Abstract
Background
Disturbances in evidence gathering and disconfirmatory evidence integration have been associated with the presence of or propensity for delusions. Previous evidence suggests that these 2 types of reasoning bias might be differentially affected by antipsychotic medication. We aimed to investigate the effects of a dopaminergic agonist (L-dopa) and a dopaminergic antagonist (haloperidol) on evidence gathering and disconfirmatory evidence integration after single-dose administration in healthy individuals.
Methods
The study used a randomized, double-blind, placebo-controlled, 3-way crossover design. Participants were healthy individuals aged 18–40 years. We administered a new data-gathering task designed to increase sensitivity to change compared with traditional tasks. The Bias Against Disconfirmatory Evidence (BADE) task was used as a measure of disconfirmatory evidence integration.
Results
We included 30 individuals in our study. In the data-gathering task, dopaminergic modulation had no significant effect on the amount of evidence gathered before reaching a decision. In contrast, the ability of participants to integrate disconfirmatory evidence showed a significant linear dopaminergic modulation pattern (highest with haloperidol, intermediate with placebo, lowest with L-dopa), with the difference between haloperidol and L-dopa marginally reaching significance.
Limitations
Although the doses used for haloperidol and L-dopa were similar to those used in previous studies, drug plasma level measurements would have added to the validity of findings.
Conclusion
Evidence gathering and disconfirmatory evidence integration might be differentially influenced by dopaminergic agents. Our findings are in support of a dual-disturbance account of delusions and provide a plausible neurobiological basis for the use of interventions targeted at improving reasoning biases as an adjunctive treatment in patients with psychotic disorders.
Introduction
Delusions are defined as erroneous beliefs that involve a misinterpretation of perceptions or experiences and are held with high conviction despite clear contradictory evidence.1 In the past decades, these characteristics of delusional ideas have been conceptualized as resulting from disruptions in the normal cognitive processes for belief generation and evaluation.2 Several such disruptions, or “reasoning biases,” have been shown to be associated with the presence of or vulnerability to delusions.3–8 One prominent construct refers to disturbed evidence gathering; patients with delusions display a jumping-to-conclusions thinking style (i.e., they make inferences based on limited evidence).4,9 A second construct pertains to a certain incorrigibility, which manifests as disturbed integration of disambiguating or disconfirmatory evidence7,10–12 and increased confidence in false judgments.13,14
Evidence gathering and incorrigibility have been shown to be largely independent from each other,15,16 though intercorrelated to some extent.16,17 Interestingly, current work suggests that they respond differently to antipsychotic medication in clinical populations. The jumping-to-conclusions bias, for example, does not appear to be affected by antipsychotic medication in a consistent manner,18–20 whereas overconfidence in errors has been suggested to improve with antipsychotic treatment.14,16,21 This issue is highly relevant to the treatment of psychotic disorders in light of the prominent account that delusions result from a dopaminergic dysfunction in the mesolimbic system.22 If delusion-associated reasoning biases are differentially affected by antipsychotic medication, it is possible that they represent different stages of delusion formation and/or maintenance, not all of which are dependent on the dopamine system. This latter point is of critical importance, because it suggests the possibility that there are additional loci of intervention in patients with delusions, different from (or complementary to) those targeted by antipsychotic medication. In the past years, there has been increased interest in the development of interventions specifically targeting cognitive biases and, through them, positive symptoms.23,24 Research into the neurobiological bases of reasoning biases might help clarify the role of such interventions as an adjunct to medication in an integrated treatment of psychotic disorders.
