mechanisms on how GATA-3 activation facilitates gastric carcinogenesis remain unclear, albeit GATA-3 direct binds to Cx43 promoters and owns negative transcriptional regulation. These uncertainties hint at the complexities along the way, but it can be anticipated that these transcriptional mechanisms will be shed light on the chronic pathological conditions and H. pylori-dependent gastric inflammation-carcinoma chain not limited to the down-regulated Cx43 molecule.
Material and Methods
Bacterial culture
A strain of malignant H. pylori (East-Asian type CagA+) which was isolated from gastric carcinoma patients during gastroscopy, was grown on Columbia blood agar plate (Sangong Biotech, Shanghai, China) at 37°C in microaerophilic conditions (5% O2, 10% CO2, 85% N2) followed by liquid culture with brucella broth supplemented with antibiotics (10 mg/L Vancomycin/, 2500 U/L Polymixins, 2 mg/L Amphotericin and 5 mg/L Trimethoprim and 10% sheep blood (Bianzhen Biotech, Nanjing, China). After incubated for 3–4 days, bacterial was harvested from a solid culture by scrape and were re-suspended in PBS (with calcium). At this time point, H. pylori was in the early log phase with good motility and action for subculture or bacterial intervention. The bacterial concentration was calculated based on an optical density (OD) of 1×108 colony-forming unit (CFU) at 660nm.
Cell culture and cells/bacterial co-culture
Human gastric epithelial cells GES-1 (Bogu Biotech, Shanghai, China) and intermediately differentiated Human Gastric Cancer cells BGC-803 (from cancer institute of Central South University, Changsha, China) were grown in high-glucose (4.5 g/liter) Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine Serum, 100U/ml penicillin, 100μg/ml streptomycin, 15 mM HEPES (PH 7.4), 2 mM L-glutamine, 1% non-essential amino acids (Hyclone, Logan, UT, USA) in a controlled humidified atmosphere in an incubator containing 5% CO2 and was passaged with 0.25% trypsin. The H. pylori was added at the ratio 35:1 of H. pylori/GES-1 cells.
Clinical/animal tissue collecting
Clinical specimens from gastric mucosa were collected from patients who underwent gastroscopy screening and diagnoses at Third Xiangya affiliated Hospital of Central South University during 2012.8–2014.8. Written informed consent was obtained from each patient. Each 5 samples from developmental stages of carcinogenesis (non-atrophic gastritis (NAG), chronic atrophic gastritis (CAG), intestinal metaplasia (IM), dysplasia (DYS) and gastric carcinoma (GC)) with H. pylori infection (H. pylori (+)) and normal gastric mucosa (NGM) without H. pylori infection (H. pylori (-)) were mixed for the transcriptional factors array. Meanwhile, every 15 tissues from CAG, GC with H. pylori infection and NGM without H. pylori infection were served as quantitative RT-PCR analysis, and additional each 15 specimens from CAG, IM, GC with and without H. pylori infection and NGM without H. pylori infection were undertaken by Western blot analysis.
When coming to animal tissues, medical ethics protocol was approved by the Ethics Committee of the hospital. Twenty male Mongolian gerbils were randomly allocated to the experimental and control groups. Once got 24 hours fasting for all gerbils, the Experimental group were administrated by H. pylori gavage (East-Asian type CagA+ from GC patients, once a day and amounted to 7 days), and the controls were carried out with Saline gavage. Gerbils were sacrificed after 48 weeks H. pylori gavage. Gastric antrum tissue were collected and kept in liquid nitrogen immediately for further detection.
