Abstract
Background
Abnormal regional cerebral blood flow (rCBF) and grey matter volume have been frequently reported in patients with major depressive disorder (MDD). However, it is unclear to what extent structural and functional change co-occurs in patients with MDD and whether markers of neural activity, such as rCBF, can be predicted by structural change.
Methods
Using MRI, we investigated resting-state rCBF and brain structure in patients with MDD and healthy controls between July 2008 and January 2013. We acquired perfusion images obtained with continuous arterial spin labelling, used voxel-based morphometry to assess grey matter volume and integrated biological parametric mapping analyses to investigate the impact of brain atrophy on rCBF.
Results
We included 43 patients and 29 controls in our study. Frontotemporal grey matter volume was reduced in patients compared with controls. In patients, rCBF was reduced in the anterior cingulate and bilateral parahippocampal areas and increased in frontoparietal and striatal regions. These abnormalities were confirmed by analyses with brain volume as a covariate. In patients with MDD there were significant negative correlations between the extent of depressive symptoms and bilateral parahippocampal rCBF. We found a positive correlation between depressive symptoms and rCBF for right middle frontal cortical blood flow.
Limitations
Medication use in patients has to be considered as a limitation of our study.
Conclusion
Our data suggest that while changes of cerebral blood flow and brain volume co-occur in patients with MDD, structural change is not sufficient to explain altered neural activity in patients at rest. Abnormal brain structure and function in patients with MDD appear to reflect distinct levels of neuropathology.
Introduction
In the past 2 decades, functional and structural neuroimaging techniques have been developing rapidly, yielding a large body of research addressing neural correlates of major depressive disorder (MDD). Neuroimaging has substantially shaped our understanding of depressive etiopathology, indicating its potential to become an objective tool of diagnostic, therapeutic and prognostic value.1 In MDD, first neuroimaging studies examined cerebral metabolism by means of positron emission tomography (PET). Predominantly left-sided decreased regional cerebral blood flow (rCBF) in the dorsolateral prefrontal cortex (DLPFC), medial prefrontal cortex (MPFC) and anterior cingulate cortex (ACC) were found.2,3 Comparing periods of acute illness with periods of recovery, remission of depressive symptoms was associated with a significant increase in rCBF in the left DLPFC cortical midline regions, such as the MPFC and ACC, suggesting state-relatedness of metabolic alterations.4 Pretreatment metabolism in the rostral (pregenual) part of the ACC was reported to predict positive treatment response,5–7 whereas hypermetabolism in the subcallosal part of the ACC was related to treatment resistance.8–11 Different treatment modalities have been shown to target distinct regions of the brain in patients with MDD. For instance, cognitive behavioural therapy was found to be associated with increased metabolism in the hippocampus and dorsal ACC together with metabolic decreases in prefrontal areas. In contrast, pharmacotherapy with paroxetine was related to prefrontal metabolism increases and subcortical decreases.12 Based on a rapidly growing body of neuroimaging evidence, specific neurobiological models of MDD have been put forward. Current concepts emphasize the pathophysiological relevance of networks involved in mood regulation, such as corticolimbic–prefrontal–amygdalar–pallidostriatal–mediothalamic pathways and amygdalar–subcallosal ACC circuits.13,14 Complementing neuroimaging data, genome-wide association studies have identified a number of candidate loci contributing to MDD susceptibility,15 such as the serotonin (5-HT) transporter gene, the brain-derived neurotrophic factor (BDNF) gene,16 the neuronal calcium channel CaV1.2 (CACNA1C) gene or the presynaptic active zone protein Piccolo (PCLO) gene.17 Genetic variation likely defines intermediate phenotypes presenting with aberrant brain function, as reflected by activity changes of a mood-regulating circuit including prefrontal regions, the ACC and basal ganglia.16
In patients with MDD both brain function and structure have been found to be abnormal. Structural MRI studies indicate widespread cortical and subcortical loss of brain volume involving prefrontal areas, particularly orbitofrontal, dorsolateral and dorsomedial regions, together with volume reductions of the ACC, hippocampus and striatum.18–24 These regions have also been found to exhibit aberrant activity at rest and during cognitive or emotional challenge,25 suggesting considerable spatial overlap between regions exhibiting both abnormal brain structure and function. Considering both MRI and PET data, a recent meta-analysis suggested that regional volume loss and regions exhibiting metabolic changes in patients with MDD partly overlap.26 At present, there is still considerable uncertainty whether abnormal neural activity is coupled to regionally confined brain volume loss or whether distinct MRI modalities may provide complementary and essentially nonredundant information on neural dysfunction in patients with MDD. For instance, it is possible that regional volume loss substantially affects levels of neural activity; in this case functional alterations in patients with MDD may be regarded as epiphenomena. Alternatively, abnormal brain volume and aberrant neural activity could reflect distinct levels of neuropathology, each differentially contributing to symptom expression during the course of the disorder. Although multimodal neuroimaging together with analysis techniques that directly integrate structural data and functional data have been shown to be suitable to address such issues,27–29 studies investigating both brain structure and neural activity in patients with MDD are scarce.
In the present study, we investigated brain structure and resting-state rCBF in patients with MDD and healthy controls using high-resolution structural data and MRI perfusion images obtained with continuous arterial spin labelling [CASL].30 This method uses magnetically labelled arterial blood water to trace and quantify regional changes in CBF as a surrogate measure reflecting underlying neural activity.30 It allows safe, economical and noninvasive repeated measurements with high stability over time.31,32 The specific aims of the present study were three-fold. First, under naturalistic treatment conditions, we were interested in identifying rCBF changes at rest in a clinically well-characterized and relatively large sample of adult patients with MDD. Second, to account for possible effects of brain volume change in the patient group, we integrated high-resolution structural data with the CBF data analyses for patients and controls, correcting for regional brain atrophy across the whole brain. Third, in patients with MDD we explored the association between rCBF and clinical features, such as the severity of depressive symptoms and the duration of illness, including the number of depressive episodes. Based on cerebral perfusion data in adolescent,33 adult34–36 and late-life depression37 and on PET findings reporting metabolic changes in patients with MDD, we predicted that patients would exhibit abnormal lateral prefrontal, anterior cingulate and medial temporal rCBF compared with controls. Given that regions exhibiting abnormal neural activity and aberrant brain volume in patients with MDD may spatially overlap, we expected that correction for brain atrophy would also affect rCBF data. Considering the extant data,26 we specifically expected to find significant associations between prefrontal volume loss and rCBF. In contrast, we expected to find ACC and basal ganglia blood flow to be unrelated to the extent of regional volume.