Studies of antipsychotic effects on reasoning biases in patients with psychotic disorders are challenging in terms of implementation; the target patient population (acutely ill, antipsychotic-naive patients) is not always easily accessible, either because these patients avoid mental health services, or because antipsychotic drug treatment is initiated very quickly upon admission. Only 1 small study was able to investigate the jumping-to-conclusions bias in patients before and after treatment with antipsychotic medication.18 Additionally, neurocognitive impairments that are typical for schizophrenia25 might constitute a confounding factor, as they have been suggested to contribute to reasoning biases.26,27 For these reasons, a previous study by our group28 used a complementary approach, investigating the effects of dopaminergic agonists (L-dopa) and antagonists (haloperidol) on delusion-associated reasoning biases in healthy individuals. We observed that, although overconfidence in errors significantly decreased with haloperidol, dopaminergic agents had no effect on evidence gathering.28 However, the paradigm used to assess the latter was a variant of the classical beads task, a very simple task consisting of a single trial.29 Although the validity of this task has been repeatedly confirmed in patients with schizophrenia,4 it is possible that its simplicity led to insufficient variance and/or practice effects30,31 that made it difficult to detect effects of single-dose medication in healthy individuals. Therefore, the present study investigated whether the pattern of differential dopaminergic modulation for data gathering and incorrigibility could be replicated using different assessment measures: a new evidence-gathering task designed to maximize sensitivity to change and a disconfirmatory evidence integration task.
Methods
Participants and design
The present study was part of a larger project investigating the effects of dopaminergic agonists and antagonists on cognitive functions associated with psychotic symptoms, such as semantic priming and reasoning biases. Participants were healthy individuals aged 18–40 years recruited through postings on university recruitment sites. The sample size was calculated based on effect sizes of a previous study by our group28 on dopaminergic modulation of reasoning biases, with which there was no participant overlap. Exclusion criteria were any past or current psychiatric or neurologic disorder, including substance use disorders; history of schizophrenia or bipolar disorder in a first-degree relative; history of craniocerebral trauma, arterial hypertension, cardiologic or serious medical conditions; pregnancy; or treatment with any psychotropic or other drugs. Eligibility for the study was confirmed by means of an interview. The study was approved by the Ethics Committee of the Medical Association Hamburg, and was performed in accordance with the Declaration of Helsinki ethical standards. All participants provided written informed consent before participating in the study.
To assess the effects of dopaminergic agents on reasoning biases, we used a randomized, double-blind, 3-way crossover design.28,32 In 3 successive visits, participants were administered either 100 mg of L-dopa and 25 mg of benserazide, 2 mg of haloperidol, or placebo in randomized order and under double-blind conditions (see Andreou and colleagues28 for dose selection rationale). The 3 visits were separated by at least 7 days to allow a complete wash-out of the drug with the longer half-time (haloperidol).33 In order to compensate for the different Tmax33 of haloperidol and L-dopa, we implemented a double-dummy design (Table 1). The testing session began at the time of maximal serum concentration of each drug and lasted 60 minutes at the maximum. Subjective psychological, somatic and motor (adverse) effects of the drugs were assessed through subjective ratings on a 42-item Likert scale at baseline, at the time of ingestion of the second capsule and after the end of the testing session; moreover, blood pressure and pulse were measured at 30-minute intervals. To assess the success of the blinding procedure, participants were asked to guess which substance they had received at the end of each session.
Table 1.
Double-dummy design of study drug administration. The 2 middle columns display the content of the 2 capsules ingested before the testing session.
| Content of capsules | |||
|---|---|---|---|
|
|
|||
| Administered substance | t0 | t1 (1.5 h after t0) | t2 (2.5 h after t0) |
| Haloperidol | Haloperidol | Placebo | Onset of testing session |
| L-dopa | Placebo | L-dopa | Onset of testing session |
| Placebo | Placebo | Placebo | Onset of testing session |
The present study did not include psychopathology assessments. However, the effects of dopaminergic agents on psychotic experiences were confirmed in a sample of healthy individuals participating in another study with the same design using a psychotic symptom self-rating scale (Appendix 1, available at jpn.ca).
Tasks and procedure
All tasks used were available in 3 parallel versions to minimize practice effects. The various versions of each task were presented in a fixed order across visits, while the order of drug administration was randomized. In this way, performance measures on each substance relied on data from all 3 parallel versions, thereby minimizing version-specific effects.
