Abstract
Background
Reduced sensitivity to positive feedback is common in patients with major depressive disorder (MDD). However, findings regarding negative feedback are ambiguous, with both exaggerated and blunted responses being reported. The ventral striatum (VS) plays a major role in processing valenced feedback, and previous imaging studies have shown that the locus of controls (self agency v. external agency) over the outcome influences VS response to feedback. We investigated whether attributing the outcome to one’s own action or to an external agent influences feedback processing in patients with MDD. We hypothesized that depressed participants would be less sensitive to the feedback attribution reflected by an altered VS response to self-attributed gains and losses.
Methods
Using functional MRI and a motion prediction task, we investigated the neural responses to self-attributed (SA) and externally attributed (EA) monetary gains and losses in unmedicated patients with MDD and healthy controls.
Results
We included 21 patients and 25 controls in our study. Consistent with our prediction, healthy controls showed a VS response influenced by feedback valence and attribution, whereas in depressed patients striatal activity was modulated by valence but was insensitive to attribution. This attribution insensitivity led to an altered ventral putamen response for SA – EA losses in patients with MDD compared with healthy controls.
Limitations
Depressed patients with comorbid anxiety disorder were included.
Conclusion
These results suggest an altered assignment of motivational salience to SA losses in patients with MDD. Altered striatal response to SA negative events may reinforce the belief of not being in control of negative outcomes contributing to a cycle of learned helplessness.
Introduction
Affective biases in emotion processing are characteristic of major depressive disorder (MDD), as indicated by a reduced ability to experience pleasure (anhedonia) and a bias toward the negative aspects of the environment.1 Depressed patients, for instance, show a blunted response to positive or pleasant stimuli2 but an enhanced memory for negative stimuli3 and a stronger responsiveness to threatening stimuli.4
Different behavioural responses to feedback have also been widely reported in patients with MDD.1 Across various cognitive tasks, depressed patients show an increased risk of committing a subsequent error after failure.5,6 Moreover, when performing a probabilistic reversal learning (PRL) task, depressed patients show an increased tendency to switch after obtaining misleading negative feedback. The higher rate of switching has been interpreted as an oversensitivity to negative feedback,7,8 an interpretation also supported by electrophysiological findings in patients with MDD demonstrating greater error-related negativity, an index of automatic error detection.9–11 However, increased sensitivity to negative feedback is not a consistent finding in depressed patients. Several electrophysiological studies have reported either no changes or even reduced error-related negativity in patients with MDD.12–14 Neuroimaging data suggest that MDD is associated with a blunted response to both negative and positive feedback. Using a gambling task, weaker responses to losses in the anterior cingulate cortex and to gains in the ventral striatum have been reported in depressed patients relative to healthy controls.15 A blunted reactivity to feedback is consistent with behavioural findings demonstrating reduced responsiveness during the anticipation and/or the presentation of rewards in depressed individuals2,16,17 and with neuroimaging studies reporting that this decreased sensitivity to positive feedback is associated with reduced striatal response.15,18–22
Hyposensitivity to positive feedback is a common finding in depressed patients, while results regarding sensitivity to negative feedback seem to be ambiguous, with both exaggerated and blunted responses being reported. A possible explanation is that reactivity to negative feedback may depend on symptom severity, with moderately depressed patients showing oversensitivity and severely depressed patients showing a blunted response.23 An alternative explanation is that feedback responsiveness in patients with MDD may be task-dependent. The learned helplessness theory hypothesizes that in individuals exposed to stressful, uncontrollable life events a generalized emotional–cognitive state of perceiving the environment as uncontrollable can develop and that this mechanism is central to the onset and maintenance of MDD.24,25 Given its theoretical importance to understanding depressed cognition, the level of control attainable within a task may be relevant. In fact, an abnormal sense of agency — the experience of controlling one’s own actions and, through them, events in the outside world26 — in the form of loss of agency and helplessness constitute a major marker of MDD.27
The ventral striatum (VS) plays a key role in the processing of valenced outcomes.28,29 In light of the centrality of agency to computational accounts of VS30 and of cognitive accounts of valence processing,31 the locus of control (self v. external agency) over an outcome should influence VS response to feedback. Consistent with this hypothesis, previous studies have shown greater VS response when gains were contingent to performance32 and when task difficulty increased.33
The goal of our study was to more directly investigate the neuronal responses to financial gains and losses attributed to one’s own action (related to response contingency) or an external agent (unrelated to response contingency) in patients with MDD. To this end, we conducted a functional MRI (fMRI) study using a task in which participants experience monetary gains or losses either due to their performance (self-attributed [SA]) or to a biased coin toss (externally attributed [EA]). We have previously shown that healthy controls modulate their VS response according to the feedback’s valence and attribution,34 and we hypothesized that, compared with healthy controls, depressed patients would show a VS response less sensitive to feedback attribution, leading to an altered response to SA gains and losses.