Detection of GATA-3 and Cx43 by RT-PCR and western blot
Total RNA was extracted from cells, clinical specimens, Mongolian gerbil gastric tissues using RNeasy Mini kit (Invitrogen, Life Technologies, Carlsbad, CA, USA). Genomic DNA was removed following DNaseI treatment (Qiagen, Valencia, CA, USA), and cDNA was synthesized using random primers and M-MLV reverse transcriptase (Qiagen). Real-time PCR reactions were set up using the iScriptTM 2-step RT-PCR kit with SYBR Green (Invitrogen) and the targeting gene primers. GATA-3: forward: 5′-CGGCAGGCAGATGAGAAAG, reverse: 3′-GGGCACATAGGGCGGATAG; Cx43: forward: 5′-GGAAGAGAAACTCAACAAAAA, reverse: 3′-GATGTACCACTGGATCAGAAG. The Ct values were normalized to β-actin: forward: 5′-GGCTGTGGAGACAAAAATGACCTC, reverse: 3′-AGGCTTGGGCTTGAATGGAGTC. Reactions were run on MyiQ Single color Real-time PCR Detection system (Bio-Rad, Hercules, CA, USA).
GATA-3 or Cx43 protein levels were tested by Western blot. Antrum tissues from Mongolian gerbil were not performed due to lack of specific antibodies. Cells and clinical specimens were washed twice with PBS (with calcium), and then homogenized in RIPA lysis buffer (150mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 50mM Tris pH 8.0) containing protease, phosphatase inhibitors and phenylmethylsulfonyl fluoride (all from Sigma, St Louis, MO, USA). Following sonication and centrifugation (14000g, 10 min, 4°C), the supernatant (soluble proteins) was collected. Protein concentration of each sample was determined by protein assay kit reagent (Pierce, Rockford, IL, USA). Samples were denatured in loading buffer, separated by polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes. Anti-GATA-3 (Santa Cruz, Dallas, TX, USA; diluted 1:200), Cx43 (Proteintech, Chicago, IL, USA; diluted 1:500), β-actin (Proteintech; diluted 1:4000) were incubated 4°C overnight followed by horseradish peroxidase conjugated secondary antibody incubation (1:5000, Jackson Immuno-Research, West Grove, PA, USA). Membranes were then visualized by the enhanced chemiluminescence Western blotting substrate (Bio-Rad).
Transcriptional factor/DNA array
Cx43 promoters (-2300bp) sequence (H. sapiens) were downloaded from UCSC.37 The candidate transcriptional factors binding sites of Cx43 promoters were predicted by TFSEARCH (http://www.cbrc.jp/research/db/TFSEARCH.html).38 Nuclear extracts of gastric mucosa biopsies were subjected to the TranSignalTM Protein/DNA Array (Panomics, Redwood City, CA, USA) according to the manufacturer's specifications. In short, Biotin-labeled DNA-binding oligonucleotides (TranSignalTM Probe Mix) were incubated with 10μg of nuclear extract at 15°C for 30 min to form the transcription factor/DNA complexes. These complexes were then separated from the free probes by 2% agarose gel electrophoresis in 0.5X TBE at 120 V for 15 min. The probes in the complexes were released, ethanol precipitated, and hybridized to the TranSignalTM Protein/DNA Array. Detection of signals was obtained using an enhanced chemiluminescence imaging system.
Transfection
Two RNAi targets sequences were selected according to stealth RNAi of the human GATA-3 mRNA (GenBank Accession No.X55037.1). The sequences of synthesized oligonucleotide were: siRNA-1: sense 5′-CCACACUCUGGAGGAGGAAUGCCAA-3′, antisense 5′-UUGGCAUUCCUCCUCCAGAGUGUGG-3′; siRNA-2: sense 5′-GGCUCUACUACAAGCUUCACAAUAU-3′, antisense 5′-AUAUUGUGAAGCUUGUAGUAGAGCC-3′. Lipofectamine RNAi reagent (Invitrogen) was used to transfect synthesized Stealth RNAi against GATA-3 into BGC-803 and H. pylori infected GES-1 cells. Stealth RNAi, fluorescent logo or negative control duplexes were delivered into cells as well. BLOCK-iT Alexa Fluor Red Fluorescent (Invitrogen) was used to confirm the transfection efficiency of each duplex siRNA.