Evidence gathering
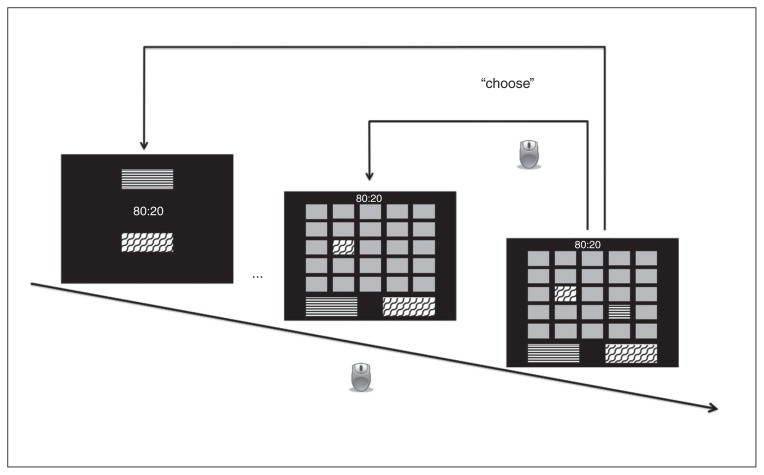
The Box Task is a new paradigm developed for the present study based on the Information Sampling Task of the CANTAB.34 The latter is a gambling task that assesses reward-related impulsivity and decision making. In the Box Task, elements related to the gambling dimension (feedback and monetary reward) have been removed to isolate the effects of data gathering to the best possible extent. In each trial, the participant is presented with a 5 × 5 array of grey boxes on the screen that can be opened per mouse-click to reveal their colour (1 of 2 colors displayed on 2 large panels at the bottom of the screen; Fig. 1). The ratio of the 2 different colours (either 80:20 or 60:40) is displayed at the beginning of a trial and thereafter at the top of the screen throughout the trial. Participants are instructed to open as many boxes as they like before making a decision about which colour is in the majority in the current block and to indicate their decision by clicking on the corresponding coloured panel at the bottom of the screen. The task includes a total of 20 trials (10 per colour ratio), and the distribution of colours across boxes is randomized at each trial. This, in addition to the possibility of opening a total of 25 boxes, allows the Box Task greater flexibility than classical data-gathering paradigms regarding the presented sequences. The variable of interest was the mean number of draws to decision (i.e., mean number of boxes opened before choosing a colour).
Fig. 1.
Box Task. The participant can open boxes with a mouse-click to reveal their colour (indicated by arrows with a mouse symbol; different black and white patterns represent different colours). When the participant selects 1 of the 2 colour panels at the bottom of the screen (upper bent arrow, “choose”), the trial is terminated and a new trial begins.
To assess the validity of the new task, the Fish Task35 was used. The exact procedure has been described elsewhere.28 In summary, participants were shown 2 lakes containing red and blue fish: lake A with 60 red and 40 blue fish, and lake B with the reverse ratio. Ten fish were successively presented in a predetermined sequence; following each draw, participants were asked to indicate whether they had arrived at a decision regarding the origin of the fish (and, if so, which lake it came from) and to provide a probability rating as to the possibility that the fish originated from lake A (data not used in the present analysis). All drawn fish remained visible throughout the task to minimize working memory demands. The task was terminated after presentation of the final fish. The variable of interest was the number of draws to decision.
In view of studies showing higher probability ratings for fish matching the lake colour12 as well as lower probability thresholds to decision35 in patients with delusions, we also conducted subsidiary analyses on these variables. Because such ratings were available only for the Fish Task, the results of these analyses (all negative) are reported in Appendix 1.
Disconfirmatory evidence integration
The Bias Against Disconfirmatory Evidence (BADE) task8 (adapted from Woodward and colleagues10) consists of the presentation of initially ambiguous scenarios that are gradually disambiguated. Each trial begins with an ambiguous statement, followed by 2 further statements that provide disambiguating information. Four possible interpretations are provided for the scenario (the true interpretation, 1 absurd interpretation and 2 plausible lures). After each statement, the participant is required to provide probability ratings for each of the 4 interpretations on a visual analogue scale from 0 to 10 and indicate whether they have reached a decision regarding the true interpretation. The task consists of 16 experimental trials, in which the initial statement favours 1 of the lure interpretations, while the 2 following statements gradually deliver evidence in favour of the true interpretation. Eight further control items, in which the true interpretation is from the beginning the most plausible one, are included to mask the rationale of the paradigm and are not considered in the analyses. In the BADE task, successful integration of disconfirmatory evidence is reflected by a decrease of plausibility ratings for lure items after each successive statement; greater change scores indicate better integration of evidence. Therefore, similar to previous studies, the variables of interest were changes in probability ratings for lure items following the 2 disambiguating statements (second and third) compared with the first statement.36
The 3 tasks were always presented in the same order (Box Task, BADE Task, Fish Task). To rule out performance differences owing to nonspecific effects of the drugs on attention, the d2-test, a letter-cancelation task with well-documented validity and excellent test–retest reliability,37 was also administered at each session.
