Abstract
Background
Published meta-analyses of resting-state regional cerebral blood flow (rCBF) studies of major depressive disorder (MDD) have included patients receiving antidepressants, which might affect brain activity and thus bias the results. To our knowledge, no meta-analysis has investigated regional homogeneity changes in medication-free patients with MDD. Moreover, an association between regional homogeneity and rCBF has been demonstrated in some brain regions in healthy controls. We sought to explore to what extent resting-state rCBF and regional homogeneity changes co-occur in the depressed brain without the potential confound of medication.
Methods
Using the effect-size signed differential mapping method, we conducted 2 meta-analyses of rCBF and regional homogeneity studies of medication-free patients with MDD.
Results
Our systematic search identified 14 rCBF studies and 9 regional homogeneity studies. We identified conjoint decreases in resting-state rCBF and regional homogeneity in the insula and superior temporal gyrus in medication-free patients with MDD compared with controls. Other changes included altered resting-state rCBF in the precuneus and in the frontal–limbic–thalamic–striatal neural circuit as well as altered regional homogeneity in the uncus and parahippocampal gyrus. Meta-regression revealed that the percentage of female patients with MDD was negatively associated with resting-state rCBF in the right anterior cingulate cortex and that the age of patients with MDD was negatively associated with rCBF in the left insula and with regional homogeneity in the left uncus.
Limitations
The analysis techniques, patient characteristics and clinical variables of the included studies were heterogeneous.
Conclusion
The conjoint alterations of rCBF and regional homogeneity in the insula and superior temporal gyrus may be core neuropathological changes in medication-free patients with MDD and serve as a specific region of interest for further studies on MDD.
Introduction
Major depressive disorder (MDD), one of the most common psychiatric disorders, involves not only profound dysregulation of affect and mood, but also cognitive dysfunction, sleep and appetite disturbance, fatigue, and metabolic, endocrine and inflammatory abnormalities.1 In the United States, 17% of people meet the criteria for MDD at least once in their lives.2 In addition to individual suffering, MDD causes substantial impairments in social and occupational functioning, resulting in a major public health and economic burden. Understanding the neural basis of MDD is therefore important.
Over the past 2 decades functional neuroimaging techniques have provided important insights. Recently, resting-state studies, in which participants are typically asked to rest quietly with their eyes closed for several minutes during acquisition of data, have become a popular tool to measure intrinsic brain activity, independent of particular tasks. Common methods applied in these studies include positron emission tomography (PET), single photon emission computed tomography (SPECT) and the MRI-based techniques of arterial spin labelling (ASL) and regional homogeneity. Positron emission tomography, SPECT and ASL all measure regional cerebral blood flow (rCBF), which reflects brain energy demand–supply dynamics.3 Regional homogeneity is based on the theory that the blood oxygen level–dependent (BOLD) signal fluctuations in a given region reflect local neuronal activity occurring at the same frequency and that this temporal synchrony is characteristic of populations of neurons performing a related function;4 thus, increased regional homogeneity represents greater temporal synchrony, whereas decreased regional homogeneity indicates lower local coherence. Since resting BOLD signals are modulated by rCBF,5 rCBF fluctuations will likely be correlated to the resting BOLD-based regional homogeneity, although this is only 1 possible causal relation between the 2 measurements.
Resting-state abnormalities in the limbic–cortical networks have been frequently observed in patients with MDD in studies of rCBF measured using PET,6,7 SPECT8,9 and ASL10 and in regional homogeneity studies.11–13 However, the detailed results typically differ, presumably as a result of interstudy differences in sample size, the sociodemographic and clinical characteristics of the participants and the technical characteristics of image acquisition and analysis. As the number of published resting-state rCBF and regional homogeneity studies in patients with MDD has grown, attention has turned to meta-analysis as a way to identify common abnormalities. Two previous meta-analyses of PET and SPECT studies found increased rCBF in the medial frontal gyrus and thalamus and decreased rCBF in the pregenual anterior and posterior cingulate, pulvinar nucleus and superior temporal gyrus in patients with MDD compared with controls.14,15 However, these meta-analyses included both patients who were medication-free and patients taking antidepressants at the time of scanning. While there are no prospective randomized controlled trials of the long-term effects of antidepressants on rCBF, there is accumulating evidence that antidepressants can affect rCBF. A serial PET study of patients with MDD treated with fluoxetine reported altered brain glucose metabolism in subcortical, limbic–paralimbic regions and in the neocortex by 1 week and in the anterior cingulate (ACC), dorsolateral prefrontal, ventral frontal and subgenual cingulate cortices by 6 weeks.16 Mixing medicated and medication-free patients with MDD might therefore have biased, or at least complicated, the interpretation of previous meta-analyses. Moreover, to our knowledge, there has been no published meta-analysis of regional homogeneity studies involving medication-free patients with MDD. Now that a large number of resting-state rCBF and regional homogeneity studies of these patients have been reported, the time is right for a specific meta-analysis to isolate the intrinsic abnormalities in patients with MDD, independent of the confounding effects of antidepressant therapy.