Methods
Participants
We recruited patients with MDD at the Department of Psychiatry and Psychotherapy III, University of Ulm, Ulm, Germany. None of the patients was recruited through study-specific advertising. Medication use did not preclude patients from participating in the study. All patients were electroconvulsive therapy (ECT)–naive at the time of scanning. No patient fulfilled criteria for treatment-resistant depression.38,39 Exclusion criteria were history or presence of any medical or neurologic disorder, drug or alcohol abuse and previous head trauma with loss of consciousness.
Healthy controls were recruited from the general community via personal communication. Individuals with a past or current diagnosis of any psychiatric disorder; drug or alcohol abuse or dependence, as documented using the Structured Clinical Interview for DSM-IV (SCID); drug treatment other than oral contraception; or a positive family history of neurologic diseases and mental disorders were not eligible for participation in the control group.
We assessed handedness using the Edinburgh Inventory.40 Diagnostic assessments using the German versions of the Structured Clinical Interview for DSM axis I and II disorders (SCID) were performed by trained raters. In addition to a detailed interview conducted by an experienced clinical psychiatrist involved in the study (N.D.W., R.C.W., N.V., Z.S.V, B.J.C), case notes were reviewed to corroborate a definitive diagnosis of MDD and exclude comorbid axis I or II disorders. Depression severity was evaluated using the Beck Depression Inventory41 (BDI) and the 21-item Hamilton Rating Scale for Depression42 (HAMD-21).
All participants were recruited for MRI scanning exclusively under resting-state conditions (i.e., no other experimental tasks were involved). The local ethics committee (Ulm University, Germany) approved our study protocol, and we obtained written informed consent from all participants.
Continuous arterial spin labelling MRI
Data acquisition
We obtained CASL brain volumes using a 3 T MRI system (Magnetom Allegra, Siemens) equipped with a head volume coil at the Department of Psychiatry and Psychotherapy III at Ulm University. Scanning was carried out in darkness, and all participants were explicitly instructed to keep their eyes closed, not to think about something special, not to fall asleep and to move as little as possible. Adherence to these instructions was verified by verbal contact immediately after the scan and as part of a postscanning exit interview.
We opted to use MRI-based perfusion CASL because this method appears better suited than blood oxygen level–dependent (BOLD) techniques for investigating slow changes in neural activity that could otherwise be biased by scanner drifts, has a high stability over longer time scales and generates data that are statistically independent over time under the null hypothesis (i.e., the data do not possess autocorrelation).43 Technical details on the CASL sequence used in this study have been described in more detail elsewhere.30 Briefly, the labelling plane was 8 cm beneath the centre of the imaging sections. We used 20 radiofrequency pulses of 100 ms duration and a gap of 7.5 ms for labelling. The mean duration of each control or labelling image acquisition was 2142.5 ms. In order to reduce transit-related effects, a delay of 1 s between the end of the labelling pulses and image acquisition was introduced. Off-resonance artifacts were controlled by a sinusoidally amplitude-modulated version of the labelling pulse. We acquired T2-weighted interleaved label and control images using a gradient-echo echo-planar imaging (EPI) sequence (matrix size 64 × 64 pixels, repetition time [TR] 4 s, echo time [TE] 16 ms, bandwidth 3.005 Hz/Px). Eighteen transversal slices were positioned along the anterior–posterior commissure (AC-PC) line (thickness 5 mm, 1.5 mm gap), and the in-plane resolution was 3.44 × 3.44 mm so that effective resolution in x, y, z direction was 3.44 × 3.44 × 6.5 mm3, respectively, to cover the entire brain. The perfusion block for all participants comprised 80 acquisitions of labelled and control images, with a total duration of 320 s.
Data analysis
Preprocessing and statistical analyses of perfusion data were performed using SPM8 (www.fil.ion.ucl.ac.uk) in combination with software implemented in MATLAB 7.3 (MathWorks) for use as a toolbox under SPM8. The toolbox code is based on a MATLAB script by H.Y. Rao and J.J. Wang from the Center for Functional Imaging at the University of Pennsylvania that implements a single compartment CASL perfusion model30 for reconstructing images of raw perfusion and quantified rCBF in millilitres/100 g tissue/min (http://cfn.upenn.edu/perfusion/software.html). The individual images underwent realignment to the first image, reslicing and generation of perfusion-weighted images by pairwise subtraction of the label and control images followed by conversion to quantified rCBF. This procedure also incorporated the calculation of a mean EPI by averaging across all acquired EPI images and the coregistration of the mean EPI to individual T1 images. The rCBF images were normalized to the canonical Montreal Neurological Institute (MNI) space by applying the transformation matrices estimated from the normalization of the individual high-resolution T1-weighted MRIs to a standard T1 template of 3 × 3 × 3 mm3 voxels. The normalized rCBF images were then smoothed with a 3-dimensional (3D) 10 mm full-width at half-maximum (FWHM) kernel.
To prepare group comparisons of rCBF data, we computed individual mean rCBF images for each participant within the framework of the general linear model (GLM) with the time course of the volumes mean as a covariate to reduce spatially coherent noise. During this analysis rCBF images were scaled to a grand mean of 50. No temporal filtering was used. We compared patients and controls at the second level using a 2-sample t test model including mean rCBF images per participant and group together with age, sex and individual grand mean CBF averages as nuisance variables. The latter were included to regress out unspecific changes in overall CBF. Inference of meaningful local group differences was based on a correction for multiple comparisons at the cluster level, using an uncorrected height threshold of p < 0.005 together with an extent threshold of p < 0.05.33 All analyses were inclusively masked by a statistical parametric map comprising voxels with positive rCBF values that were significantly (p < 0.05) different from zero when averaged across both groups of patients and controls.
Structural MRI
Data acquisition
For CASL, high-resolution structural data were acquired using the same 3 T head MRI system (Magnetom Allegra, Siemens). The MRI parameters of the 3D magnetization-prepared rapid gradient-echo sequences were as follows: TE 3.93 ms, TR 2080 ms, inversion time (TI) 1100 ms, field of view (FOV) 256 mm, slice thickness 1 mm, resolution 1.0 × 1.0 × 1.0 mm3, 256 slices.