Methods
Participants
We recruited healthy controls without any psychiatric, neurologic, or medical illness and unmedicated patients with MDD for participation in the study. Exclusion criteria for both groups included age younger than 18 years or older than 65 years, current or past psychosis or mania, major medical or neurologic illness, current drug or alcohol abuse and MRI contraindications (as assessed by an MRI safety questionnaire). Inclusion in the MDD group was contingent on a diagnosis of current MDD based on a semistructured interview: the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I). Control participants were required to have no history of MDD in their lifetime, have no history of MDD in first-degree relatives and have no Axis I disorders based on a SCID-I interview. The University of Zurich’s Institutional Review Board approved our study protocol, and all participants gave written informed consent. They were paid a modest compensation for participation in the study in addition to the gains they could make during the experimental tasks.
Psychometric measures
All participants completed the German version of the Positive and Negative Affect Schedule (PANAS35), the German version of the Hopelessness Scale originally developed by Beck (H-Scale36), the State-Trait Anxiety Inventory (STAI37), and the Snaith-Hamilton Pleasure Scale (SHAPS38). In addition, depressed participants completed the Beck Depression Inventory II (BDI-II39) and the Inventory for Depressive Symptomatology (IDS40,41).
Motion prediction task
The motion prediction task has been described previously34 and is reported in details in Appendix 1, available at jpn.ca. In brief, each trial started with 2 balls moving with different speeds and from different starting points toward a finish line. The task on every trial of the experiment was to predict which ball would cross the finish line first and to indicate the decision by a left or right button press. Only after this decision were participants instructed whether the response was relevant or irrelevant to the upcoming feedback. Specifically, participants were told that on each trial they could gain or lose 50 cents, indicated by “+50” or “−50” feedback. On a random 50% of the trials feedback was performance-dependent (SA gains or losses; correct v. error). On the other 50% of trials feedback was dependent on chance and was randomly selected by the computer with a probability tailored to match their success rate in performance-dependent trials (EA negative or positive feedback was determined by a biased virtual coin flip). A picture with the words “You” and “Coin” and an arrow pointing toward either word was presented on the screen 750 ms after the response to indicate if the feedback was associated with the participant’s performance or not. Finally, feedback about winning (+50) or losing (−50) was presented (Appendix 1, Fig. S1). The next trial started after a fixation period of 2000 ± 500 ms. If the participant failed to respond, the arrow pointed toward the word “You” and was followed by the feedback “Missed.” Two healthy controls and 1 patient with MDD performed a shorter version of the task (100 trials), and all other participants completed 130 trials. To keep uncertainty about performance high, task difficulty was adapted for each participant (error rate > 30%) using a training session of 100 trials in which the participants received only performance feedback (correct: happy face; incorrect: unhappy face). Participants were unaware that performance was manipulated and were told to do their best.
Image acquisition
Images were acquired on a Philips Achieva 3 T whole-body MRI unit equipped with an 8-channel head coil. Functional time series were acquired with a sensitivity encoded single shot echo-planar sequence. We placed 36 contiguous axial slices along the anterior–posterior commissure plane covering the entire brain. We used the following parameters: repetition time 2000 ms, echo time 35 ms, ascending acquisition order, voxel matrix 80 × 80 interpolated to 128 × 128, voxel size 2.75 × 2.75 × 4 mm, SENSE acceleration factor R = 2.0. The first 4 acquisitions were discarded owing to T1 saturation effects. T1-weighted high-resolution images were also acquired for each participant.
Data analysis
Demographic and psychometric data were analyzed with an unpaired t test and sex with a χ2 test using StatView version 5.0.1 (SAS Institute). We analyzed mean reaction time (RT) differences for correct and incorrect trials (independently of attribution) with repeated-measures analyses of variance (RM-ANOVA). We considered results to be significant at p < 0.05, 2-tailed.
To accurately attribute the financial outcome, participants were required to attend to contextual information presented after their performance but before the outcome (Appendix 1, Fig. S1). It is possible that depressed patients had a reduced ability to discriminate the 2 attribution contexts. However, we have previously shown that in healthy controls mean RT in trials following an SA feedback is longer than in trials following an EA feedback (independent of valence).34 This post-SA feedback RT slowing is a behavioural measure indicating that participants were able to discriminate feedback attribution. Therefore, RTs for post-SA and post-EA gains and losses were also analyzed using RM-ANOVA, with valence and attribution as within-subjects factors and diagnosis as an independent factor.