It is also timely to consider the association between the 2 kinds of measurement. In separate studies of patients with treatment-refractory depression (TRD) compared with healthy controls, we found decreases in both regional homogeneity17 and rCBF10 in the inferior frontal gyrus of patients with TRD. Two resting-state studies in healthy participants have reported significantly correlated spatial variation in rCBF and regional homogeneity, both measures being relatively high in the insula, superior temporal gyrus, posterior cingulate cortex, medial prefrontal cortex and thalamus18 and the cortical–limbic network.19
We therefore set out to conduct meta-analyses of both rCBF and regional homogeneity studies to explore the extent to which resting-state rCBF and regional homogeneity changes co-occur in medication-free patients with MDD without the confounding effects of antidepressant therapy. As resting-state CBF reflects brain energy demand, relating regional homogeneity to CBF provides a way to understand its physiologic underpinnings in abnormal brain metabolism in patients with MDD. We used a relatively new voxel-based meta-analytic tool (effect size signed differential mapping [ES-SDM]; www.sdmproject.com/), which allows the results of individual studies to be weighted and controlled for several moderator variables, including demographic, clinical and imaging factors. Of relevance to the present application, ES-SDM has been previously applied with success in neuropsychiatric populations.20–22
Methods
Inclusion of studies
We conducted our meta-analysis according to “Preferred reporting items for systematic reviews and meta-analyses“ (PRISMA) guidelines.23 Studies that were peer-reviewed and published between January 1947 and February 2014 were selected from the search results in 8 databases (PubMed, Web of Knowledge, Embase, PsycINFO, MEDLINE, ERIC, CINAHL and Google Scholar) using a systematic search process. Keyword searches used the following terms: 1) “ReHo” < OR > “regional homogeneity” < OR > “CBF” < OR > “cerebral blood flow” < OR > “PET” < OR > “positron emission tomography” < OR > “SPECT” < OR > “single photon emission computed tomography” < OR > “arterial spin labeling” < OR > “ASL” < OR > “neuroimaging”; 2) “rest” < OR > “resting state”; and 3) “depression” < OR > “unipolar depression” < OR > “depressive disorder” < OR > “major depression” < OR > “major depressive disorder” < OR > “depressed.” In addition, we manually checked the reference lists of retrieved articles for additional relevant studies. Studies were selected for inclusion according to the following criteria: 1) they compared resting-state rCBF or regional homogeneity between patients with MDD and healthy controls at the whole brain level, 2) the patients either had never taken antidepressants or underwent a medication washout period before scanning and 3) the studies reported Montreal Neurological Institute (MNI) or Talairach coordinates of the whole brain contrast comparing patients and controls. For studies containing multiple independent patient samples, the appropriate coordinates were included as separate studies. Studies reporting only region of interest (ROI) findings or using seed voxel–based analysis procedures were excluded. We also excluded studies on patients with geriatric depression; depressed adolescents; and patients with psychiatric comorbid disorders, including bipolar disorder, panic disorder and posttraumatic stress disorder. Finally, studies were also excluded if there was sample overlap with other included studies. To minimize the possibility of a biased sample set, we contacted the authors of studies in which the Talairach or MNI coordinates (necessary for the voxel-level quantitative meta-analysis) were not explicitly reported. Two of us (Z.Q.C. and M.Y.D.) independently conducted the literature search. The results were compared, and any inconsistent results were discussed and resolved by consensus.
Quality assessment
We assessed the quality of the included studies using a 10-point checklist that focused on both the clinical and demographic aspects of individual study samples and the imaging-specific methodology (see the Appendix, Table S1, available at jpn.ca). This checklist was based on previous meta-analytic studies.24–26 Although the checklist was not designed as an assessment tool, it provided some objective indication of the rigour of individual studies. At least 2 authors reviewed every paper and independently determined a completeness rating. Any paper for which the 2 ratings disagreed was discussed, and a consensus quality score was obtained.
Voxel-wise meta-analysis
Group differences between patients and controls were analyzed using ES-SDM software, a voxel-based meta-analytic approach. The SDM methods have been described in detail elsewhere.27–30 Briefly, we selected the reported peak coordinates of all functional differences that were statistically significant at the whole brain level. We checked that each included study used the same statistical threshold throughout the whole brain to avoid potential bias toward liberally thresholded regions. Studies reporting no group differences were also included. Second, we recreated peak coordinates for each study with a map of the effect size of group differences. The recreation was based on converting the peak t value to Hedges’ effect size and then applying a non-normalized Gaussian kernel to the voxels near the peak, which assigns higher values to the voxels closer to peaks. In the assignment, a relatively wide full-width at half-maximum (FWHM; 20 mm) was used to control false-positive results.30 Unlike with previous meta-analytic methods, such as activation likelihood estimation31 and multilevel kernel density analysis,32 in the present method, both positive (i.e., increased rCBF) and negative (i.e., decreased rCBF) coordinates were reconstructed in the same map to avoid any voxel erroneously appearing to be positive and negative at the same time.27 Findings from studies reporting no group differences were recreated with effect size and variance maps as in any other study, with the only difference being that all voxels in the effect size group were estimated to have a null effect size; these were included in the meta-analysis as any other effect size, thus modifying the meta-analytic effect size. Third, as in standard meta-analyses, studies were combined with a random-effects model taking into account sample size, intrastudy variability and between-study heterogeneity. The null hypothesis assumed that effect sizes (rather than only peaks) are randomly distributed throughout the brain.30 To optimally balance the sensitivity and specificity, uncorrected for false discovery rate (FDR), we used a p value of 0.005 as the main threshold with an additional peak height of z = 1 and a cluster extent of 10 voxels.30 To assess the robustness of the findings we used jackknife sensitivity analyses, iteratively repeating the analysis excluding 1 data set at a time to establish whether the results could be replicated.27