Data analysis
We conducted a voxel-based morphometry (VBM) analysis using Christian Gaser’s VBM toolbox (http://dbm.neuro.uni-jena.de/vbm8/) running within SPM8. The segmentation algorithm used by this toolbox is based on an adaptive “Maximum A Posterior” (MAP) technique. This approach does not require a priori information about tissue probabilities (i.e., the tissue probability maps are used for spatial normalization only).44 During the MAP estimation local parameter variations are modelled as varying spatial functions, thus accounting for intensity inhomogeneity and other local intensity variations. During the data segmentation step, each participant’s original T1 image was spatially normalized and segmented into grey and white matter and cerebrospinal fluid (CSF). This segmentation procedure is followed by partial volume estimation,45 data denoising based on a spatially adaptive nonlocal means filter,46 and the application of Markov Random Fields.44 The toolbox also integrates normalization using DARTEL. With DARTEL, deformations are produced by exponentiation of a velocity field thus ensuring that the Jacobian determinants always remain positive. This precondition guarantees that the transformations are inversely consistent through generating both forward and inverse transformations from the same flow field.47 After data preprocessing, the modulated normalized grey matter segments were smoothed using a 10 mm FWHM Gaussian kernel before between-group analyses at the second level. To test for grey matter volume differences between groups, we used 2-sample t tests with age and sex included as nuisance variables. Using VBM8 we saved modulated images by correcting for nonlinear warping only, which results in data corrected for head size. Given that total intracranial volume (TIV) significantly differed between controls and patients, we nevertheless chose to include TIV as an additional nuisance variable to remove any potential residual effect of TIV differences in the modulated images. For completeness, results derived from between-group comparisons not covaried for TIV are provided in Appendix 1, Fig. S1, available at jpn.ca.
In second-level analysis models we used an absolute threshold of 0.2 to prevent effects located at tissue border regions. Local group differences were considered significant when surviving a correction for multiple comparisons at the cluster level. To achieve this significance we used an uncorrected height threshold of p < 0.005 together with an extent threshold of p < 0.05.33
Regional CBF data with brain volume as covariate
In order to investigate perfusion changes controlled for voxel-wise regional atrophy we used the Biological Parametric Mapping (BPM) toolbox (www.ansir.wfubmc.edu).48 The BPM analysis algorithm is based on a voxel-wise use of the general linear model (GLM)49 and permits integration of multiple imaging modalities (e.g., functional and structural data) as regressors in voxel-based analyses. The BPM algorithm solves a GLM analysis where imaging data can be used as a covariate. Prior to BPM individual normalized and modulated grey matter volume maps were interpolated to the voxel size of the functional data to achieve the same spatial resolution for both modalities. We investigated between-groups rCBF differences using an analysis of covariance (ANCOVA) model, as implemented in BPM. Within this model, individual grey matter volume maps (as obtained by the VBM analysis) were included as covariates, together with age, sex, TIV and individual grand mean rCBF averages. We considered between-group differences to be significant when surviving a correction for multiple comparisons at the cluster level (uncorrected height threshold of p < 0.005, extent threshold of p < 0.05).
For all analyses (structural data and rCBF data, both uncorrected and corrected for brain atrophy), stereotaxic coordinates obtained by between-group comparisons are reported as coordinates of cluster-maxima in MNI space. Anatomic regions were labelled according to the Talairach Daemon labels and the Automatic Anatomic Labelling atlas (AAL50), implemented in the Wake Forest University (WFU) PickAtlas toolbox (http://fmri.wfubmc.edu/software/PickAtlas). To facilitate comparisons between the different analyses, Appendix 1, Table S1 displays stereotaxic coordinates and z scores for both SPM8 and BPM outputs, and Appendix 1, Fig. S2 displays results of both SPM8 and BPM analyses. To evaluate potential effects of medication in patients, we reanalyzed all the imaging data using a medicated subsample of patients with MDD (demographic characteristics and clinical scores are shown in Appendix 1 Table S2). Detailed results of these analyses are shown in Appendix 1, Table S1 and Fig. S3. Potential effects of psychotropic treatment were additionally investigated using psychotropic medication status as nuisance variable. Grey matter volume and rCBF between-group analyses on the second level were recalculated using antidepressant monotherapy or drug combinations as distinct covariates.
Brain structure and function: region-of-interest analyses
In addition to BPM, we performed exploratory region of interest (ROI) analyses to investigate associations between grey matter volume and rCBF in patients. Based on meta-analyses of volumetric abnormalities in patients with MDD,19,22,23,26 we first identified regions consistently reported to exhibit lower grey matter volume in patients with MDD (i.e., MPFC, ACC, hippocampus and striatum [putamen and caudate nucleus]). Subsequently, we defined ROIs using the AAL, including the following regions: medial orbitofrontal cortex, ACC, hippocampus, caudate and putamen. Since we did not have specific hypotheses on lateralization, we chose a bilateral spatial distribution. We applied these masks to VBM and CASL within-group analyses based on 1-sample t test models adjusted for age and sex and thresholded at p < 0.005, uncorrected for height. For each ROI, we extracted mean grey matter volume parameter estimates and mean rCBF values. Exploratory correlation analyses (p < 0.05, uncorrected) between grey matter volume and rCBF were calculated offline using the Statistica software package version 6.0.
Correlations with clinical measures
We chose a nonparametric analysis to minimize potential effects of data outliers and of a non-Gaussian distribution of the data. Spearman rank correlations within the entire MDD patient group were calculated using mean rCBF values from clusters showing significant differences between patients and controls and clinical measures. Using MarsBar,51 we extracted individual participants’ voxelwise rCBF values averaged across clusters. These values were subsequently analyzed using the Statistica software package version 6.0 (StatSoft Inc.). As a nominal level of significance, we identified a level of p < 0.05 adjusted for multiple comparisons using false-discovery rate (FDR) correction52 according to the number of ROIs (clusters showing between-group differences) and clinical measures (BDI, HAMD, duration of illness, number of episodes) for which correlations were computed (adjusted to p < 0.0260).
Results
Participants
We included a total of 43 patients with MDD and 29 healthy controls, matched for age, education and right-handedness in this study (Table 1). Thirty-three patients were treated as inpatients, 7 patients were treated in our hospital’s day clinic and 3 were outpatients. Twenty-nine patients were on a stable psychotropic drug regime at the time of the scanning (Appendix 1, Table S3). Patients who were on medication were treated for a mean period of 1.33 months before scanning. Nine of 29 patients received a new drug treatment regime for approximately 1 week without showing clinical improvement within this period. The others were on medication for at least 2 weeks but did not show signs of substantial clinical improvement under their respective treatment regimes.
Table 1.
Demographic and clinical characteristics of the study sample
| Group, mean ± SD* | |||
|---|---|---|---|
|
|
|||
| Characteristic | Control, n = 29 | MDD, n = 43 | p value |
| Age, yr | 34.5 ± 10.7 | 37.1 ± 10.9 | 0.32 |
| Education, yr | 14.9 ± 2.6 | 10.4 ± 1.5 | 0.009 |
| Sex, male:female | 11:18 | 17:26 | — |
| Duration of illness, yr | — | 7.2 ± 7.1 | — |
| No. of episodes | — | 3.1 ± 1.9 | — |
| BDI score | 1.5 ± 2.4 | 29.0 ± 9.3 | < 0.001 |
| HAMD score | 0.9 ± 1.7 | 20.9 ± 5.1 | < 0.001 |
| TIV, mL | 1417.5 ± 125.9 | 1352.2 ± 114.6 | 0.026 |
BDI = Beck Depression Inventory; HAMD = Hamilton Rating Scale for Depression; MDD = major depressive disorder; SD = standard deviation; TIV = total intracranial volume.