Participants were paid a total of 80€ (or 40€ plus course credit for students) for their participation in the study, or a proportional amount in case of drop-out before all 3 sessions were completed.
Statistical analysis
To assess the validity of the Box Task, we correlated participant performance (mean number of boxes opened) to the number of draws-to-decision in the Fish Task. Because there was more than 1 observation per participant, we calculated correlation coefficients both between-subjects (assessing whether individuals with high values in one variable also displayed high values in another), and within-subjects (assessing whether increase of one variable within a participant was associated with increase in another variable), according to the procedures proposed by Bland and Altman.38,39 We assessed test–retest reliability for both the Fish Task and the Box Task with the intraclass correlation coefficient (ICC).
Preliminary analyses (repeated-measures analyses of variance [ANOVA]) indicated practice effects in all tasks. In the data-gathering tasks, significant main effects of session (Fish Task: F2,58 = 5.018, p = 0.01; Box Task: F2,58 = 3.187, p = 0.05) indicated a decrease of draws to decision from the first to the second session; this effect was somewhat more pronounced in the 60:40 condition in the Box Task [session × ratio interaction F2,58 = 2.566, p = 0.09). In the BADE task, there was also some evidence of practice effects; the session × statement interaction was not significant (F4,116 = 1.700, p = 0.16), but a significant quadratic contrast (F1,29 = 7.880, p = 0.01) indicated that in the third session initial ratings for lure items (i.e., following the first statement) were lower than for the first session. For the above reason, statistical analyses were conducted using linear mixed models, which allowed simultaneous assessment of both time (session) and substance effects. Linear mixed models carry additional advantages compared with traditional repeated-measures designs, as they can accommodate departures from the assumptions of homogeneity of regression slopes and independence and thus are better suited to model interindividual variability.40,41
For the evidence-gathering tasks, the dependent variable was the mean number of draws to decision. The linear mixed models included the predictors substance (haloperidol, L-dopa, or placebo), session (first, second, third) and, in the case of the Box Task, ratio (60:40 or 80:20); substance and ratio were modelled as repeated-measures predictors. The interactions of ratio × session and ratio × substance were originally also included as predictors, but were removed again as they were nonsignificant. For the BADE task, the dependent variable was change in plausibility scores for lure items following the second and third statement of the scenario. Predictors were substance, session and statement (second, third); substance and statement were modelled as repeated-measures predictors. To adjust for the impact of the initial belief strength on belief change, initial plausibility ratings (which, as detailed above, differed across sessions) were used as a covariate, in accordance with previous studies.11,36 Participant ID was entered as a random factor in all models. We determined the optimal covariance structure for each linear mixed model using goodness-of-fit criteria (Akaike’s information criterion). Significant substance effects were followed-up with post-hoc pairwise comparisons. We applied the Bonferroni correction to correct for multiple comparisons; adjusted p values (corresponding to observed p values × number of comparisons) are reported. In addition, polynomial contrasts were conducted to investigate the hypothesis that participant performance would show a linear trend from haloperidol (lowest bias) to placebo to L-dopa (highest bias).
Results
We included 30 healthy individuals aged 18–40 years (13 men, mean age 25.1 ± 5.66 yr) in our analyses. All 30 participants completed all 3 testing sessions. There were no significant differences among the 3 substances in d2-scores (F2,58 = 1.534, p = 0.22) in a repeated-measures ANOVA with substance as the within-subjects factor. A 3 (substance) × 3 (time: baseline, ingestion of second capsule, end of testing session) analysis showed no significant differences regarding adverse effects (main effect of substance: F2,54 = 2.113, p = 0.15; time × substance interaction: F4,108 = 0.669, p = 0.46). There were no drop-outs and no premature session terminations owing to adverse effects. There was also no association between ingested and guessed substance (χ26 = 5.67, p = 0.46). Performance patterns for each substance and task are presented in Figure 2 and Figure 3.