Analysis of heterogeneity and publication bias
The statistical (between-studies) heterogeneity of individual clusters was examined using a random-effects model with Q statistics (χ2 distribution converted to z values and tested with a permutation approach (p < 0.005, uncorrected for FDR; peak height z = 1; cluster extent = 10 voxels). We examined the possibility of publication bias for brain regions showing conjoint alteration of rCBF and regional homogeneity using the Egger test.25
Meta-regression analysis
We examined the potential effect of several relevant sociodemographic and clinical variables by means of simple linear regression using ES-SDM.30 To minimize the detection of spurious relationships we decreased the probability threshold to 0.0005, required abnormalities to be detected both in the slope and in 1 of the extremes of the regressor, and discarded findings in regions other than those detected in the main analyses.27 Finally, we inspected the regression plots to discard fits driven by too few studies.22
Results
Studies included in the meta-analyses
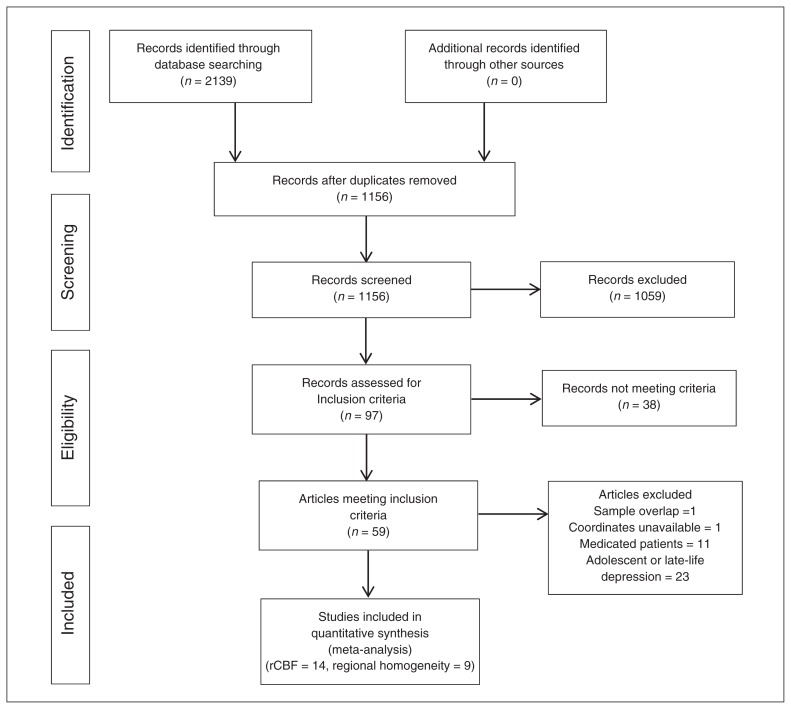
Figure 1 shows the identification and attrition of the studies. Our search yielded 1156 studies. Though we did not apply any language restriction in the literature search, the abstracts of all studies yielded by our search were written in English. If the articles were written in other languages, they were translated into English for assessment. In all, 5 SPECT studies,8,9,33–35 8 PET studies,6,7,36–41 1 ASL study10 and 9 regional homogeneity studies11–13,17,42–46 met our inclusion criteria (Table 1). All these papers were written in English. The ASL study10 and the regional homogeneity study17 were both performed based on 2 subgroups of patients with MDD, namely refractory MDD and nonrefractory MDD, which were compared with the same healthy control group. Each subgroup comparison was included as a data set in the meta-analysis. Since PET, SPECT and ASL all provide measurements of rCBF, 14 rCBF (PET, SPECT and ASL) studies (15 data sets) comparing 336 medication-free patients with MDD and 357 healthy controls were included in the rCBF meta-analysis. Given the different modalities applied in rCBF studies, we also conducted subgroup meta-analyses of non-ASL rCBF studies and of rCBF PET studies; a subgroup meta-analysis of SPECT studies was precluded owing to their small number. Nine regional homogeneity studies (10 data sets) comparing 187 medication-free patients with MDD and 188 healthy controls were included in the regional homogeneity meta-analysis. All patients with MDD were currently depressed and medication-free, having either never taken antidepressants or having undergone a washout period before scanning.
Fig. 1.
Meta-analysis of resting-state rCBF and regional homogeneity studies in medication-free patients with major depressive disorder. Study selection was done according to “Preferred reporting items for systematic reviews and meta-analysis” (PRISMA) guidelines. rCBF = regional cerebral blood flow.
Table 1.
Demographic and clinical characteristics of participants in the 25 functional neuroimaging data sets included in the meta-analyses
| No. (%) female | Mean age, yr | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Study | Imaging | MDD | Control | MDD | Control | Illness duration, mo | HAM-D score | Clinical details | Medication status | Quality score* |
| rCBF | ||||||||||
| Lui et al.10 | ASL | 24 (33%) | 42 (36%) | 35 | 37 | 192 | 22 | Refractory | Drug-naive | 9.5 |
| Lui et al.10 | ASL | 37 (30%) | 42 (36%) | 33 | 37 | 24 | 24 | Nonrefractory | Drug-naive | 9.5 |
| Krausz et al.8 | 99mTc-HMPAO SPECT | 10 (90%) | 10 (90%) | 49 | 50 | NA | NA | Treatment-sensitive | > 3-wk washout | 9.5 |
| Kohn et al.33 | 99mTc-HMPAO SPECT | 33 (58%) | 25 (52%) | 53 | 49 | NA | NA | — | > 2-wk washout | 9.5 |
| Périco et al.34 | 99mTc-ECD SPECT | 15 (80%) | 15 (60%) | 35 | 33 | 29 | 27 | — | > 4-wk washout | 8.5 |
| Skaf et al.35 | 99mTc-ECD SPECT | 9 (44%) | 12 (50%) | 41 | 34 | 127 | 35 | — | > 4-wk washout | 8 |
| Vardi et al.9 | 99mTc-HMPAO SPECT | 37 (57%) | 27 (58%) | 55 | 50 | NA | NA | — | > 2-wk washout | 9.5 |
| Aihara et al.36 | 18F-FDG PET | 24 (63%) | 23 (65%) | 52 | 55 | NA | NA | — | Drug-naive | 10 |
| Brody et al.37 | 18F-FDG PET | 24 (54%) | 16 (50%) | 39 | 36 | 228 | 19 | — | > 2-wk washout | 9.5 |
| Germain et al.39 | 18F-FDG PET | 12 (83%) | 13 (77%) | 38 | 37 | NA | NA | — | > 2-wk washout | 8.5 |
| Kennedy et al.6 | 18F-FDG PET | 13 (0%) | 24 (0%) | 36 | 32 | NA | 22 | — | > 4-wk washout | 9.5 |
| Kimbrell et al.40 | 18F-FDG PET | 38 (66%) | 37 (65%) | 43 | 43 | 322 | NA | — | > 2-wk washout | 9.5 |
| Saxena et al.7 | 18F-FDG PET | 27 (50%) | 17 (50%) | 38 | 33 | NA | 21 | — | > 4-wk washout | 9.5 |
| Drevets et al.38 | H215O PET | 13 (54%) | 33 (61%) | 36 | 30 | NA | NA | MDD plus first-degree relatives | > 3-wk washout | 9.5 |
| Monkul et al.41 | H215O PET | 20 (75%) | 21 (67%) | 37 | 35 | NA | NA | — | > 2-wk washout | 9.5 |
| Regional homogeneity | ||||||||||
| Chen et al.11 | — | 15 (40%) | 15 (47%) | 24 | 24 | 3.3 | 23 | First-episode | Drug-naive | 9.5 |
| Guo et al.42 | — | 24 (50%) | 19 (47%) | 28 | 24 | 30 | 25 | TRD | 1-wk washout | 9.5 |
| Guo et al.43 | — | 22 (46%) | 19 (47%) | 28 | 24 | 3.0 | 26 | Treatment-sensitive, first-episode | Drug-naive | 8.5 |
| Liang et al.44 | — | 16 (50%) | 16 (50%) | 36 | 35 | 28 | 26 | — | > 2-wk washout | 9.5 |
| Liu et al.12 | — | 14 (50%) | 15 (47%) | 29 | 30 | 26 | NA | First-episode | Drug-naive | 8.5 |
| Peng et al.13 | — | 16 (63%) | 16 (63%) | 34 | 34 | 3.1 | NA | First-episode | Drug-naive | 9.5 |
| Wang et al.45 | — | 14 (36%) | 14 (36%) | 33 | 34 | 6 | 26 | First-episode | Drug-naive | 9.5 |
| Wu et al.17 | — | 22 (55%) | 26 (38%) | 35 | 33 | 32 | 23 | Treatment-sensitive | 8-wk washout | 9.5 |
| Wu et al.17 | — | 22 (32%) | 26 (38%) | 35 | 33 | 103 | 22 | TRD | 8-wk washout | 9.5 |
| Yao et al.46 | — | 22 (55%) | 22 (55%) | 28 | 39 | 120 | NA | Single episode, recurrent | > 2-wk washout | 9.5 |
ASL = arterial spin labelling; HAM-D = 17-item Hamilton Rating Scale for Depression; MDD = major depressive disorder; NA = not available; PET = positron emission tomography; rCBF = regional cerebral blood flow; SPECT = single photon emission computed tomography; TRD = treatment-resistant MDD.