Plasmid construction and dual-luciferase assay
To verify the GATA-3 binding sites in Cx43 promoters, fragment of the human Cx43 promoter harboring the putative GATA-3 binding site was amplified from primer DNA polymerase (Genestar, Florham Park, NJ, USA) (sense, 5′-gcgagctcGCATTGCATTTCAGTGCC-3′; antisense, 5′-gcaagcttGGTAGGGAGGAGGCTGGT-3′) with restriction cutting sites Sac I and Hind III. The amplified PCR products were confirmed by DNA sequencing and ligated to the pGL3 Basic vector (Promega, Madison, WI, USA). To construct the GATA-3 plasmid, GATA-3 cDNA was amplified by PCR with additional Nhe I and Xho I digestion sites (sense, 5′- cgctagcgATGGAGGTGACGGCGGA-3′; antisense, 5′-gcctcgagCTAACCCATGGCGGTGAC-3′). The constructed CX43-pGL3 Basic and GATA-3 plasmids were co-transfected into HEK293T cells. The firefly and Renilla luciferase activities in the cell lysates were triplicated and determined using a dual-luciferase assay system (Promega) and normalized data were calculated as the ratio of the Renilla/firefly luciferase activities.
Statistical analysis
Statistical Package for Social Sciences software 12 (SPSS Inc., Chicago, IL, USA) was used for analysis. Data were shown as the mean and SD. Multiple group comparisons were determined using one-way analysis of variance (ANOVA) with Dunnett's multiple comparison tests. Correlations between groups were employed by 2-sided t-tests and Spearmen's correlation test. A value of P <0.05 was considered to be statistically significant.
Supplementary Material
Funding Statement
This research is supported by a grant from the National Natural Science Foundation of China (No. 81172301) and National Innovative Training Program (No.201310533059).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to the Endoscopic Unit of Third Xiangya Hospital of Central South University for the supply of clinic samples.
References
- 1. Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 2003; 83:1359-400; PMID:14506308; http://dx.doi.org/ 10.1152/physrev.00007.2003 [DOI] [PubMed] [Google Scholar]
- 2. Panani AD. Cytogenetic and molecular aspects of gastric cancer: clinical implications. Cancer Lett 2008; 266:99-115; PMID:18381231; http://dx.doi.org/ 10.1016/j.canlet.2008.02.053 [DOI] [PubMed] [Google Scholar]
- 3. Tang B, Peng ZH, Yu PW, Yu G, Qian F, Zeng DZ, Zhao YL, Shi Y, Hao YX, Luo HX. Aberrant expression of Cx43 is associated with the peritoneal metastasis of gastric cancer and Cx43-mediated gap junction enhances gastric cancer cell diapedesis from peritoneal mesothelium. Plos One 2013; 11(9):274527; 8:PMID:24040271; http://dx.doi.org/ 10.1371/journal.pone.0074527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu CX, Jia Y, Yang WB, Wang F, Shen SR. [Relationship between Helicobacter pylori infection and expression of connexin (Cx) 32 and Cx43 genes in gastric cancer and gastric precancerous lesions]. Zhonghua yi xue za zhi 2008; 88:1523-7; PMID:18956631 [PubMed] [Google Scholar]
- 5. Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. New Engl J Med 1991; 325:1127-31; PMID:1891020; http://dx.doi.org/ 10.1056/NEJM199110173251603 [DOI] [PubMed] [Google Scholar]
- 6. Yokozaki H. Molecular characteristics of eight gastric cancer cell lines established in Japan. Pathol Int 2000; 50:767-77; PMID:11107048; http://dx.doi.org/17478938 10.1046/j.1440-1827.2000.01117.x [DOI] [PubMed] [Google Scholar]