Image processing was carried out using Statistical Parametric Mapping software version 8 (Wellcome Department of Imaging Neuroscience). The preprocessing of functional images included motion correction, coregistration to a standard template, alignment to the first volume for each participant, spatial normalization to the Montreal Neurological Institute (MNI) template (voxel size 2 × 2 × 2 mm), and smoothing using an 8 mm full-width at half-maximum Gaussian kernel. Statistical analysis was performed by modelling the different conditions convolved with a hemodynamic response function and its temporal derivative as explanatory variables within the context of the general linear model on a voxel-by-voxel basis. Our 2 × 2 factorial design independently manipulated the agency and valence of feedback. Four regressors corresponding to the 4 feedback conditions of interest (SA losses, EA losses, SA gains, EA gains) were modelled as well as several covariates of no interest (missed feedback, realignment parameters, time of the motor response; Appendix 1). Feedback was modelled as an event (duration = 0) and occurred 4.13 s after the onset of the trial. These regressors of no interest explained additional variance in the blood oxygen level–dependent (BOLD) signal.42
A fixed-effect model at a single-participant level was specified, giving images of parameter estimates for each contrast that were then used for a second-level random effects analysis (Appendix 1). We entered individual participant contrasts into a second-level analysis to examine group differences in activation using 2-sample t tests with age, sex and years of education as covariates. Activation differences were identified with a global height threshold of p < 0.001, uncorrected, and then family-wise error (FWE)–corrected at peak level for multiple comparisons.43 Peak level inference assesses the amplitude of parameter contrasts over space (i.e., the chance of finding an activation with this amplitude or a greater amplitude in a manner that controls for multiple comparisons over space; Appendix 1). For the small volume–corrected analyses, we created a functional mask of the left and right VS using the gains – losses contrast across all participants (Appendix 1, Fig. S2). We calculated the mean percent signal change using MarsBar from the SPM toolbox.
Results
Participants
We included 25 healthy controls and 21 unmedicated patients with MDD in our study. All participants were right-handed and had normal or corrected-to-normal vision. Groups did not differ in sex distribution, age, years of education or performance in the motion prediction task (Table 1). Fourteen patients with MDD were medication-naive, and 3 stopped treatment with antidepressant medications 6 weeks or more before the study. All other patients had been free from medications for at least 2 years. Six patients with MDD had comorbid anxiety disorders: 2 had social phobia (1 of whom also had an eating disorder), 1 had generalized anxiety disorder (GAD) and 3 had panic disorder (1 of whom also had GAD).
Table 1.
Demographic and clinical characteristics of the study sample
| Group; no. (%) or mean ± SD | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | MDD, n = 21 | Control, n = 25 | Statistic | p value |
| Male sex | 14 (67) | 12 (52) | χ2 = 1.0 | 0.37 |
| Age, yr | 39.3 ± 13.1 | 33.5 ± 10.4 | t = 1.7 | > 0.99 |
| Education, yr | 16.4 ± 2.6 | 15.9 ± 2.6 | t = 0.6 | 0.58 |
| BDI-II score | 25.4 ± 8.4 | — | — | — |
| IDS score | 33.4 ± 8.3 | — | — | — |
| Single MDD episode | 5 (24) | — | — | — |
| Never medicated | 14 (67) | — | — | — |
| Comorbid anxiety disorder | 6 (29) | — | — | — |
| STAI-Trait score | 56.3 ± 10.7 | 33.0 ± 9.0 | t = 7.9 | < 0.001 |
| SHAPS score | 3.4 ± 3.0 | 0.5 ± 1.1 | t = 4.5 | < 0.001 |
| H-Scale score | 73.9 ± 12.9 | 44.3 ± 10.2 | t = 8.7 | < 0.001 |
| PANAS positive score | 10.5 ± 3.2 | 13.6 ± 3.9 | t = −2.9 | 0.006 |
| PANAS negative score | 7.7 ± 2.2 | 5.6 ± 0.9 | t = 4.7 | < 0.001 |
| %SA gains | 61.2 ± 7.8 | 59.4 ± 6.7 | t = 0.8 | 0.41 |
| %EA gains | 59.3 ± 6.1 | 58.3 ± 6.9 | t = 0.5 | 0.60 |
| %Miss | 2.1 ± 3.3 | 1.5 ± 3.3 | t = 0.6 | 0.53 |
| RT correct | 542.3 ± 162.7 | 516.2 ± 97.2 | t = 0.6 | 0.50 |
| RT incorrect | 578.5 ± 139.8 | 547.8 ± 112.8 | t = 0.8 | 0.41 |
| RT post-SA gain | 566.6 ± 149.2 | 529.7 ±111.8 | t = 1.0 | 0.34 |
| RT post-SA loss | 560.8 ± 174.5 | 525.1 ± 101.2 | t = 0.9 | 0.39 |
| RT post-EA gain | 531.5 ± 139.4 | 519.8 ± 109.3 | t = 0.3 | 0.75 |
| RT post-EA loss | 542.2 ± 155.5 | 512.2 ± 100.0 | t = 0.8 | 0.43 |
BDI-II = Beck Depression Inventory II; EA = externally attributed; H-Scale = Hopelessness Scale; IDS = Inventory of Depressive Symptomatology; MDD = major depressive disorder; PANAS = Positive and Negative Affect Scale; RT = reaction time; SA = self-attributed; SD = standard deviation; SHAPS = Snaith–Hamilton Pleasure Scale; STAI = Spielberger Trait Anxiety Inventory.