Unless otherwise indicated.
Continuous arterial spin labelling MRI
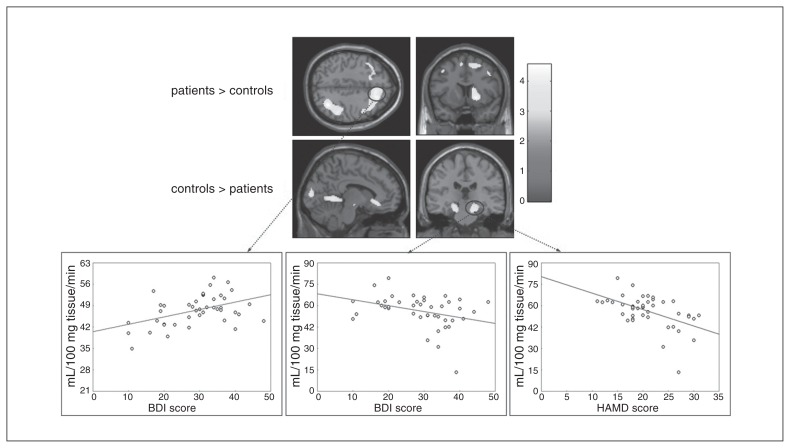
Patients showed lower rCBF than controls in the cuneus, bilateral parahippocampal cortex (PHC) and subgenual ACC. Higher rCBF in patients was found in right temporoparietal regions, in a cluster comprising predominantly right frontal regions (middle, medial and superior frontal gyri) and in right striatal areas (lentiform nucleus and caudate; Fig. 1, Table 2 and Appendix 1, Table S1). Abnormal rCBF in these regions was confirmed in the medicated patient subsample (Appendix 1, Fig. S3 and Table S1). In rCBF between-group analyses adjusted for medication status patients showed lower rCBF than controls in the cuneus, bilateral PHC and left superior temporal cortex. The subgenual ACC did not survive correction for spatial extent. Compared with controls, patients showed higher rCBF in right frontoparietal regions (superior and inferior parietal cortices; superior, middle and medial frontal cortices), left superior and middle frontal gyri and right lentiform nucleus (Appendix 1, Table S4).
Fig. 1.
Regions with abnormal regional cerebral blood flow (rCBF) in patients with major depressive disorder (MDD; n = 43) compared with healthy controls (n = 29). Results of the second-level between-group analysis (p < 0.005, uncorrected at the voxel level; p < 0.05, corrected for spatial extent). The color bar indicates t values. Correlation plots show the association between rCBF and depression scores in patients with MDD (p < 0.05, false discovery rate–corrected). Left: association between Beck Depression Inventory (BDI) scores and right middle frontal cortical blood flow (ρ = 0.38, p = 0.012). Middle: association between BDI scores and right parahippocampal blood flow (ρ = −0.37 and p = 0.016). Right: association between HAMD scores and right parahippocampal blood flow (ρ = −0.45 and p = 0.003).
Table 2.
Brain areas showing brain perfusion changes at rest in unmedicated and medicated patients with MDD (n=43) compared to controls (n=29)*
| SPM analysis | BPM analysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Comparison | Region | x | y | z | Z | No. of voxels | Region | x | y | z | Z | No. of voxels |
| Controls > patients | Right cuneus | 16 | −90 | 8 | 4.57 | 2703 | Right cuneus | 15 | −90 | 8 | 4.55 | 2725 |
| Right parahippocampal gyrus | 24 | −24 | −16 | 3.21 | Right parahippocampal gyrus | 22 | −26 | −14 | 3.45 | |||
| Left parahippocampal gyrus | −18 | −26 | −18 | 3.54 | 449 | Left parahippocampal gyrus | −18 | −26 | −18 | 3.40 | 389 | |
| Left anterior cingulate | −8 | 26 | −6 | 3.44 | 214 | Left anterior cingulate | −8 | 28 | −6 | 3.48 | 238 | |
| Patients > controls | Right inferior parietal lobule | 34 | −62 | 44 | 4.23 | 1955 | Right inferior parietal lobule | 34 | −62 | 44 | 4.31 | 1933 |
| Right middle temporal gyrus | 52 | −45 | 8 | 3.43 | Right middle temporal gyrus | 52 | −45 | 8 | 3.47 | |||
| Right middle frontal gyrus | 20 | 28 | 46 | 4.07 | 2905 | Right middle frontal gyrus | 20 | 28 | 46 | 4.09 | 3242 | |
| Right medial frontal gyrus | 10 | 25 | 44 | 3.91 | Right superior frontal gyrus | 25 | 32 | 38 | 3.95 | |||
| Right superior frontal gyrus | 8 | 30 | 48 | 3.91 | Right medial frontal gyrus | 8 | 25 | 44 | 3.72 | |||
| Right middle frontal gyrus | 38 | 15 | 45 | 3.72 | Right middle frontal gyrus | 38 | 15 | 45 | 3.53 | |||
| Left middle frontal gyrus | −24 | 20 | 50 | 3.25 | Right middle frontal gyrus | 40 | −2 | 52 | 3.47 | |||
| Right superior frontal gyrus | 20 | 55 | 10 | 3.38 | ||||||||
| Left middle frontal gyrus | −24 | 20 | 50 | 3.22 | ||||||||
| Right lentiform nucleus | 20 | 10 | −2 | 4.03 | 848 | Right lentiform nucleus | 22 | 10 | −4 | 4.30 | 896 | |
| Right caudate | 15 | 10 | 12 | 3.58 | Right caudate | 16 | 10 | 12 | 3.55 | |||
BPM = Biological Parametric Mapping; MDD = major depressive disorder; SPM = Statistical Parametric Mapping.
Results of second-level t tests (standard SPM8 analysis) and analysis of covariance (BPM analysis), p < 0.005 uncorrected at the voxel level, p < 0.05 corrected for spatial extent.