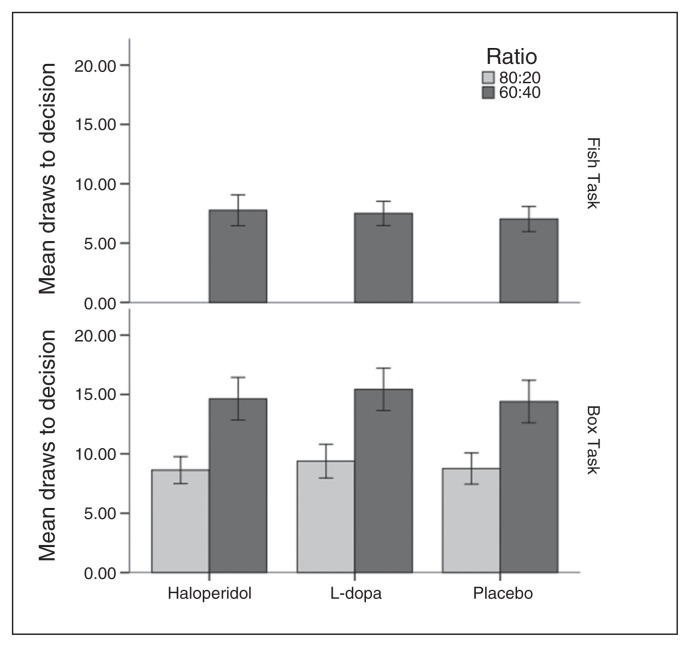
Fig. 2.
Performance in the Box Task and the Fish Task (draws to decision) depending on substance and (for the Box Task) on colour ratio. Error bars represent 95% confidence intervals.
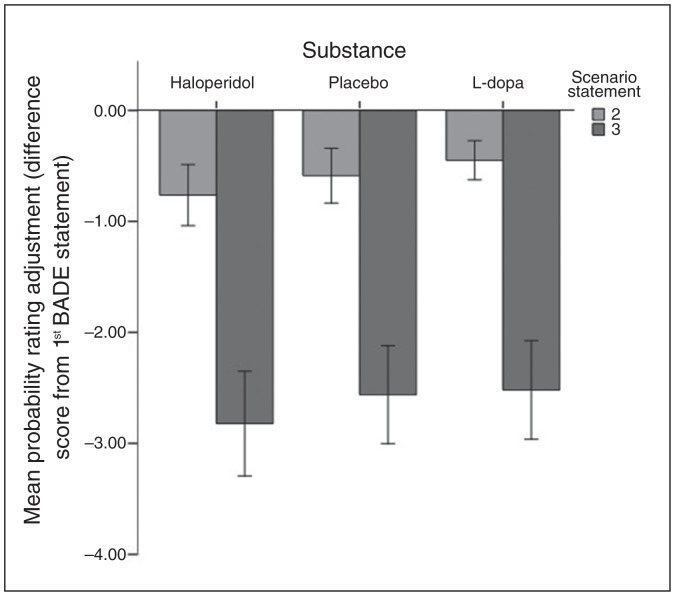
Fig. 3.
Mean change in probability ratings for lure items following the second and third statements of Bias Against Disconfirmatory Evidence (BADE) task scenarios, for each of the 3 substances. Bars represent the difference from baseline (first BADE statement). Error bars represent 95% confidence intervals.
Box Task validity and reliability
Between-subjects analyses showed that number of draws to decision in the Fish Task (60:40 ratio) correlated significantly with the mean number of draws to decision in the 60:40 condition of the Box Task (r = 0.451, p = 0.012), but not with the 80:20 condition of the Box Task (r = 0.231, p = 0.22). Within participants, Fish Task performace significantly correlated with both the 60:40 (r = 0.296, p = 0.012) and the 80:20 (r = 0.266, p = 0.040) condition of the Box Task. Test–retest reliability was excellent for both conditions of the Box Task (60:40 condition: ICC 0.770, 95% confidence interval [CI] 0.626–0.873, F = 11.056, p < 0.001; 80:20 condition: ICC 0.786, 95% CI 0.649–0.883, F = 12.016, p < 0.001) and adequate for the Fish Task (ICC 0.507, 95% CI 0.291–0.701, F = 4.088, p < 0.001).