Behavioural data
Demographic, psychometric and behavioural data, including mean RT for correct and incorrect trials (across SA and EA trials) and mean RT for trials following SA or EA feedback are presented in Table 1.
The mean RT for incorrect trials was significantly longer than for correct trials (F1,44 = 23.1, p < 0.001), but we found no effect of diagnosis (p = 0.45) or diagnosis × correctness interaction (p = 0.74). Moreover, on trials following SA feedback (post-SA losses/gains), mean RT was longer than for trials following EA feedback (post-EA losses/gains; F1,44 = 11.81, p = 0.002). However, we found no effect of diagnosis (p = 0.44) or diagnosis × attribution (p = 0.17), diagnosis × valence (p = 0.54) or diagnosis × attribution × valence interactions (p = 0.38).
Imaging data
Whole-brain analyses investigating the agency × valence interaction effects
In healthy controls, the 1-sample t test for the contrast (SA gains – EA gains) – (SA losses – EA losses) showed a significant activation in the right ventral putamen (p = 0.012FWE at peak level, t = 7.59, MNI space x, y, z = 20, 12, −14). The reported coordinates index the location of the peak (local maxima). In contrast, we found no significant activation in depressed patients even when a lower statistical threshold (uncorrected p < 0.005) was investigated.
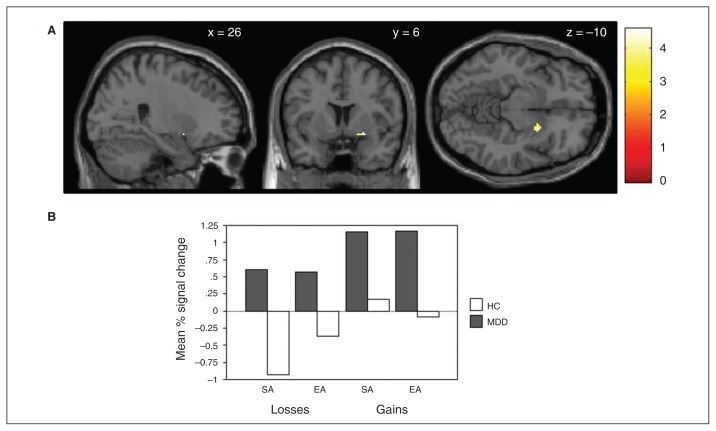
When comparing depressed patients and healthy controls, the 2-sample t test revealed a trend toward a significant whole-brain difference in the right ventral putamen (p = 0.05FWE at peak level, t = 5.51, MNI space x, y, z = 28, 8, −8). When we conducted a small volume–corrected analysis we found a significant difference in the right VS (p = 0.002FWE at peak level, t = 4.58, MNI space x, y, z = 26, 6, −10) but no significant difference in the left VS. Figure 1 displays the significant 15 voxels (p < 0.05FWE at peak level) in the right ventral putamen along with the mean percent signal change for each feedback condition.
Fig. 1.
Small volume–corrected analysis of the attribution × valence interaction comparing healthy controls (HC) and patients with major depressive disorder (MDD). (A) The small volume–corrected analysis of the attribution (SA/EA) × valence (gains/losses) interaction comparing healthy controls and patients with MDD shows differences in blood oxygen level–dedpendent signal change in the right ventral putamen. (B) Mean percent signal change within the right ventral putamen for each feedback condition in healthy controls and patients with MDD. EA = externally attributed; SA = self-attributed.
When we repeated the analysis excluding all the depressed patients with comorbid anxiety disorders, a significant effect in the right ventral putamen was still present (Appendix 1).
No correlations emerged between mean percent signal change in any of the 4 feedback conditions and depression severity (BDI, IDS) or anhedonia and hopelessness scores (SHAPS, H-Scale).
Small volume–corrected analyses investigating the simple effects of attribution in the VS
To further investigate the interaction effect reported previously, we conducted post hoc between-groups analyses of the simple effects of attribution. The 2-sample t test showed a significant effect for the contrast SA losses – EA losses (p = 0.049FWE at peak level, t = 3.33, MNI space x, y, z = 22, 4, −8). We found no effect for the contrast SA gains – EA gains.
Whole-brain analyses investigating the main effect of valence and attribution
We found no significant between-group differences, and the results for each diagnostic group separately are reported in Appendix 1, Tables S1 and S2.