Structural MRI
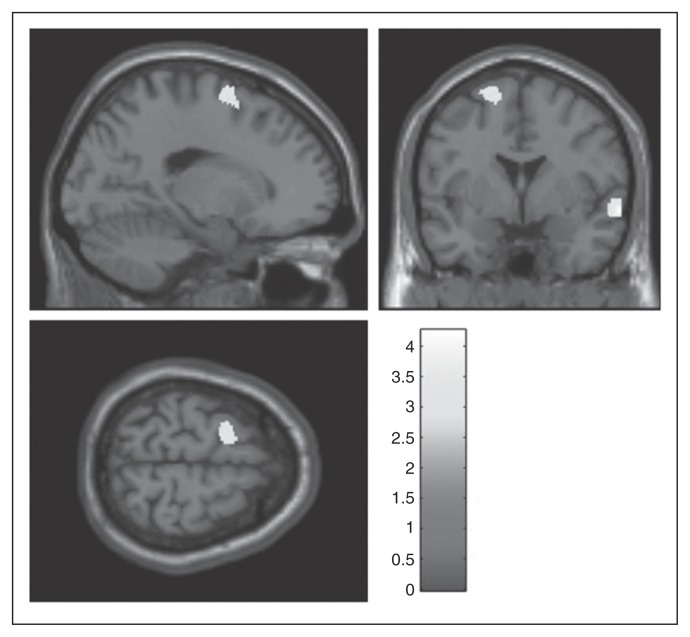
Patients had lower grey matter volume than controls in the right middle and superior temporal cortices (MNI space x, y, z = 65, 1, −9; z score 3.99 v. x, y, z = 57, −48, 21; z score 3.13; k = 501) and in the left superior frontal/middle frontal gyrus (x, y, z = −17, 1, 67; z score 3.13; k = 320; Fig. 2 and Table 2).
Fig. 2.
Brain regions with decreased grey matter volume in patients with major depressive disorder (MDD; n = 43) compared with healthy controls (n = 29). Results of the second-level between-group analysis (p < 0.005, uncorrected at the voxel level; p < 0.05, corrected for spatial extent). The shade bar indicates t values.
Considering the medicated patient subsample (n = 29), these participants showed lower grey matter volume than controls in inferior and middle temporal regions, the right inferior frontal gyrus, left middle and superior frontal gyrus, precentral cortex and bilateral cingulate gyrus (Appendix 1, Fig. S4 and supplementary Table S5). In between-group analyses of grey matter volume adjusted for medication status patients showed lower grey matter volume than controls in the right middle frontal (MNI space x, y, z = 30, 39, 43; z score 3.11; k = 259), right middle temporal (x, y, z = 57, −42, −5; z score 2.97; k = 218) and right medial frontal cortex (x, y, z = 4, 64, 12; z score 2.77; k = 228).
Regional CBF data covaried for brain structure
The BPM essentially aligned with the findings from the analysis uncorrected for structural change: patients showed lower rCBF than controls in the cuneus, bilateral PHC and subgenual ACC. Higher rCBF in patients was found in the right striatum, right temporoparietal areas and in a cluster comprising predominantly right middle, medial and superior frontal regions (Appendix 1, Table S1 and Fig. S2).
Considering the medicated patient subsample, these participants also showed higher rCBF in the left middle frontal gyrus in addition to those regions of abnormal rCBF that were already detected by analyses uncorrected for structural change (Appendix 1, Table S1 and Fig. S3).
Region of interest analyses
The ROI correlations between grey matter volume and rCBF did not yield significant findings (correlation plots available on request from the authors).
Correlations with clinical measures
In the patient group there were significant negative correlations between right parahippocampal rCBF and HAMD scores (ρ = −0.45, p = 0.003) and BDI scores (ρ = −0.37, p = 0.016). We found a positive correlation between right middle frontal cortical blood flow and BDI scores (ρ = 0.38, p = 0.012; Fig. 1). We found trends toward significance (not surviving the FDR-corrected threshold for multiple comparisons) when correlating HAMD scores with ACC rCBF (ρ = −0.32, p = 0.04) or with left parahippocampal blood flow (ρ = −0.34, p = 0.027). We found no significant correlations between rCBF and duration of illness or number of episodes.
Discussion
We investigated rCBF at rest and brain structure in patients with MDD. Five main findings emerged. First, in patients with MDD rCBF was reduced in subgenual anterior cingulate and bilateral parahippocampal areas and increased in frontoparietal and striatal regions. Second, grey matter volume was reduced in patients with MDD in temporal and frontal regions. Third, the between-group differences in rCBF did not substantially change when voxel-wise correction for brain volume was applied, nor did ROI-based analyses reveal significant associations between grey matter volume and rCBF in patients with MDD. Fourth, changes in rCBF but not in grey matter volume correlated with individual expressions of symptom severity. Finally, we found no significant associations between rCBF and illness duration or number of depressive episodes.
In contrast to previous PET findings, we detected hypo- but not hyperperfusion of the subgenual ACC. Patient sample characteristics may account for this divergence (for example, see the review by Lorenzetti and colleagues23), but methodological aspects of the selected neuroimaging technique should also be considered. For instance, perfusion measures in healthy controls indicate both regional and individual differences with sometimes higher ASL-CBF values than [15O]PET-CBF values in cortical areas despite overall similar results between ASL and PET.53 Also, in neuropsychiatric diseases such as Alzheimer disease, ASL and FDG-PET have been shown to detect similar, but not identical, perfusion/metabolism abnormalities,50,51 suggesting complementary and nonredundant information provided by both imaging modalities. Modality-dependent characteristics, however, cannot fully account for our finding of lower rCBF in the subgenual part of the ACC. Concomitantly, previous MRI-based perfusion studies in adults did not find rCBF differences between healthy controls and patients with MDD in this region.37, 54 In another report, only patients with poor treatment response exhibited hypoperfusion of the ACC,36 indicating that lower cingulate blood flow may be associated with drug-related clinical outcomes. In accordance with our present findings, a recent transdiagnostic study reported lower ACC blood flow in adult patients with MDD than in healthy controls and patients with schizophrenia.55 It appears that patterns of blood flow or metabolism may be critically linked to clinical aspects of the disorder, especially when studied cross-sectionally. Patients in our sample were acutely ill but have not yet been characterized treatment-refractory, for whom hyperperfusion35 or hypermetabolism56 in the subcallosal regions of the ACC have been reported.57
Decreased rCBF in the PHC in the acute phase of the illness is consistent with reported lower mean medial temporal lobe blood flow in adolescent depression.33 Perfusion deficits of medial temporal lobe structures may partly explain cognitive deficits, particularly mnemonic and executive functions, in patients with MDD.58–61 Higher striatal perfusion revealed in our sample might be part of the overall increased activity/responsiveness of limbic structures according to currently hypothesized MDD models.62 Different forms of antidepressant therapy modulate activity in key regions associated with mood regulation and affective processing, most notably in lateral prefrontal cortices and the ACC.63 Accordingly, when considering psychopharmacological therapy, increased striatal perfusion might be more pronounced in unmedicated patients, being attenuated by antidepressant treatment. Hyperperfusion in the frontoparietal regions is in accordance with some previous CBF findings in patients with MDD, particularly derived from single-photon emission computed tomography (SPECT) investigations.54,64 This may be explained by the fact that most patients were on antidepressants. Nonmedicated patients may present prefrontal hypoperfusion when measured with ASL.33, 36