Effects of dopaminergic modulation
In the Fish Task, there was a significant effect of session (F2,48.62 = 3.564, p = 0.036), but no significant effect of substance (F2,38.34 = 1.087, p = 0.35) (mean differences: haloperidol v. placebo 0.830, 95% CI −0.639 to 2.299; L-dopa v. placebo 0.431, 95% CI −0.708 to 1.571; haloperidol v. L-dopa 0.398, 95% CI −1.031 to 1.828).
Session was also significant in the case of the Box Task (F2,92.86 = 3.271, p = 0.042), as was ratio (there were significantly more draws to decision in the 60:40 than in the 80:20 condition [F1,43.96 = 223.886, p < 0.001]), but not substance (F2,67.89 = 1.877, p = 0.16). Mean differences for pairwise comparisons between substances were as follows: haloperidol versus placebo −0.055, 95% CI −0.788 to 0.679; L-dopa versus placebo 0.660, 95% CI −0.270 to 1.590; haloperidol versus L-dopa −0.715, 95% CI −1.689 to 0.259 (all CIs based on Bonferroni correction).
In the BADE Task, there was a significant effect of substance (F2,80.87 = 3.568, p = 0.033); a significant linear effect was observed (t = 2.436, p = 0.017) indicating a gradual increase in disconfirmatory evidence integration capacity from L-dopa to placebo to haloperidol, while the quadratic effect was not significant (p = 0.34). Post hoc pairwise comparisons with Bonferroni correction indicated that changes in probability ratings for lure items were more pronounced for haloperidol than L-dopa at a level of marginal significance (mean difference 0.200, 95% CI −0.001 to 0.401, Bonferroni-corrected p = 0.05). There were no significant differences between placebo and the other 2 substances (haloperidol v. placebo: mean difference 0.166, 95% CI −0.025 to 0.358, corrected p = 0.11; placebo v. L-dopa: mean difference 0.034, 95% CI −0.170 to 0.237, corrected p = 0.99). The main effect of statement (F1,94.32 = 598.027, p < 0.001) and the initial probability rating entered as a covariate (F1,74.45 = 66.751, p < 0.001) were also significant.
Discussion
The present study investigated the effects of dopaminergic manipulation on 2 conceptually distinct delusion-associated reasoning biases pertaining to evidence gathering and incorrigibility. Single-dose haloperidol, L-dopa and placebo were administered to healthy participants within a randomized, double-blind, 3-way crossover design. In accordance with our previous results,28 data gathering was not affected by dopaminergic agents. In contrast, the ability of participants to integrate disconfirmatory evidence showed a significant linear dopaminergic modulation pattern (highest with haloperidol, intermediate with placebo, lowest with L-dopa), with the difference between haloperidol and L-dopa marginally reaching significance. These results suggest successful modulation of disconfirmatory evidence integration when dopaminergic transmission was enhanced compared with when it was decreased from normal levels, consistent with our hypothesis and our previous results regarding overconfidence in errors.
With regard to evidence gathering, our findings corroborate those of previous studies that have failed to find significant changes following administration of psychotomimetic substances directly or indirectly associated with the dopamine system, such as ketamine42 and methamphetamine.43 Interestingly, another study noted more cautious behaviour (i.e., increased number of draws to decision) in the Information Sampling Task in healthy participants after administration of pramipexole, a D2/D3 dopamine receptor agonist.44 Given the well-established association between reward processing and the dopamine system,45 the fact that the Information Sampling Task entails an explicit reward dimension that is absent in the Box Task might account for the differences between these 2 studies.