Quality score out of 10.
Meta-analysis of rCBF studies
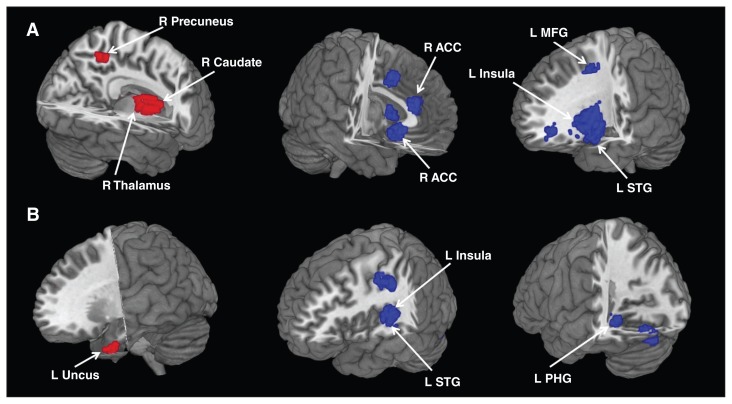
Relative to controls, resting-state rCBF in patients with MDD was increased in the right thalamus, right caudate and right precuneus and decreased in the left middle frontal gyrus, right ACC, left insula and left superior temporal gyrus (Fig. 2A, Table 2). Whole brain jackknife sensitivity analysis revealed these results were highly replicable, and this finding was preserved in all 15 combinations of data sets.
Fig. 2.
Areas of increased (red) and decreased (blue) resting-state brain activity in medication-free patients with major depressive disorder compared with healthy controls in the meta-analyses of (A) regional cerebral blood flow studies and (B) regional homogeneity studies. ACC = anterior cingulate cortex; L = left; MFG = middle frontal gyrus; PHG = parahippocampal gyrus; R = right; STG = superior temporal gyrus.
Table 2.
Regional differences between medication-free patients with MDD and healthy controls in rCBF and regional homogeneity meta-analyses*
| Talairach coordinates | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Study type, region | x | y | z | SDM z score | p value | No. voxels | Cluster breakdown |
| rCBF | |||||||
| MDD > control | |||||||
| R thalamus, ventral anterior nucleus | 12 | −6 | 12 | 1.886 | < 0.001 | 234 | R thalamus, ventral anterior nucleus (92) |
| — | — | — | — | — | — | R thalamus, anterior nucleus (26) | |
| — | — | — | — | — | — | R thalamus, ventral lateral nucleus (27) | |
| — | — | — | — | — | — | R lentiform nucleus, putamen (60) | |
| — | — | — | — | — | — | R lentiform nucleus, lateral globus pallidus (21) | |
| — | — | — | — | — | — | R lentiform nucleus, medial globus pallidus (8) | |
| R caudate | 6 | 2 | 4 | 1.413 | < 0.001 | 201 | R caudate body (193) R caudate head (8) |
| R precuneus | 14 | −54 | 50 | 1.289 | 0.001 | 68 | R precuneus (68) |
| MDD < control | |||||||
| L middle frontal gyrus | −28 | 2 | 46 | −1.327 | 0.001 | 50 | L middle frontal gyrus (50) |
| R anterior cingulate | 6 | 20 | −8 | −1.788 | < 0.001 | 204 | Bilateral anterior cingualte (94) |
| — | — | — | — | — | — | Bilateral subcallosal gyrus (50) | |
| — | — | — | — | — | — | R caudate head (26) | |
| — | — | — | — | — | — | Bilateral medial frontal gyrus (34) | |
| R anterior cingulate | 4 | 30 | 18 | −1.336 | 0.001 | 118 | Bilateral anterior cingulate (107) |
| — | — | — | — | — | — | Bilateral cingulate gyrus (11) | |
| L insula | −44 | 12 | 2 | −1.738 | < 0.001 | 147 | L insula (147) |
| L superior temporal gyrus | −48 | 10 | −4 | −1.754 | < 0.001 | 80 | L superior temporal gyrus (80) |
| Regional homogeneity | |||||||
| MDD > control | |||||||
| L uncus | −24 | −2 | −38 | 1.286 | 0.002 | 28 | L uncus (28) |
| MDD < control | |||||||
| L insula | −44 | −26 | 14 | −1.816 | < 0.001 | 99 | L insula (43) |
| — | — | — | — | — | — | L transvers temporal gyrus (45) | |
| — | — | — | — | — | — | L postcentral gyrus (11) | |
| L superior temporal gyrus | −50 | −30 | 4 | −1.436 | 0.004 | 86 | L superior temporal gyrus (86) |
| L parahippocampal gyrus | −28 | −42 | −10 | −1.634 | 0.001 | 73 | L parahippocampal gyrus (43) |
| — | — | — | — | — | — | L culmen (24) | |
| — | — | — | — | — | — | L fusiform gyrus (6) | |
FWHM = full width at half-maximum; L = left; MDD = major depressive disorder; R = right; rCBF = regional cerebral blood flow; SDM = signed differential mapping.
Regions identified by meta-analyses of coordinates from 15 rCBF data sets and 10 regional homogeneity data sets separately (voxelwise p < 0.005 and FWHM 20 mm).