Major depressive disorder has been repeatedly associated with brain volume change.19,20, 21,22–24 Volume reduction of the ACC, as found in our medicated patient subsample, is a robust finding in patients with MDD.18,19,21 Furthermore, volumetric decrease of this brain structure correlated significantly with both cross-sectional symptom burden in acute depression65 and with MDD illness duration.66 To our knowledge, only 1 study so far has combined ASL with measures of cortical thickness,54 whereas a recent transdiagnostic study investigating patients with MDD and schizophrenia used whole-brain grey matter volumes to account for partial volume effects in ASL data.55 Perfusion changes observed in these studies were not related to structural change.54,55 Similarly, although based on correlations between brain volume and brain activity at rest, a recent study conducted in medication-naive patients with MDD reported no overlap between functional abnormalities and volume reduction.67 In synopsis with these data, our present findings support the notion that abnormal patterns of brain activity in patients with MDD could occur in the absence of structural deficits, at least within the limits given by predefined detection thresholds of whole-brain VBM and cortical thickness comparisons. Thus, functional and structural change might differentially contribute to the development, maintenance and individual expression of affective symptoms. This said, we are aware that the neural abnormalities observed in our patient sample may not be specific to MDD. Prefrontal, anterior cingulate and temporoparietal regions have been shown to exhibit abnormal volume and activity in patients with other affective disorders (e.g., bipolar disorder68,69) or in patients with depressive comorbidity and affective dysregulation, such as borderline personality disorder (BPD).70 It is possible that rCBF changes in our patient sample and the relationships between rCBF and depressive symptoms could reflect associations between neural activity and psychopathology independent from a diagnosis of MDD. Future transdiagnostic studies are warranted to identify structural and functional signatures that are associated with specific symptom dimensions and psychopathology independent of disorder category. Such investigations essentially acknowledge the limited associations between neurobiology and current clinical diagnoses.71
Limitations
We acknowledge several limitations to our study. It is important to note that CASL is a relatively new technique compared with PET, SPECT or even BOLD fMRI,72 which might partly account for the lack of clinically relevant data in psychiatric samples73 acquired with this specific method. Also, findings obtained under resting-state conditions do not necessarily imply either intact or deficient function of distinct brain regions when active cognitive, social or affective processing is required. While we sought to acquire data unbiased by explicit stimulus manipulation, we acknowledge that resting-state functional neuroimaging data are conducted in a poorly controlled experimental environment, so we cannot directly relate CBF changes in patients to specific cognitive or affective events occurring during the scan. Recently, in patients with MDD reciprocal associations between neural activity and rumination have been reported for hippocampal, inferior and superior frontal and anterior cingulate regions.74–77 Contrasting our findings with the extant literature, it is noteworthy that we observed very limited overlap between regions exhibiting abnormal rCBF in patients and brain regions that were found to be associated with rumination in patients with MDD. Yet given that we didn’t explicitly assess these symptoms, definitive conclusions cannot be drawn from the present findings. Furthermore, most of the included patients were on antidepressant treatment, and it is now well-recognized that drug treatment can modulate activity in several regions of the depressed brain, most markedly in lateral prefrontal and anterior cingulate regions.63,78 The substantially lower proportion of unmedicated patients and the associated loss of power prevented us from computing separate analyses for medicated and unmedicated individuals. As an attempt to differentiate between medicated and unmedicated patients, we performed subsample analyses and additionally controlled for psychotropic drug treatment as covariates of no interest. In line with previous reports,19,23 we found differences in grey matter volume depending on medication status. In the medicated patient subsample grey matter volume loss was found to be more widespread, and covariation for drug treatment did not fully replicate findings obtained across the entire patient population. For rCBF, however, results obtained across the entire sample were replicated in the medicated patient subgroup, and adding medication status as a covariate did not substantially alter the main findings of our study. In analyses covaried for drug treatment the ACC did not survive correction for spatial extent, yet the reduction of degrees of freedom in this type of analysis could have contributed to this result. Although patterns of rCBF perfusion change in the MDD group appear to generalize across medicated and unmedicated patients, further research in larger samples of unmedicated individuals is needed to characterize medication effects on brain perfusion in more detail. Aberrant brain structure and function has been reported in euthymic individuals with MDD,76,79–81 suggesting a pattern of neural dysfunction that persists after clinical remission, although in patients with MDD brain activity can change over time as a function of treatment.63 Given our cross-sectional study design, we cannot sufficiently address the question whether the observed rCBF changes in patients with MDD reflect state or trait aspects of the disease. The correlations between rCBF and depressive symptoms scores (HAMD, BDI) in the absence of a significant association between rCBF and duration of illness suggest correlates of acute symptom exacerbation, yet this notion needs to be confirmed by follow-up assessments.
Conclusion
Keeping the limitations of our study in mind, our findings support the notion of abnormal frontolimbic neural activity in patients with MDD during conditions of absent experimental stimulation. Although several cortical regions were found to exhibit abnormal grey matter volume, the pattern of perfusion deficits in patients remained stable after correction for regional atrophy across the whole brain. This indicates that volume loss in patients with MDD does not have a substantial impact on brain perfusion at rest and that volume loss and abnormal rCBF may differentially contribute to symptom expression, psychopathology and course of the illness. Future longitudinal studies integrating multimodal neuroimaging data are needed to disentangle these effects on neural structure and function and to generate the predictive value of distinct neural markers for treatment monitoring and outcome.
Acknowledgements
The authors thank all patients for their time and interest in this study and Kathrin Brändle for excellent technical support.
Footnotes
Competing interests: N. Vasic and M. Dudek declare symposium support from Lilly, Janssen-Cilag, Trommsdorf and Lundbeck. N. Vasic also declares receiving speaker fees from Otsuka and Lundbeck and consulting fees from Servier. F. Sambataro is a full-time employee of Hoffman-La Roche Ltd. in Basel, Switzerland. R. Wolf declares receiving speaker fees from Lundbeck and Otsuka and consulting fees from Trommsdorff. No other competing interests declared.
Contributors: N. Vasic, N. Wolf, B. Connermann and R. Wolf designed the study. N. Vasic, N. Wolf, Z. Sosic-Vasic, A. von Shrombeck, D. Lang and R. Wolf acquired the data, which N. Vasic, N. Wolf, G. Grön, Z. Sosic-Vasic, F. Sambataro, D. Lang, S. Otte, M. Dudek and R. Wolf analyzed. N. Vasic, G. Grön, Z. Sosic-Vasic, S. Otte, M. Dudek and R. Wolf wrote the article, which all authors reviewed and approve for publication.