- 7. Xu CX, Qi YM, Yang WB, Wang F, Zhou JD, Shen SR. ; [Effect of CagA(+) helicobacter pylori strain on the expression of connexin 43 and cell proliferation in BGC-823 cells]. Zhong nan da xue xue bao Yi xue ban 2007; 32:288-94; PMID:17478938 [PubMed] [Google Scholar]
- 8. Leithe E, Rivedal E. Ubiquitination of gap junction proteins. J Membrane Biol 2007; 217:43-51; PMID:21866551; http://dx.doi.org/ 10.1007/s00232-007-9050-z [DOI] [PubMed] [Google Scholar]
- 9. Cottrell GT, Lin R, Warn-Cramer BJ, Lau AF, Burt JM. Mechanism of v-Src- and mitogen-activated protein kinase-induced reduction of gap junction communication. Am J Physiol Cell Physiol 2003; 284:C511-20; PMID:12388103; http://dx.doi.org/ 10.1152/ajpcell.00214.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matesic DF, Sidorova TS, Burns TJ, Bell AM, Tran PL, Ruch RJ, May SW. p38 MAPK activation, JNK inhibition, neoplastic growth inhibition, and increased gap junction communication in human lung carcinoma and Ras-transformed cells by 4-Phenyl-3-butenoic acid. J Cell Biochem 2012; 113:269-81; PMID:21898549; http://dx.doi.org/ 10.1002/jcb.23353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maneechotesuwan K, Xin Y, Ito K, Jazrawi E, Lee KY, Usmani OS, Barnes PJ, Adcock IM. Regulation of Th2 cytokine genes by p38 MAPK-mediated phosphorylation of GATA-3. J Immunol 2007; 178:2491-8; PMID:17277157; http://dx.doi.org/ 10.4049/jimmunol.178.4.2491 [DOI] [PubMed] [Google Scholar]
- 12. Ko JA, Yanai R, Morishige N, Takezawa T, Nishida T. Upregulation of connexin43 expression in corneal fibroblasts by corneal epithelial cells. Invest Ophthal Visual Sci 2009; 50:2054-60; PMID:19117926; http://dx.doi.org/ 10.1167/iovs.08-2418 [DOI] [PubMed] [Google Scholar]
- 13. Ghosh S, Kumar A, Tripathi RP, Chandna S. Connexin-43 regulates p38-mediated cell migration and invasion induced selectively in tumour cells by low doses of gamma-radiation in an ERK-1/2-independent manner. Carcinogenesis 2014; 35:383-95; PMID:24045413; http://dx.doi.org/ 10.1093/carcin/bgt303 [DOI] [PubMed] [Google Scholar]
- 14. Xu C, Chen Y, Chen X, Wang F. Effects of different types of helicobacter pylori on the gap junction intercellular communication in GES-1 cells. Zhong nan da xue xue bao Yi xue ban 2011; 36:294-300; PMID:21566279; http://dx.doi.org/ 10.3969/j.issn.1672-7347.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 15. Jimenez T, Fox WP, Naus CCG, Galipeau J, Belliveau DJ. Connexin over-expression differentially suppresses glioma growth and contributes to the bystander effect following HSV-thymidine kinase gene therapy. Cell Commun Adhes 2006; 13:79-92; PMID:16613782; http://dx.doi.org/ 10.1080/15419060600631771 [DOI] [PubMed] [Google Scholar]
- 16. Kretz M, Euwens C, Hombach S, Eckardt D, Teubner B, Traub O, Willecke K, Ott T. Altered connexin expression and wound healing in the epidermis of connexin-deficient mice. J Cell Sci 2003; 116:3443-52; PMID:12840073; http://dx.doi.org/ 10.1242/jcs.00638 [DOI] [PubMed] [Google Scholar]