A subgroup meta-analysis of non-ASL rCBF studies (8 PET and 5 SPECT studies) included 13 data sets comparing 275 medication-free patients with MDD and 273 healthy controls. Relative to controls, resting-state rCBF in patients with MDD was increased in the right thalamus and right caudate and decreased in the right ACC, left insula and left superior temporal gyrus (Appendix, Table S2). Whole brain jackknife sensitivity analysis revealed these results were highly replicable, and this finding was preserved in all 13 combinations of data sets.
The subgroup meta-analysis of rCBF PET studies included 8 data sets comparing 171 medication-free patients with MDD and 184 healthy controls. Relative to controls, resting-state rCBF in patients with MDD was increased in the right thalamus and right caudate and decreased in the left middle frontal gyrus and right ACC (Appendix, Table S2). Whole brain jackknife sensitivity analysis revealed these results were highly replicable, and this finding was preserved in all 8 combinations of data sets.
Meta-analysis of regional homogeneity studies
Relative to controls, resting-state regional homogeneity in patients with MDD was increased in the left uncus (temporal pole) and decreased in the left insula, left superior temporal gyrus and left parahippocampal gyrus (Fig. 2B, Table 2). Whole brain jackknife sensitivity analysis revealed these results were highly replicable, and this finding was preserved in all 10 combinations of data sets.
Analysis of heterogeneity and publication bias
Analysis of heterogeneity revealed that a number of regions with altered rCBF (thalamus, caudate, insula, cingulate gyrus, middle and superior frontal gyrus) or regional homogeneity (middle and superior frontal gyrus, cerebellum, angular gyrus and superior temporal gyrus) had significant statistical heterogeneity among studies (p < 0.005; Appendix, Table S3). Analysis of publication bias revealed that the Egger test was not significant for the left insula (p = 0.80) and left superior temporal gyrus (p = 0.65) in the rCBF meta-analysis and for the left insula (p = 0.16) in the regional homogeneity meta-analysis; the Egger test was significant in the left superior temporal gyrus (p = 0.040) in the regional homogeneity meta-analysis.
Meta-regression
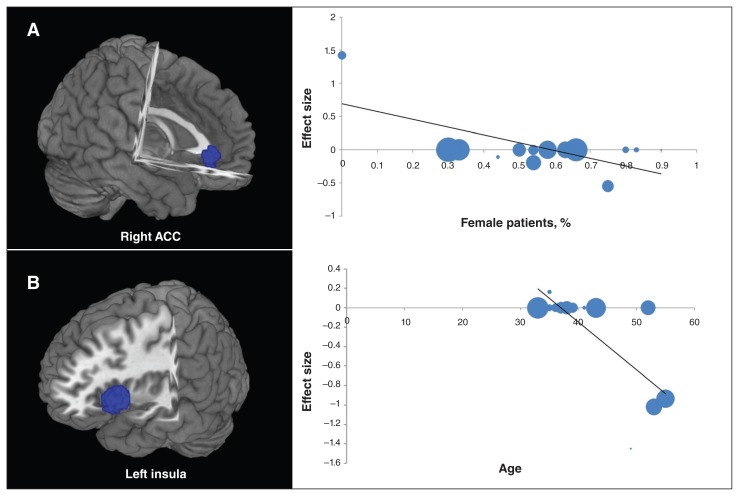
Variables explored by regression are the percentage of female patients, mean age and illness duration. Meta-regression analyses of rCBF studies showed that the percentage of medication-free women with MDD (available in all rCBF studies) was negatively associated with resting-state rCBF in the right ACC (Fig. 3A), with predicted rCBF decrease in studies with a greater proportion of female patients. The analyses also showed that the age of medication-free patients with MDD (available in all rCBF studies) was negatively associated with resting-state rCBF in the left insula (Fig. 3B), with predicted rCBF decrease in studies with a greater proportion of older patients. The 17-item Hamilton Rating Scale for Depression (HAM-D) score and illness duration in rCBF studies could not be examined because data were available for fewer than 9 studies.27 Meta-regression analyses of regional homogeneity studies showed that the age of medication-free patients with MDD (available in all regional homogeneity studies) was negatively associated with resting-state regional homogeneity in the left uncus, with predicted regional homogeneity increase in studies with a greater proportion of younger patients. No effect of the percentage of female patients and illness duration (available in all regional homogeneity studies) was detected. The 17-item HAM-D score in regional homogeneity studies could not be examined owing to the insufficient data.
Fig. 3.
Results of the meta-regression analyses of regional cerebral blood flow (rCBF) studies for resting-state rCBF against the percentage of female patients and age of medication-free patients with major depressive disorder. (A) The percentage of female patients was negatively associated with resting-state rCBF in the right anterior cingulate cortex (ACC). (B) Mean age was negatively associated with resting-state rCBF in the left insula. Each study is represented as a dot, with larger dots symbolizing larger sample sizes. The regression line (meta-regression signed differential mapping slope) is presented as a straight line.
Discussion
To our knowledge this is the first whole brain voxel-wise meta-analysis evaluating resting-state brain rCBF and local synchrony in patients with MDD compared with healthy controls without the potentially confounding interference of antidepressant therapy. We identified conjoint decreases in resting-state rCBF and regional homogeneity in the insula and superior temporal gyrus in medication-free patients with MDD compared with controls. Other differences include altered resting-state rCBF in the precuneus and frontal–limbic–thalamic–striatal neural circuit as well as altered regional homogeneity in the uncus (temporal pole) and parahippocampal gyrus. Furthermore, the percentage of female patients was negatively associated with rCBF in the right ACC, and the age of the patients was negatively associated with rCBF in the left insula and with regional homogeneity in the left uncus. There was no detectable effect of the percentage of female patients and illness duration on resting-state regional homogeneity in medication-free patients with MDD.