Model-based prediction error analyses
As the VS is considered to signal reward prediction error44 and altered prediction error signal in the striatum has been reported in patients with MDD,45,46 we also considered a model-based prediction error analysis to further investigate whether the diagnosis × agency × valence interaction effect found in the ventral putamen was due to prediction error differences in patients with MDD (Appendix 1). For each feedback condition, we included a parametric modulator that coded trial-by-trial prediction errors (as a first-level covariate). However, the diagnosis × agency × valence interaction effect described previously remained significant when the prediction errors were included as covariates. Conversely we found no significant differences in prediction error magnitude between conditions. This suggests that all interesting variance was captured by a qualitative comparison of our factorial conditions.
Discussion
The present study investigated how feedback attribution influences neuronal feedback processing in patients with MDD. We hypothesized that healthy controls would show a VS response modulated by valence and attribution, whereas depressed patients would have a VS response less sensitive to feedback attribution, leading to an altered processing of SA gains and losses. Consistent with our hypothesis, we found altered striatal response in depressed patients that seems to reflect a failure to modulate the putamen activity according to the feedback’s attribution, leading to an altered putamen activity during the processing of SA losses – EA losses in patients compared with controls. In contrast, we found no evidence that MDD was associated with an altered VS response to gains.
The VS is involved in several processes that are relevant when evaluating feedback, including signalling reward prediction error, incentive motivation and motivational salience.44,47–49 Disentangling the contribution of these different processes was not the aim of our study. However, we conducted a supplementary analysis that strongly suggests prediction error signals did not contribute to our VS results (Appendix 1). Consistent with our results in healthy controls, personal responsibility for positive outcomes has been associated with greater VS response.32,33 Moreover, in our tasks only SA feedback provided information about performance, thus it is likely that controls processed SA feedback as more salient than EA feedback, leading to a VS response modulated by feedback valence and attribution. In contrast, the internal bias of having no control over the environment may have led depressed patients to an altered salience attribution and consequently a VS response insensitive to feedback attribution. This interpretation is consistent with recent findings showing altered functional connectivity between the salience network and the striatum during the processing of emotional stimuli in unmedicated depressed patients.50 More importantly, we have recently investigated functional alterations present at rest and during performance of the motion prediction task in a subgroup of individuals involved in the present study using independent component analysis. We found that 1 intrinsic network showed greater amplitude of low-frequency fluctuations specifically during task performance in depressed patients compared with healthy controls (unpublished data, 2014). This network included the anterior insula and the ventrolateral prefrontal cortex. Considering the role of these brain regions in processing salient events,51,52 these results strongly suggest that differences in motivational salience may contribute to the altered VS response in patients with MDD in the present study.
Interestingly, we have recently reported an altered striatal response to feedback attribution in healthy controls homozygous for the Val allele of the brain-derived neurotrophic factor (BDNF) Val66Met polymorphism.53 Whereas Met carriers showed a VS response modulated by feedback valence and attribution, Val/Val carriers showed a VS response insensitive to feedback attribution that led to an altered VS response to SA losses – EA losses, which is similar to our findings in depressed patients. Moreover, increased striatal response to performance-dependent monetary losses has been reported in healthy adolescents characterized as behaviourally inhibited,54 a temperamental trait associated with an increased risk for anxiety disorder early in life.55 As trait anxiety and neuroticism, known risk factors for MDD, are reportedly higher in Val/Val than in Met carriers,56–59 these results may lead to the speculation that altered motivational salience processing of SA losses is present in populations at risk for MDD. Future studies are warranted to investigate this hypothesis.
Reduced striatal response to positive feedback has previously been reported in depressed patients.15,18,19 In contrast, we found no evidence of a blunted striatal response to gains (but rather a potential increase for EA gains). However, greater activity in the right putamen has been also found by Smoski and colleagues22 during the anticipation of monetary rewards compared with pleasant images in patients with mild depression. Since financial rewards have a higher incentive than pleasant images or than simply being correct, it is possible that differences in incentive motivation in the present study compared with previous studies contributed to our VS findings. Reduced reward responsiveness has also been correlated with anhedonia scores in depressed and healthy individuals and with anhedonia symptoms 1 month later.2,17 Moreover, reduced striatal reward prediction error signals have been found in patients with MDD45,46 and have been shown to correlate with anhedonia scores.46 However, anhedonia scores in our study were not particularly high on average but were highly variable among patients (Table 1). It remains possible that blunted response to positive feedback, be they reward prediction errors or not, is more prevalent in depressed individuals with high anhedonia scores.