- 17. Aasen T, Hodgins MB, Edward M, Graham SV. The relationship between connexins, gap junctions, tissue architecture and tumour invasion, as studied in a novel in vitro model of HPV-16-associated cervical cancer progression. Oncogene 2003; 22:7969-80; PMID:12970745; http://dx.doi.org/ 10.1038/sj.onc.1206709 [DOI] [PubMed] [Google Scholar]
- 18. Pai SY, Truitt ML, Ting CN, Leiden JM, Glimcher LH, Ho IC. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity 2003; 19:863-75; PMID:14670303; http://dx.doi.org/ 10.1016/S1074-7613(03)00328-5 [DOI] [PubMed] [Google Scholar]
- 19. Ellis CL, Chang AG, Cimino-Mathews A, Argani P, Youssef RF, Kapur P, Montgomery EA, Epstein JI. GATA-3 Immunohistochemistry in the differential diagnosis of adenocarcinoma of the urinary bladder. Am J Surg Pathol 2013; 37:1756-60; PMID:24061521; http://dx.doi.org/ 10.1097/PAS.0b013e31829cdba7 [DOI] [PubMed] [Google Scholar]
- 20. Jacquemier J, Charafe-Jauffret E, Monville F, Esterni B, Extra JM, Houvenaeghel G, Xerri L, Bertucci F, Birnbaum D. Association of GATA3, P53, Ki67 status and vascular peritumoral invasion are strongly prognostic in luminal breast cancer. Breast Cancer Res 2009; 11(2):R23; PMID:19405945; http://dx.doi.org/ 10.1186/bcr2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Molenaar JJ, Ebus ME, Koster J, Santo E, Geerts D, Versteeg R, Caron HN. Cyclin D1 is a direct transcriptional target of GATA3 in neuroblastoma tumor cells. Oncogene 2010; 29:2739-45; PMID:20154722; http://dx.doi.org/ 10.1038/onc.2010.21 [DOI] [PubMed] [Google Scholar]
- 22. Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 2007; 9:201-U103; PMID:17187062; http://dx.doi.org/ 10.1038/ncb1530 [DOI] [PubMed] [Google Scholar]
- 23. Kouros-Mehr H, Bechis SK, Slorach EM, Littlepage LE, Egeblad M, Ewald AJ, Pai SY, Ho IC, Werb Z. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell 2008; 13:141-52; PMID:18242514; http://dx.doi.org/ 10.1016/j.ccr.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. P Natl Acad Sci USA 2003; 100:2645-50; PMID:12598651; http://dx.doi.org/ 10.1073/pnas.0437939100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jackson JA, Hall AJ, Friberg IM, Ralli C, Lowe A, Zawadzka M, Turner AK, Stewart A, Birtles RJ, Paterson S. et al. An immunological marker of tolerance to infection in wild rodents. Plos Biol 2014; 12(7):e1001901; PMID:25004450; http://dx.doi.org/ 10.1371/journal.pbio.1001901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stellato C, Gubin MM, Magee JD, Fang X, Fan JS, Tartar DM, Chen J, Dahm GM, Calaluce R, Mori F., et al. Coordinate regulation of GATA-3 and Th2 cytokine gene expression by the RNA-binding protein HuR. J Immunol 2011; 187:441-9; PMID:21613615; http://dx.doi.org/ 10.4049/jimmunol.1001881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Apte SH, Groves P, Olver S, Baz A, Doolan DL, Kelso A, Kienzle N. IFN-gamma inhibits IL-4-induced type 2 cytokine expression by CD8 T cells in vivo and modulates the anti-tumor response. J Immunol 2010; 185:998-1004; PMID:20562261; http://dx.doi.org/ 10.4049/jimmunol.0903372 [DOI] [PubMed] [Google Scholar]
- 28. Lindgren A, Yun CH, Sjoling A, Berggren C, Sun JB, Jonsson E, Holmgren J, Svennerholm AM, Lundin SB. Impaired IFN-gamma production after stimulation with bacterial components by natural killer cells from gastric cancer patients. Exp Cell Res 2011; 317:849-58; PMID:21255568; http://dx.doi.org/ 10.1016/j.yexcr.2011.01.006 [DOI] [PubMed] [Google Scholar]