Findings in the rCBF meta-analysis
The pooled analyses and the 2 subgroup meta-analyses of rCBF studies all revealed increased resting-state rCBF in the right thalamus and right caudate and decreased resting-state rCBF in the right ACC in medication-free patients with MDD relative to controls. The thalamus receives the majority of incoming sensory information and relays it to the appropriate part of the cerebral cortex, which directs high-level functions, such as speech, behavioural reactions, movement, thinking and learning.47 The ACC plays an role in cognitive and affective regulation, involving attention, problem solving, error detection, motivation, decision making and social behaviour.48,49 It is therefore tempting to link the altered thalamus and ACC resting-state rCBF in patients with MDD to their deficits in these functions. This link is supported by the reported association between changes of resting-state brain glucose metabolism in the thalamus and ACC and successful antidepressant treatment in patients with MDD.6,37 There is evidence that altered resting-state rCBF in these patients may be related to changes of the neuron number in these regions.50
Findings in the regional homogeneity meta-analysis
The regional homogeneity meta-analysis revealed increased regional homogeneity in the left uncus (temporal pole) and decreased regional homogeneity in the left insula, left superior temporal gyrus and left parahippocampal gyrus. Such findings indicate an abnormal degree of functional similarity in these regions with regard to neural activation.4 The local synchrony indexed by regional homogeneity makes a unique contribution to the baseline BOLD fluctuations underlying fMRI connectivity.21 While an agreed-upon systems-level interpretation of regional homogeneity or local synchrony changes remains elusive, developmental studies have shown that a reduction in local synchrony (or segregation) occurs alongside an increase in distributed connectivity (integration) during late neurodevelopment.20 Thus, altered resting-state local synchrony in these regions accompanied by distributed connectivity changes with distant hubs may reflect a substrate of the onset of depression. Notably, most patients in the regional homogeneity studies included in this meta-analysis were experiencing a first episode and were medication-naive, suggesting that our findings were local synchrony alterations in the early stage of MDD and may be a trait for the disorder (i.e., directly related to MDD).
Conjoint findings between the rCBF and regional homogeneity meta-analyses
The rCBF and regional homogeneity meta-analyses both revealed decreased resting-state rCBF and regional homogeneity in the left insula and left superior temporal gyrus in medication-free patients with MDD compared with controls. Since resting BOLD signals are modulated by rCBF,5 rCBF fluctuations will likely be correlated to the resting BOLD-based regional homogeneity though an agreed-upon interpretation of regional homogeneity changes remains elusive. That this may be the mechanism of the conjoint alteration of rCBF and regional homogeneity in the left insula and left superior temporal gyrus is supported by a resting-state study in healthy participants that defined the insula, superior temporal gyrus, posterior cingulate cortex, medial prefrontal cortex and thalamus as ROIs; these regions showed higher rCBF and regional homogeneity in close spatial correlation.18 The insula is uniquely situated at the interface of the cognitive, homeostatic and affective systems, linking stimulus-driven processing and regions that are involved in monitoring the internal environment51 and in interoceptive awareness, high-level cognitive control and attentional processes,52 some of which are impaired in patients with MDD. The insula has been reported to show decreased activation during affective switching and cognitive control demands53 and decreased grey matter volume54 in patients with MDD compared with controls. These functional and structural changes in the insula may contribute to the cognitive and affective deficits in patients with MDD. One explanation of our conjoint rCBF and regional homogeneity findings in the insula and superior temporal gyrus is that decreased rCBF has caused decreased regional homogeneity. However, regions with lower regional homogeneity are not always accompanied by decreased rCBF (as in the left parahippocampal gyrus), which suggests that the alterations in regional homogeneity cannot be attributed solely to changes in rCBF. Notably, the rCBF studies included mainly Western samples, whereas regional homogeneity studies mainly included Eastern China samples; this difference may have complicated our exploration of the association between regional homogeneity and rCBF.
Influence of sex: rCBF in the right ACC
Meta-regression analysis revealed that rCBF in the right ACC (decreased overall in patients with MDD compared with controls) was negatively associated with the percentage of female patients. This disorder is more prevalent in women than in men,2 and it is widely accepted that women generally respond more expressively to emotional stimuli and feel more emotion than men.55 This sex difference may have a neurophysiological basis. A meta-analysis reported greater ACC activation in women than men responding to negative emotional stimuli.56 Enhanced response to negative emotional stimuli may contribute to the greater prevalence of depression and anxiety disorders in women.57 Moreover, there is a consensus that serotonin is a key neurotransmitter associated with mood regulation, and alterations in serotonin neurotransmission are implicated in the pathogenesis of MDD.58 A PET study has reported that medication-free women with MDD had higher serotonin synthesis in the ACC than men.59 Thus structural and physiologic differences of the ACC may contribute to the sex differences in patients with MDD.
Influence of age: rCBF in the left insula
Meta-regression analysis of rCBF studies revealed that resting-state rCBF in the left insula (decreased overall in patients compared with controls) was negatively associated with the mean age of patients. Consistent with this finding, a longitudinal PET study reported decreased rCBF in the insula in healthy elderly people at rest and during verbal and figural recognition tasks.60 In healthy adults a cross-sectional study found that grey matter volume in the insula negatively correlated with age,61 and a longitudinal MRI study found that the bilateral insula showed accelerated grey matter volume loss with age.62 A longitudinal study of depression that directly investigates the association between age and rCBF in the insula would clearly be of interest.
Limitations
The present study had some limitations. First, our voxel-wise meta-analyses were based on the coordinates from published studies instead of raw statistical brain maps, which limits accuracy.63 Second, we included studies in which the patients experienced a medication washout period before scanning, so longer-term influences of medication on brain function could not be completely excluded; however, a recent study reported no detectable differences in resting-state brain activity between medication-naive and medication-washout patients with depression as long as both groups were currently unmedicated and acutely depressed.64 Third, in our meta-analyses the rCBF studies included mainly Western samples, whereas regional homogeneity studies mainly included Eastern China samples. Furthermore, the rCBF meta-regression results may be applicable only to Western populations, whereas regional homogeneity meta-regression results may be applicable only to Eastern China populations. Fourth, the heterogeneity analysis showed that a number of regions with altered rCBF or regional homogeneity had significant statistical heterogeneity among studies. To examine the moderator variables contributing to heterogeneity of the findings, we conducted meta-regression analyses and subgroup analyses.27 Fifth, the effect of age on the resting-state rCBF in the insula analyzed by meta-regression should be interpreted with caution, as it was driven by only a few studies. Finally, our inclusion of data acquired and analyzed using 4 different functional neuroimaging modalities may have decreased the sensitivity of the analysis. As more resting-state studies on medication-naive patients with MDD are published, a modality-specific meta-analysis will be able to address the pathophysiological questions more directly.