Limitations
Several limitations need to be mentioned. First, we included patients with comorbid anxiety disorders, thus it is possible our findings are not representative of functional abnormalities occurring in the entire MDD population. However, when all the depressed patients with comorbid anxiety disorders were excluded from the analysis, a significant valence × attribution effect in the VS was still present (Appendix 1), indicating that comorbid anxiety disorder cannot account for our results. Second, to accurately attribute the financial outcome, participants were required to attend to contextual information presented after their performance but before the outcome (Appendix 1, Fig. S1). Although our study was conducted in mildly depressed patients, it is still possible that patients had a reduced ability to discriminate the 2 attribution contexts. We have previously shown in healthy individuals that RTs in trials following SA feedback are longer than in trials following EA feedback.34,54 This post-SA feedback slowing is a behavioural measure indicating that participants are able to differentiate feedback attribution. In the present study, we observed post-SA feedback slowing in patients and controls, strongly indicating that patients were able to accurately discriminate feedback attribution. Third, although the target of learning (i.e., 60% expected reward rate) was identical for both agency conditions (SA and EA were matched), the nature of learning was different. During the processing of SA feedback participants learned whether their actions led to a reward (instrumental learning), whereas during EA feedback participants learned the probability of receiving a reward independent of their actions (noninstrumental learning; Appendix 1). Fourth, imaging studies have also demonstrated a clear dissociation between regions involved in the processing of feedback anticipation and outcome,21,60,61 and differences in the hemodynamic response between depressed patients and healthy controls have been reported during both phases of feedback processing.18 Although this dissociation could not be investigated with our task’s design, a better understanding of the neural network involved in anticipation of controllable and uncontrollable feedback is important to understand altered feedback processing in patients with MDD. Finally, we used an 8 mm smoothing kernel because this is more commonly used in fMRI studies and thus leads to coordinates being more easily comparable with those of previous reports. However, this is likely to bias the spatial localization of ventral striatal responses posteriorly.62
Conclusion
Our results show that in depressed patients the striatal response to feedback was insensitive to feedback attribution, leading to an altered VS response during SA losses – EA losses compared with healthy controls. Our findings suggest an altered assignment of motivational salience to SA losses in patients with MDD. This altered sensitivity to feedback attribution may reinforce the belief of not being in control of negative events. This cycle is compatible with mechanisms leading to learned helplessness and, translated into clinical terms, appears to be relevant for psychotherapy. A combination of mindfulness and cognitive interventions may raise the awareness for self-determined gains and help correct the depressed individual’s bias in processing of feedback.
Acknowledgments
The authors thank Dr. Philipp Stämpfli for his invaluable assistance in study procedures. S. Spinelli is funded by a Swiss National Science Foundation Ambizione Fellowship (grants PZ00P3_126363/1 and PZ00P3_146001/1). J. Chumbley is funded by a grant from FAN of the Zurich University Association (ZUNIV). M. Grosse Holtforth and N. Doerig are funded by a grant from the Swiss National Science Foundation (grant PP00P1-123377/1) and a research grant by the Foundation for Research in Science and the Humanities at the University of Zurich. We acknowledge support by the Clinical Research Priority Program “Molecular Imaging” at the University of Zurich.
Footnotes
Competing interests: None declared.
Contributors: J. Chumbley and S. Spinelli designed the study. J. Späti, N. Doerig and J. Brakowski acquired the data, which J. Chumbley, M. Grosse Holtforth, E. Seifritz and S. Spinelli analyzed. S. Spinelli wrote the article. All authors reviewed and approved it for publication.