- 29. Sayi A, Kohler E, Toller IM, Flavell RA, Muller W, Roers A, Müller A. TLR-2-activated B cells suppress helicobacter-induced preneoplastic gastric immunopathology by inducing T regulatory-1 cells. J Immunol 2011; 186:878-90; PMID:21149607; http://dx.doi.org/ 10.4049/jimmunol.1002269 [DOI] [PubMed] [Google Scholar]
- 30. Khan MM, Chatterjee S, Dwivedi VP, Pandey NK, Singh Y, Tousif S, Bhavesh NS, Van Kaer L, Das J, Das G. CD4(+) T cell-derived novel peptide Thp5 induces interleukin-4 production in CD4(+) T cells to direct T helper 2 cell differentiation. J Biol Chem 2012; 287:2830-5; PMID:22130674; http://dx.doi.org/ 10.1074/jbc.M111.319947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hou L, El-Omar EM, Chen J, Grillo P, Rabkin CS, Baccarelli A, Yeager M, Chanock SJ, Zatonski W, Sobin LH., et al. Polymorphisms in Th1-type cell-mediated response genes and risk of gastric cancer. Carcinogenesis 2007; 28:118-23; PMID:16885196; http://dx.doi.org/ 10.1093/carcin/bgl130 [DOI] [PubMed] [Google Scholar]
- 32. Straubinger RK, Greiter A, McDonough SP, Gerold A, Scanziani E, Soldati S, et al. Quantitative evaluation of inflammatory and immune responses in the early stages of chronic Helicobacter pylori infection. Infect Immun 2003; 71:2693-703; PMID:12704144; http://dx.doi.org/ 10.1128/IAI.71.5.2693-2703.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Enarsson K, Lundgren A, Kindlund B, Hermansson M, Roncador G, Banham AH, et al. Function and recruitment of mucosal regulatory T cells in human chronic Helicobacter pylori infection and gastric adenocarcinoma. Clin Immunol 2006; 121:358-68; PMID:16934529; http://dx.doi.org/ 10.1016/j.clim.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 34. Yang P, Qiu G, Wang S, Su Z, Chen J, Wang S, et al. The mutations of Th1 cell-specific T-box transcription factor may be associated with a predominant Th2 phenotype in gastric cancers. Int J Immunogenet 2010; 37:111-5; PMID:20193034; http://dx.doi.org/ 10.1111/j.1744-313X.2010.00899.x [DOI] [PubMed] [Google Scholar]
- 35. Liu Z, Xu X, Chen L, Li W, Sun Y, Zeng J, et al. Helicobacter pylori CagA inhibits the expression of Runx3 via Src/MEK/ERK and p38 MAPK pathways in gastric epithelial cell. J Cell Biochem 2012; 113:1080-6; PMID:22266963; http://dx.doi.org/ 10.1002/jcb.23440 [DOI] [PubMed] [Google Scholar]
- 36. King TJ, Gurley KE, Prunty J, Shin JL, Kemp CJ, Lampe PD. Deficiency in the gap junction protein connexin32 alters p27Kip1 tumor suppression and MAPK activation in a tissue-specific manner. Oncogene 2005; 24:1718-26; PMID:15608667; http://dx.doi.org/ 10.1038/sj.onc.1208355 [DOI] [PubMed] [Google Scholar]
- 37. Goldman M, Craft B, Swatloski T, Ellrott K, Cline M, Diekhans M, Dailidiene D, Dailide G, Berg DE, Simpson KW. The UCSC cancer genomics browser: update 2013. Nucleic Acids Res 2013; 41:D949-D54; PMID:23109555; http://dx.doi.org/ 10.1093/nar/gks1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wingender E. The TRANSFAC project as an example of framework technology that supports the analysis of genomic regulation. Brief Bioinform 2008; 9:326-32; PMID:18436575; http://dx.doi.org/ 10.1093/bib/bbn016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.