References
- 1.Mayberg HS. Neuroimaging and psychiatry: the long road from bench to bedside. Hastings Cent Rep. 2014;(Spec No):S31–6. doi: 10.1002/hast.296. [DOI] [PubMed] [Google Scholar]
- 2.Bench CJ, Friston KJ, Brown RG, et al. The anatomy of melancholia–focal abnormalities of cerebral blood flow in major depression. Psychol Med. 1992;22:607–15. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- 3.Bench CJ, Friston KJ, Brown RG, et al. Regional cerebral blood flow in depression measured by positron emission tomography: the relationship with clinical dimensions. Psychol Med. 1993;23:579–90. doi: 10.1017/s0033291700025368. [DOI] [PubMed] [Google Scholar]
- 4.Bench CJ, Frackowiak RS, Dolan RJ. Changes in regional cerebral blood flow on recovery from depression. Psychol Med. 1995;25:247–61. doi: 10.1017/s0033291700036151. [DOI] [PubMed] [Google Scholar]
- 5.Mayberg HS, Brannan SK, Mahurin RK, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–61. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 6.Brannan SK, Mayberg HS, McGinnis S. Cingulate metabolism predicts treatment response: a replication. Biol Psychiatry. 2000;47:107S. [Google Scholar]
- 7.Holthoff VA, Beuthien-Baumann B, Zundorf G, et al. Changes in brain metabolism associated with remission in unipolar major depression. Acta Psychiatr Scand. 2004;110:184–94. doi: 10.1111/j.1600-0447.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- 8.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Lozano AM, Mayberg HS, Giacobbe P, et al. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–7. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 10.Holtzheimer PE, Kelley ME, Gross RE, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69:150–8. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldapple K, Segal Z, Garson C, et al. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- 13.Mayberg HS. Modulating limbic-cortical circuits in depression: targets of antidepressant treatments. Semin Clin Neuropsychiatry. 2002;7:255–68. doi: 10.1053/scnp.2002.35223. [DOI] [PubMed] [Google Scholar]
- 14.Matthews SC, Strigo IA, Simmons AN, et al. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J Affect Disord. 2008;111:13–20. doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Flint J, Kendler KS. The genetics of major depression. Neuron. 2014;81:484–503. doi: 10.1016/j.neuron.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience. 2009;164:300–30. doi: 10.1016/j.neuroscience.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schott BH, Assmann A, Schmierer P, et al. Epistatic interaction of genetic depression risk variants in the human subgenual cingulate cortex during memory encoding. Transl Psychiatry. 2014;4:e372. doi: 10.1038/tp.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai CH. Gray matter volume in major depressive disorder: a meta-analysis of voxel-based morphometry studies. Psychiatry Res. 2013;211:37–46. doi: 10.1016/j.pscychresns.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Bora E, Fornito A, Pantelis C, et al. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 20.Koolschijn PC, Cédric MP, van Haren N, et al. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–35. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du MY, Wu Q, Yue Q, et al. Voxelwise meta-analysis of gray matter reduction in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:11–6. doi: 10.1016/j.pnpbp.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Arnone D, McIntosh AM, Ebmeier KP, et al. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur Neuropsychopharmacol. 2012;22:1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzetti V, Allen NB, Fornito A, et al. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Kempton MJ, Salvador Z, Munafò MR, et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–90. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 25.Diener C, Kuehner C, Brusniak W, et al. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage. 2012;61:677–85. doi: 10.1016/j.neuroimage.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Sacher J, Neumann J, Fünfstück T, et al. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J Affect Disord. 2012;140:142–8. doi: 10.1016/j.jad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Smieskova R, Allen P, Simon A, et al. Different duration of at-risk mental state associated with neurofunctional abnormalities. A multimodal imaging study. Hum Brain Mapp. 2012;33:2281–94. doi: 10.1002/hbm.21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Werner CJ, Dogan I, Saß C, et al. Altered resting-state connectivity in Huntington’s disease. Hum Brain Mapp. 2014;35:2582–93. doi: 10.1002/hbm.22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf RC, Sambataro F, Vasic N, et al. Visual system integrity and cognition in early Huntington’s disease. Eur J Neurosci. 2014;40:2417–26. doi: 10.1111/ejn.12575. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Wang J, Connick TJ, et al. Continuous ASL (CASL) perfusion MRI with an array coil and parallel imaging at 3T. Magn Reson Med. 2005;54:732–7. doi: 10.1002/mrm.20574. [DOI] [PubMed] [Google Scholar]
- 31.Floyd TF, Ratcliffe SJ, Wang J, et al. Precision of the CASL-perfusion MRI technique for the measurement of cerebral blood flow in whole brain and vascular territories. J Magn Reson Imaging. 2003;18:649–55. doi: 10.1002/jmri.10416. [DOI] [PubMed] [Google Scholar]
- 32.Petersen ET, Mouridsen K, Golay X. The QUASAR reproducibility study, Part II: Results from a multi-center Arterial Spin Labeling test-retest study. Neuroimage. 2010;49:104–13. doi: 10.1016/j.neuroimage.2009.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho TC, Wu J, Shin DD, et al. Altered cerebral perfusion in executive, affective, and motor networks during adolescent depression. J Am Acad Child Adolesc Psychiatry. 2013;52:1076–1091.e2. doi: 10.1016/j.jaac.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orosz A, Jann K, Federspiel A, et al. Reduced cerebral blood flow within the default-mode network and within total gray matter in major depression. Brain Connect. 2012;2:303–10. doi: 10.1089/brain.2012.0101. [DOI] [PubMed] [Google Scholar]
- 35.Duhameau B, Ferré J, Jannin P, et al. Chronic and treatment-resistant depression: a study using arterial spin labeling perfusion MRI at 3 Tesla. Psychiatry Res. 2010;182:111–6. doi: 10.1016/j.pscychresns.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Lui S, Parkes L, Huang X, et al. Altered cerebral perfusion measured with arterial spin-labeling MR imaging. Radiology. 2009;251:476–84. doi: 10.1148/radiol.2512081548. [DOI] [PubMed] [Google Scholar]
- 37.Colloby SJ, Firbank MJ, He J, et al. Regional cerebral blood flow in late-life depression: arterial spin labelling magnetic resonance study. Br J Psychiatry. 2012;200:150–5. doi: 10.1192/bjp.bp.111.092387. [DOI] [PubMed] [Google Scholar]
- 38.McIntyre RS, Filteau M, Martin L, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014;156:1–7. doi: 10.1016/j.jad.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 39.Berlim MT, Turecki G. Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can J Psychiatry. 2007;52:46–54. doi: 10.1177/070674370705200108. [DOI] [PubMed] [Google Scholar]
- 40.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 41.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 42.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Detre JA, Wang J. Technical aspects and utility of fMRI using BOLD and ASL. Clin Neurophysiol. 2002;113:621–34. doi: 10.1016/s1388-2457(02)00038-x. [DOI] [PubMed] [Google Scholar]