Conclusion
Using the ES-SDM method, we identified conjoint decreased resting-state rCBF and regional homogeneity in the insula and superior temporal gyrus in medication-free patients with MDD, avoiding the potentially confounding influence of antidepressant therapy. The conjoint alterations of rCBF and local synchrony in the insula and superior temporal gyrus may be core neuropathological changes in MDD and serve as a specific ROI for further study on MDD. Other changes include altered resting-state rCBF in the precuneus and frontal–limbic–thalamic–striatal neural circuit as well as altered regional homogeneity in the uncus (temporal pole) and parahippocampal gyrus. The altered rCBF and local synchrony in these brain regions may be related to impaired cognitive and affective function in depression. Furthermore, meta-regression analyses showed that the percentage of female patients was negatively associated with rCBF in the right ACC, and the age of the patients was negatively associated with rCBF in the left insula and with regional homogeneity in the left uncus — all findings that merit further investigation.
Acknowledgments
This study was supported by the National Natural Science Foundation (grants # 81227002 and 81220108013) and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, grant # IRT1272) of China. Q. Gong acknowledges support from his Changjiang Scholar Professorship Award (#T2014190) of China and the CMB Distinguished Professorship Award (# F510000/G16916411) adnibistered by the Institute of International Education, USA.
Footnotes
Competing interests: None declared.
Contributors: Z. Chen, M. Du and Q. Gong designed the study. Z. Chen, M. Du, Y. Zhao, J. Li and S. Lui acquired the data, which Z. Chen, M. Du, X. Huang, J. Hu, H. Sun, J. Liu, G. Kemp and Q. Gong analyzed. Z. CHen and M. Du wrote the article, which all authors reviewed and approved for publication.
References
- 1.Fitzgerald PJ. Gray colored glasses: Is major depression partially a sensory perceptual disorder? J Affect Disord. 2013;151:418–22. doi: 10.1016/j.jad.2013.06.045. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wintermark M, Sesay M, Barbier E, et al. Comparative overview of brain perfusion imaging techniques. J Neuroradiol. 2005;32:294–314. doi: 10.1016/s0150-9861(05)83159-1. [DOI] [PubMed] [Google Scholar]
- 4.Zang Y, Jiang T, Lu Y, et al. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 5.Kannurpatti SS, Biswal BB, Kim YR, et al. Spatio-temporal characteristics of low-frequency BOLD signal fluctuations in isoflurane-anesthetized rat brain. Neuroimage. 2008;40:1738–47. doi: 10.1016/j.neuroimage.2007.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy SH, Evans KR, Kruger S, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- 7.Saxena S, Brody AL, Ho ML, et al. Cerebral metabolism in major depression and obsessive-compulsive disorder occurring separately and concurrently. Biol Psychiatry. 2001;50:159–70. doi: 10.1016/s0006-3223(01)01123-4. [DOI] [PubMed] [Google Scholar]
- 8.Krausz Y, Freedman N, Lester H, et al. Brain SPECT study of common ground between hypothyroidism and depression. Int J Neuropsychopharmacol. 2007;10:99–106. doi: 10.1017/S1461145706006481. [DOI] [PubMed] [Google Scholar]
- 9.Vardi N, Freedman N, Lester H, et al. Hyperintensities on T2-weighted images in the basal ganglia of patients with major depression: cerebral perfusion and clinical implications. Psychiatry Res. 2011;192:125–30. doi: 10.1016/j.pscychresns.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Lui S, Parkes LM, Huang X, et al. Depressive disorders: focally altered cerebral perfusion measured with arterial spin-labeling MR imaging. Radiology. 2009;251:476–84. doi: 10.1148/radiol.2512081548. [DOI] [PubMed] [Google Scholar]
- 11.Chen JD, Liu F, Xun GL, et al. Early and late onset, first-episode, treatment-naive depression: same clinical symptoms, different regional neural activities. J Affect Disord. 2012;143:56–63. doi: 10.1016/j.jad.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Xu C, Xu Y, et al. Decreased regional homogeneity in insula and cerebellum: a resting-state fMRI study in patients with major depression and subjects at high risk for major depression. Psychiatry Res. 2010;182:211–5. doi: 10.1016/j.pscychresns.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Peng DH, Jiang KD, Fang YR, et al. Decreased regional homogeneity in major depression as revealed by resting-state functional magnetic resonance imaging. Chin Med J (Engl) 2011;124:369–73. [PubMed] [Google Scholar]
- 14.Fitzgerald PB, Laird AR, Maller J, et al. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–95. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton JP, Etkin A, Furman DJ, et al. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–43. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 17.Wu QZ, Li DM, Kuang WH, et al. Abnormal regional spontaneous neural activity in treatment-refractory depression revealed by resting-state fMRI. Hum Brain Mapp. 2011;32:1290–9. doi: 10.1002/hbm.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou Q, Wu CW, Stein EA, et al. Static and dynamic characteristics of cerebral blood flow during the resting state. Neuroimage. 2009;48:515–24. doi: 10.1016/j.neuroimage.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Zhu Y, Childress AR, et al. Relations between BOLD fMRI-derived resting brain activity and cerebral blood flow. PLoS ONE. 2012;7:e44556. doi: 10.1371/journal.pone.0044556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Pan P, Huang R, et al. A meta-analysis of voxel-based morphometry studies of white matter volume alterations in Alzheimer’s disease. Neurosci Biobehav Rev. 2012;36:757–63. doi: 10.1016/j.neubiorev.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Nortje G, Stein DJ, Radua J, et al. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J Affect Disord. 2013;150:192–200. doi: 10.1016/j.jad.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 22.Radua J, Borgwardt S, Crescini A, et al. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav Rev. 2012;36:2325–33. doi: 10.1016/j.neubiorev.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du M, Liu J, Chen Z, et al. Brain grey matter volume alterations in late-life depression. J Psychiatry Neurosci. 2014;39:397–406. doi: 10.1503/jpn.130275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shepherd AM, Matheson SL, Laurens KR, et al. Systematic meta-analysis of insula volume in schizophrenia. Biol Psychiatry. 2012;72:775–84. doi: 10.1016/j.biopsych.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Brambilla P, Hardan A, di Nemi SU, et al. Brain anatomy and development in autism: review of structural MRI studies. Brain Res Bull. 2003;61:557–69. doi: 10.1016/j.brainresbull.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira LK, Busatto GF. Heterogeneity of coordinate-based meta-analyses of neuroimaging data: an example from studies in OCD. Br J Psychiatry. 2010;197:76–7. doi: 10.1192/bjp.197.1.76a. [DOI] [PubMed] [Google Scholar]
- 29.Radua J, Via E, Catani M, et al. Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychol Med. 2011;41:1539–50. doi: 10.1017/S0033291710002187. [DOI] [PubMed] [Google Scholar]