The 2 investigated reasoning biases could not be assessed in the context of a single task, such that it was not possible to directly assess interactions between the factors substance and bias type (i.e., incorrigibility v. jumping-to-conclusions bias). Still, the present results suggest that different reasoning biases are differentially associated with dopaminergic processes. This conclusion is of some consequence for the treatment of patients with psychotic disorders, as it opens the way for research into additional treatment approaches that are not dependent on dopamine system modulation. For example, reasoning biases are amenable to specific cognitive interventions;23,24 hence, it is possible that such interventions might have an independent and/or complementary effect to that of antipsychotic medication when treating patients with psychotic disorders. Indeed, it has been shown that interventions that explicitly address reasoning biases lead to significantly greater reductions in delusional severity and/or conviction23 when applied as an adjunct to pharmacological treatment compared with antipsychotic treatment alone.
Research into the neurobiological substrates of reasoning biases might help refine psychopathological models of psychosis. A considerable amount of evidence suggests that dysregulated dopaminergic activity results in psychotic symptoms through disturbed information processing within the reward system, which leads to a state of aberrant allocation of salience to random stimuli.46 Early accounts proposed that delusion-associated reasoning biases are simply a result of this state of aberrant salience.47 Our findings suggest that this might be the case for incorrigibility, but not for evidence gathering. Emerging neuroimaging evidence supports this distinction. Subjective response confidence in the context of difficult tasks48 and prediction errors on subjective confidence tasks49 have been associated with activations in the ventral striatum (a part of the reward system). It has also been suggested that the latter activations reflect self-generated dopaminergic signals that assume the role of feedback in the absence of an external outcome.49 In contrast, a core feature of the jumping-to-conclusions bias, the willingness to seek more information, was associated with activations of the inferior parietal cortex,50 a region not related to the reward/salience system. These findings are best accounted for by a “dual-disturbance” theory of delusions,2 suggesting that delusions result from the combined effect of 2 distinct types of cognitive disturbance — 1 leading to the generation of an implausible thought and 1 contributing to the uncritical acceptance of this thought. These 2 cognitive disturbances could correspond to aberrant salience and disturbed evidence gathering, respectively. Indirect evidence from studies in patients with schizophrenia also points to a dissociation between the jumping-to-conclusions bias and aberrant salience.18,51 However, the exact association between salience and reasoning biases remains to be assessed in future studies.
Limitations
Certain limitations of the study need to be acknowledged. First, drug plasma-level assessments were not possible. However, the drug doses used were similar to previous studies of dopaminergic modulation,52,53 and the same doses had produced the expected results in an indirect validity check in our previous study.28 Second, it should be pointed out that the psychotic state includes several changes in dopaminergic system function,54 which cannot be fully approximated by single-dose administration of dopaminergic substances to healthy individuals. As a related point, the observed changes in disconfirmatory evidence integration after administration of L-dopa in this study may have been in the expected direction, but it is not certain that their extent was similar to the impairment reported in delusional patients, as the present study did not include a comparison to a demographically matched patient group. Moreover, although single-dose administration of haloperidol and L-dopa led to the expected changes on a self-rated scale of psychotic experiences in another sample of healthy individuals (Appendix 1), no such ratings were available for the present sample, making it difficult to draw any parallels between dopaminergic modulations of reasoning biases and symptoms. It should also be noted that, although comparisons of the 2 evidence-gathering tasks support the validity of the Box Task, there are as yet no data on the performance of patients with delusions compared with healthy controls on this task. Thus, although single-dose administration of dopaminergic substances in healthy individuals provides useful insights into the association of reasoning biases with dopaminergic activity, uncritical generalizations of findings to clinical populations are unwarranted.
Conclusion
The present study was able to replicate previous results, suggesting that reasoning biases pertaining to evidence gathering and incorrigibility are differentially influenced by dopaminergic modulation in healthy participants. This finding supports a dual-disturbance account of delusions and provides a plausible neurobiological basis for the use of interventions targeted at improving reasoning biases as an adjunctive treatment in patients with psychotic disorders.
Acknowledgments
This word was supported by a grant to C. Andreou by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG, Project-Nr: AN970/1-1). The authors thank Yanis Konstantinou and Mihail Mihov for programming the Box Task.
Footnotes
Competing interests: J. Gallinat reports receiving speaker fees from Janssen and a grant from Astra outside the submitted work. No other competing interests declared.