References
- 1.Eshel N, Roiser JP. Reward and punishment processing in depression. Biol Psychiatry. 2010;68:118–24. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Pizzagalli DA, Iosifescu D, Hallett LA, et al. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy FC, Sahakian BJ, Rubinsztein JS, et al. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–21. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- 4.Fales CL, Barch DM, Rundle MM, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–84. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas KM, Porter RJ, Frampton CM, et al. Abnormal response to failure in unmedicated major depression. J Affect Disord. 2009;119:92–9. doi: 10.1016/j.jad.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Elliott R, Sahakian BJ, McKay AP, et al. Neuropsychological impairments in unipolar depression: the influence of perceived failure on subsequent performance. Psychol Med. 1996;26:975–89. doi: 10.1017/s0033291700035303. [DOI] [PubMed] [Google Scholar]
- 7.Murphy FC, Michael A, Robbins TW, et al. Neuropsychological impairment in patients with major depressive disorder: the effects of feedback on task performance. Psychol Med. 2003;33:455–67. doi: 10.1017/s0033291702007018. [DOI] [PubMed] [Google Scholar]
- 8.Taylor Tavares JV, Clark L, Furey ML, et al. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage. 2008;42:1118–26. doi: 10.1016/j.neuroimage.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu PH, Deldin PJ. Neural evidence for enhanced error detection in major depressive disorder. Am J Psychiatry. 2007;164:608–16. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- 10.Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch Gen Psychiatry. 2008;65:179–88. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes AJ, Pizzagalli DA. Effects of task-relevant incentives on the electrophysiological correlates of error processing in major depressive disorder. Cogn Affect Behav Neurosci. 2010;10:119–28. doi: 10.3758/CABN.10.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olvet DM, Klein DN, Hajcak G. Depression symptom severity and error-related brain activity. Psychiatry Res. 2010;179:30–7. doi: 10.1016/j.psychres.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Ruchsow M, Herrnberger B, Beschoner P, et al. Error processing in major depressive disorder: evidence from event-related potentials. J Psychiatr Res. 2006;40:37–46. doi: 10.1016/j.jpsychires.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Schrijvers D, de Bruijn ER, Maas Y, et al. Action monitoring in major depressive disorder with psychomotor retardation. Cortex. 2008;44:569–79. doi: 10.1016/j.cortex.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Steele JD, Kumar P, Ebmeier KP. Blunted response to feedback information in depressive illness. Brain. 2007;130:2367–74. doi: 10.1093/brain/awm150. [DOI] [PubMed] [Google Scholar]
- 16.McFarland BR, Klein DN. Emotional reactivity in depression: diminished responsiveness to anticipated reward but not to anticipated punishment or to nonreward or avoidance. Depress Anxiety. 2009;26:117–22. doi: 10.1002/da.20513. [DOI] [PubMed] [Google Scholar]
- 17.Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–27. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pizzagalli DA, Holmes AJ, Dillon DG, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–10. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson OJ, Cools R, Carlisi CO, et al. Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. Am J Psychiatry. 2012;169:152–9. doi: 10.1176/appi.ajp.2011.11010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smoski MJ, Felder J, Bizzell J, et al. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knutson B, Bhanji JP, Cooney RE, et al. Neural responses to monetary incentives in major depression. Biol Psychiatry. 2008;63:686–92. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smoski MJ, Rittenberg A, Dichter GS. Major depressive disorder is characterized by greater reward network activation to monetary than pleasant image rewards. Psychiatry Res. 2011;194:263–70. doi: 10.1016/j.pscychresns.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tucker DM, Luu P, Frishkoff G, et al. Frontolimbic response to negative feedback in clinical depression. J Abnorm Psychol. 2003;112:667–78. doi: 10.1037/0021-843X.112.4.667. [DOI] [PubMed] [Google Scholar]
- 24.Maier SF, Seligman MEP. Learned helplessness: theory and evidence. [accessed 2015 June 15];J Exp Psychol. 1976 105:3–46. Available: http://psycnet.apa.org/index.cfm?fa=buy.optionToBuy&id=1976-20159-001. [Google Scholar]
- 25.Abramson LY, Seligman ME, Teasdale JD. Learned helplessness in humans: critique and reformulation. J Abnorm Psychol. 1978;87:49–74. [PubMed] [Google Scholar]
- 26.Haggard P, Chambon V. Sense of agency. Curr Biol. 2012;22:R390–2. doi: 10.1016/j.cub.2012.02.040. [DOI] [PubMed] [Google Scholar]
- 27.Pryce CR, Azzinnari D, Spinelli S, et al. Helplessness: a systematic translational review of theory and evidence for its relevance to understanding and treating depression. Pharmacol Ther. 2011;132:242–67. doi: 10.1016/j.pharmthera.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Studer B, Apergis-Schoute AM, Robbins TW, et al. What are the odds? The neural correlates of active choice during gambling. Front Neurosci. 2012;6:46. doi: 10.3389/fnins.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ullsperger M, von Cramon DY. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J Neurosci. 2003;23:4308–14. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dayan P, Niv Y. Reinforcement learning: the good, the bad and the ugly. Curr Opin Neurobiol. 2008;18:185–96. doi: 10.1016/j.conb.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Weiner B. The development of an attribution-based theory of motivation: a history of ideas. Educ Psychol. 2010;45:28–36. [Google Scholar]