- 44.Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans Med Imaging. 1997;16:176–86. doi: 10.1109/42.563663. [DOI] [PubMed] [Google Scholar]
- 45.Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23:84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Manjón JV, Coupe P, Marti-Bonmati L, et al. Adaptive non-local means denoising of MR images with spatially varying noise levels. J Magn Reson Imaging. 2010;31:192–203. doi: 10.1002/jmri.22003. [DOI] [PubMed] [Google Scholar]
- 47.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Casanova R, Srikanth R, Baer A, et al. Biological parametric mapping: a statistical toolbox for multimodality brain image analysis. Neuroimage. 2007;34:137–43. doi: 10.1016/j.neuroimage.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friston KJ, Holmes A, Worsley KJ, et al. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 50.Chen Y, Wolk DA, Reddin JS, et al. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology. 2011;77:1977–85. doi: 10.1212/WNL.0b013e31823a0ef7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musiek ES, Chen Y, Korczykowski M, et al. Direct comparison of fluorodeoxyglucose positron emission tomography and arterial spin labeling magnetic resonance imaging in Alzheimer’s disease. Alzheimers Dement. 2012;8:51–9. doi: 10.1016/j.jalz.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–88. [Google Scholar]
- 53.Zhang K, Herzog H, Mauler J, et al. Comparison of cerebral blood flow acquired by simultaneous [(15)O]water positron emission tomography and arterial spin labeling magnetic resonance imaging. J Cereb Blood Flow Metab. 2014;24:1373–80. doi: 10.1038/jcbfm.2014.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Järnum H, Eskildsen SF, Steffensen EG, et al. Longitudinal MRI study of cortical thickness, perfusion, and metabolite levels in major depressive disorder. Acta Psychiatr Scand. 2011;124:435–46. doi: 10.1111/j.1600-0447.2011.01766.x. [DOI] [PubMed] [Google Scholar]
- 55.Ota M, Noda T, Sato N, et al. Characteristic distributions of regional cerebral blood flow changes in major depressive disorder patients: a pseudo-continuous arterial spin labeling (pCASL) study. J Affect Disord. 2014;165:59–63. doi: 10.1016/j.jad.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 56.McGrath CL, Kelley ME, Dunlop BW, et al. Pretreatment brain states identify likely nonresponse to standard treatments for depression. Biol Psychiatry. 2014;76:527–35. doi: 10.1016/j.biopsych.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest. 2009;119:717–25. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toki S, Okamoto Y, Onoda K, et al. Hippocampal activation during associative encoding of word pairs and its relation to symptomatic improvement in depression: a functional and volumetric MRI study. J Affect Disord. 2014;152–154:462–7. doi: 10.1016/j.jad.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 59.Frodl T, Schaub A, Banac S, et al. Reduced hippocampal volume correlates with executive dysfunctioning in major depression. J Psychiatry Neurosci. 2006;31:316–23. [PMC free article] [PubMed] [Google Scholar]
- 60.Vasic N, Wolf RC, Walter H. Executive functions in patients with depression. The role of prefrontal activation. Nervenarzt. 2007;78:628–40. doi: 10.1007/s00115-006-2240-6. [DOI] [PubMed] [Google Scholar]
- 61.Vasic N, Walter H, Hose A, et al. Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: a voxel-based morphometry study. J Affect Disord. 2008;109:107–16. doi: 10.1016/j.jad.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 62.Disner SG, Beevers CG, Haigh, Emily AP, et al. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–77. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 63.Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33:699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brockmann H, Zobel A, Joe A, et al. The value of HMPAO SPECT in predicting treatment response to citalopram in patients with major depression. Psychiatry Res. 2009;173:107–12. doi: 10.1016/j.pscychresns.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 65.Chen CH, Ridler K, Suckling J, et al. Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biol Psychiatry. 2007;62:407–14. doi: 10.1016/j.biopsych.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 66.Yucel K, McKinnon MC, Chahal R, et al. Anterior cingulate volumes in never-treated patients with major depressive disorder. Neuropsychopharmacology. 2008;33:3157–63. doi: 10.1038/npp.2008.40. [DOI] [PubMed] [Google Scholar]
- 67.Guo W, Liu F, Yu M, et al. Functional and anatomical brain deficits in drug-naive major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;3:1–6. doi: 10.1016/j.pnpbp.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 68.Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–16. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 69.Arnone D, Cavanagh J, Gerber D, et al. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- 70.Krause-Utz A, Winter D, Niedtfeld I, et al. The latest neuroimaging findings in borderline personality disorder. Curr Psychiatry Rep. 2014;16:438. doi: 10.1007/s11920-014-0438-z. [DOI] [PubMed] [Google Scholar]
- 71.Cuthbert BN. The RDoC framework:facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13:28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang DJ, Chen Y, Fernández-Seara M, et al. Potentials and challenges for arterial spin labeling in pharmacological magnetic resonance imaging. J Pharmacol Exp Ther. 2011;337:359–66. doi: 10.1124/jpet.110.172577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Théberge J. Perfusion magnetic resonance imaging in psychiatry. Top Magn Reson Imaging. 2008;19:111–30. doi: 10.1097/RMR.0b013e3181808140. [DOI] [PubMed] [Google Scholar]
- 74.Mandell D, Siegle GJ, Shutt L, et al. Neural substrates of trait ruminations in depression. J Abnorm Psychol. 2014;123:35–48. doi: 10.1037/a0035834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Connolly CG, Wu J, Ho TC, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry. 2013;74:898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schiller CE, Minkel J, Smoski MJ, et al. Remitted major depression is characterized by reduced prefrontal cortex reactivity to reward loss. J Affect Disord. 2013;151:756–62. doi: 10.1016/j.jad.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kühn S, Gallinat J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr Bull. 2013;39:358–65. doi: 10.1093/schbul/sbr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Y, Wan HI, O’Reardon JP, et al. Quantification of cerebral blood flow as biomarker of drug effect: arterial spin labeling phMRI after a single dose of oral citalopram. Clin Pharmacol Ther. 2011;89:251–8. doi: 10.1038/clpt.2010.296. [DOI] [PubMed] [Google Scholar]
- 79.Smoski MJ, Keng S, Schiller CE, et al. Neural mechanisms of cognitive reappraisal in remitted major depressive disorder. J Affect Disord. 2013;151:171–7. doi: 10.1016/j.jad.2013.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kerestes R, Bhagwagar Z, Nathan PJ, et al. Prefrontal cortical response to emotional faces in individuals with major depressive disorder in remission. Psychiatry Res. 2012;202:30–7. doi: 10.1016/j.pscychresns.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delaloye C, Moy G, de Bilbao F, et al. Neuroanatomical and neuropsychological features of elderly euthymic depressed patients with early- and late-onset. J Neurol Sci. 2010;299:19–23. doi: 10.1016/j.jns.2010.08.046. [DOI] [PubMed] [Google Scholar]