- 30.Radua J, Mataix-Cols D, Phillips ML, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. 2012;27:605–11. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Laird AR, Fox PM, Price CJ, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–64. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wager TD, Lindquist MA, Nichols TE, et al. Evaluating the consistency and specificity of neuroimaging data using meta-analysis. Neuroimage. 2009;45(Suppl):S210–21. doi: 10.1016/j.neuroimage.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohn Y, Freedman N, Lester H, et al. 99mTc-HMPAO SPECT study of cerebral perfusion after treatment with medication and electroconvulsive therapy in major depression. J Nucl Med. 2007;48:1273–8. doi: 10.2967/jnumed.106.039354. [DOI] [PubMed] [Google Scholar]
- 34.Périco CA, Skaf CR, Yamada A, et al. Relationship between regional cerebral blood flow and separate symptom clusters of major depression: a single photon emission computed tomography study using statistical parametric mapping. Neurosci Lett. 2005;384:265–70. doi: 10.1016/j.neulet.2005.04.088. [DOI] [PubMed] [Google Scholar]
- 35.Skaf CR, Yamada A, Garrido GE, et al. Psychotic symptoms in major depressive disorder are associated with reduced regional cerebral blood flow in the subgenual anterior cingulate cortex: a voxel-based single photon emission computed tomography (SPECT) study. J Affect Disord. 2002;68:295–305. doi: 10.1016/s0165-0327(00)00365-7. [DOI] [PubMed] [Google Scholar]
- 36.Aihara M, Ida I, Yuuki N, et al. HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry Res. 2007;155:245–56. doi: 10.1016/j.pscychresns.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Brody AL, Saxena S, Stoessel P, et al. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Arch Gen Psychiatry. 2001;58:631–40. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- 38.Drevets WC, Videen TO, Price JL, et al. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–41. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Germain A, Nofzinger EA, Meltzer CC, et al. Diurnal variation in regional brain glucose metabolism in depression. Biol Psychiatry. 2007;62:438–45. doi: 10.1016/j.biopsych.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimbrell TA, Ketter TA, George MS, et al. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biol Psychiatry. 2002;51:237–52. doi: 10.1016/s0006-3223(01)01216-1. [DOI] [PubMed] [Google Scholar]
- 41.Monkul ES, Silva LA, Narayana S, et al. Abnormal resting state corticolimbic blood flow in depressed unmedicated patients with major depression: a (15)O-H(2)O PET study. Hum Brain Mapp. 2012;33:272–9. doi: 10.1002/hbm.21212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo WB, Sun XL, Liu L, et al. Disrupted regional homogeneity in treatment-resistant depression: a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1297–302. doi: 10.1016/j.pnpbp.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Guo WB, Liu F, Chen JD, et al. Abnormal neural activity of brain regions in treatment-resistant and treatment-sensitive major depressive disorder: a resting-state fMRI study. J Psychiatr Res. 2012;46:1366–73. doi: 10.1016/j.jpsychires.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Liang MJ, Zhou Q, Yang KR, et al. Identify changes of brain regional homogeneity in bipolar disorder and unipolar depression using resting-state FMRI. PLoS ONE. 2013;8:e79999. doi: 10.1371/journal.pone.0079999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Li K, Zhang Q, et al. Short-term effects of escitalopram on regional brain function in first-episode drug-naive patients with major depressive disorder assessed by resting-state functional magnetic resonance imaging. Psychol Med. 2014;44:1417–26. doi: 10.1017/S0033291713002031. [DOI] [PubMed] [Google Scholar]
- 46.Yao Z, Wang L, Lu Q, et al. Regional homogeneity in depression and its relationship with separate depressive symptom clusters: a resting-state fMRI study. J Affect Disord. 2009;115:430–8. doi: 10.1016/j.jad.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 47.Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst. 2002;18:386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- 48.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 49.Rushworth MF, Behrens TE, Rudebeck PH, et al. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci. 2007;11:168–76. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Young KA, Holcomb LA, Yazdani U, et al. Elevated neuron number in the limbic thalamus in major depression. Am J Psychiatry. 2004;161:1270–7. doi: 10.1176/appi.ajp.161.7.1270. [DOI] [PubMed] [Google Scholar]
- 51.Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 52.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diener C, Kuehner C, Brusniak W, et al. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage. 2012;61:677–85. doi: 10.1016/j.neuroimage.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Lee HY, Tae WS, Yoon HK, et al. Demonstration of decreased gray matter concentration in the midbrain encompassing the dorsal raphe nucleus and the limbic subcortical regions in major depressive disorder: an optimized voxel-based morphometry study. J Affect Disord. 2011;133:128–36. doi: 10.1016/j.jad.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Shields SA. Speaking from the heart: gender and the social meaning of emotion. Cambridge University Press; 2002. [Google Scholar]
- 56.Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50:1578–93. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 57.Lithari C, Frantzidis CA, Papadelis C, et al. Are females more responsive to emotional stimuli? A neurophysiological study across arousal and valence dimensions. Brain Topogr. 2010;23:27–40. doi: 10.1007/s10548-009-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maes M, Meltzer H. The serotonin hypothesis of major depression. Psychopharmacology (Berl) 1995;10:933–4. [Google Scholar]
- 59.Frey BN, Skelin I, Sakai Y, et al. Gender differences in alpha-[(11)C]MTrp brain trapping, an index of serotonin synthesis, in medication-free individuals with major depressive disorder: a positron emission tomography study. Psychiatry Res. 2010;183:157–66. doi: 10.1016/j.pscychresns.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Beason-Held LL, Kraut MA, Resnick SMI. Longitudinal changes in aging brain function. Neurobiol Aging. 2008;29:483–96. doi: 10.1016/j.neurobiolaging.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Resnick SM, Goldszal AF, Davatzikos C, et al. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10:464–72. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- 62.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 63.Salimi-Khorshidi G, Smith SM, Keltner JR, et al. Meta-analysis of neuroimaging data: a comparison of image-based and coordinate-based pooling of studies. Neuroimage. 2009;45:810–23. doi: 10.1016/j.neuroimage.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 64.Fountoulakis KN, Gonda X, Andreoulakis E, et al. No differences between drug naive and drug experienced unipolar depressed patients in terms of neurobiological testing: a cross sectional study. J Psychiatr Res. 2013;47:1984–90. doi: 10.1016/j.jpsychires.2013.09.004. [DOI] [PubMed] [Google Scholar]