- 32.Zink CF, Pagnoni G, Martin-Skurski ME, et al. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–17. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]
- 33.Satterthwaite TD, Ruparel K, Loughead J, et al. Being right is its own reward: load and performance related ventral striatum activation to correct responses during a working memory task in youth. Neuroimage. 2012;61:723–9. doi: 10.1016/j.neuroimage.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Späti J, Chumbley J, Brakowski J, et al. Functional lateralization of the anterior insula during feedback processing. Hum Brain Mapp. 2014;35:4428–39. doi: 10.1002/hbm.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 36.Krampen G. Skalen zur Erfassung von Hoffnungslosigkeit (H-Skalen) Deutsche Bearbeitung und Weiterentwicklung der H-Skala von Aaron T. Beck. Göttingen; Hogrefe: 1994. [Google Scholar]
- 37.Laux L, Glanzmann P, Schaffner P, et al. Das State-Trait-Angstinventar. In: Beltz, editor. Theoretische Grundlagen und Handanweisungen. Weinheim: 1981. [Google Scholar]
- 38.Franz M, Lemke MR, Meyer T, et al. German version of the Snaith-Hamilton-Pleasure Scale (SHAPS-D). Anhedonia in schizophrenic and depressive patients. Fortschr Neurol Psychiatr. 1998;66:407–13. doi: 10.1055/s-2007-995279. [DOI] [PubMed] [Google Scholar]
- 39.Beck AT, Steer RA, Ball R, et al. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 40.Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–86. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 41.Helmreich I, Wagner S, Mergl R, et al. The Inventory Of Depressive Symptomatology (IDS-C(28)) is more sensitive to changes in depressive symptomatology than the Hamilton Depression Rating Scale (HAMD(17)) in patients with mild major, minor or subsyndromal depression. Eur Arch Psychiatry Clin Neurosci. 2011;261:357–67. doi: 10.1007/s00406-010-0175-1. [DOI] [PubMed] [Google Scholar]
- 42.Penny WD, Henson L. Analysis of variance. In: Friston K, Ashburner J, Kiebel S, et al., editors. Statistical Parametric Mapping: the analysis of functional brain images. London: Elsevier; 2006. [Google Scholar]
- 43.Friston KJ, Worsley KJ, Frackowiak RS, et al. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:210–20. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 44.Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur J Neurosci. 2012;35:1124–43. doi: 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar P, Waiter G, Ahearn T, et al. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–93. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- 46.Gradin VB, Kumar P, Waiter G, et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134:1751–64. doi: 10.1093/brain/awr059. [DOI] [PubMed] [Google Scholar]
- 47.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–34. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gläscher J, Daw N, Dayan P, et al. States versus rewards: dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron. 2010;66:585–95. doi: 10.1016/j.neuron.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haruno M, Kawato M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. J Neurophysiol. 2006;95:948–59. doi: 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- 50.van Tol MJ, Veer IM, van der Wee NJ, et al. Whole-brain functional connectivity during emotional word classification in medication-free major depressive disorder: abnormal salience circuitry and relations to positive emotionality. Neuroimage Clin. 2013;2:790–6. doi: 10.1016/j.nicl.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chumbley J, Spati J, Dorig N, et al. The BDNF Val66Met polymorphism influence on striatal BOLD response to monetary feedback depends on valence and agency. Neuroscience. 2014;280:130–41. doi: 10.1016/j.neuroscience.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 54.Helfinstein SM, Benson B, Perez-Edgar K, et al. Striatal responses to negative monetary outcomes differ between temperamentally inhibited and non-inhibited adolescents. Neuropsychologia. 2011;49:479–85. doi: 10.1016/j.neuropsychologia.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chronis-Tuscano A, Degnan KA, Pine DS, et al. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. J Am Acad Child Adolesc Psychiatry. 2009;48:928–35. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sen S, Nesse RM, Stoltenberg SF, et al. A BDNF coding variant is associated with the NEO personality inventory domain neuroticism, a risk factor for depression. Neuropsychopharmacology. 2003;28:397–401. doi: 10.1038/sj.npp.1300053. [DOI] [PubMed] [Google Scholar]
- 57.Lang UE, Hellweg R, Kalus P, et al. Association of a functional BDNF polymorphism and anxiety-related personality traits. Psychopharmacology (Berl) 2005;180:95–9. doi: 10.1007/s00213-004-2137-7. [DOI] [PubMed] [Google Scholar]
- 58.Hünnerkopf R, Strobel A, Gutknecht L, et al. Interaction between BDNF Val66Met and dopamine transporter gene variation influences anxiety-related traits. Neuropsychopharmacology. 2007;32:2552–60. doi: 10.1038/sj.npp.1301383. [DOI] [PubMed] [Google Scholar]
- 59.Frustaci A, Pozzi G, Gianfagna F, et al. Meta-analysis of the brain-derived neurotrophic factor gene (BDNF) Val66Met polymorphism in anxiety disorders and anxiety-related personality traits. Neuropsychobiology. 2008;58:163–70. doi: 10.1159/000182892. [DOI] [PubMed] [Google Scholar]
- 60.Kerr DL, McLaren DG, Mathy RM, et al. Controllability modulates the anticipatory response in the human ventromedial prefrontal cortex. Front Psychol. 2012;3:557. doi: 10.3389/fpsyg.2012.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knutson B, Fong GW, Adams CM, et al. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–7. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 62.Sacchet MD, Knutson B. Spatial smoothing systematically biases the localization of reward-related brain activity. Neuroimage. 2013;66:270–7. doi: 10.1016/j.neuroimage.2012.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]