Contributors: C. Andreou, J. Gallinat and S. Moritz designed the study. V. Braun and K. Kolbeck acquired the data, which C. Andreou, B. Schneider, J. Gallinat and S. Moritz analyzed. C. Andreou wrote the article, which all authors reviewed and approved for publication.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edition. Washington, DC: 2000. Text revision. [Google Scholar]
- 2.Langdon R, Ward PB, Coltheart M. Reasoning anomalies associated with delusions in schizophrenia. Schizophr Bull. 2010;36:321–30. doi: 10.1093/schbul/sbn069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broome MR, Johns LC, Valli I, et al. Delusion formation and reasoning biases in those at clinical high risk for psychosis. Br J Psychiatry Suppl. 2007;51:s38–42. doi: 10.1192/bjp.191.51.s38. [DOI] [PubMed] [Google Scholar]
- 4.Fine C, Gardner M, Craigie J, et al. Hopping, skipping or jumping to conclusions? Clarifying the role of the JTC bias in delusions. Cogn Neuropsychiatry. 2007;12:46–77. doi: 10.1080/13546800600750597. [DOI] [PubMed] [Google Scholar]
- 5.Zawadzki JA, Woodward TS, Sokolowski HM, et al. Cognitive factors associated with subclinical delusional ideation in the general population. Psychiatry Res. 2012;197:345–9. doi: 10.1016/j.psychres.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Menon M, Quilty LC, Zawadzki JA, et al. The role of cognitive biases and personality variables in subclinical delusional ideation. Cogn Neuropsychiatry. 2013;18:208–18. doi: 10.1080/13546805.2012.692873. [DOI] [PubMed] [Google Scholar]
- 7.Sanford N, Veckenstedt R, Moritz S, et al. Impaired integration of disambiguating evidence in delusional schizophrenia patients. Psychol Med. 2014;44:2729–38. doi: 10.1017/S0033291714000397. [DOI] [PubMed] [Google Scholar]
- 8.Veckenstedt R, Randjbar S, Vitzthum F, et al. Incorrigibility, jumping to conclusions, and decision threshold in schizophrenia. Cogn Neuropsychiatry. 2011;16:174–92. doi: 10.1080/13546805.2010.536084. [DOI] [PubMed] [Google Scholar]
- 9.Garety PA, Freeman D. The past and future of delusions research: from the inexplicable to the treatable. Br J Psychiatry. 2013;203:327–33. doi: 10.1192/bjp.bp.113.126953. [DOI] [PubMed] [Google Scholar]
- 10.Woodward TS, Buchy L, Moritz S, et al. A bias against disconfirmatory evidence is associated with delusion proneness in a nonclinical sample. Schizophr Bull. 2007;33:1023–8. doi: 10.1093/schbul/sbm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodward TS, Moritz S, Cuttler C, et al. The contribution of a cognitive bias against disconfirmatory evidence (BADE) to delusions in schizophrenia. J Clin Exp Neuropsychol. 2006;28:605–17. doi: 10.1080/13803390590949511. [DOI] [PubMed] [Google Scholar]
- 12.Speechley WJ, Whitman JC, Woodward TS. The contribution of hypersalience to the “jumping to conclusions” bias associated with delusions in schizophrenia. J Psychiatry Neurosci. 2010;35:7–17. doi: 10.1503/jpn.090025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Köether U, Veckenstedt R, Vitzthum F, et al. “Don’t give me that look” — overconfidence in false mental state perception in schizophrenia”. Psychiatry Res. 2012;196:1–8. doi: 10.1016/j.psychres.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Moritz S, Woodward TS, Jelinek L, et al. Memory and metamemory in schizophrenia: a liberal acceptance account of psychosis. Psychol Med. 2008;38:825–32. doi: 10.1017/S0033291707002553. [DOI] [PubMed] [Google Scholar]
- 15.Moritz S, Veckenstedt R, Hottenrott B, et al. Different sides of the same coin? Intercorrelations of cognitive biases in schizophrenia. Cogn Neuropsychiatry. 2010;15:406–21. doi: 10.1080/13546800903399993. [DOI] [PubMed] [Google Scholar]
- 16.So SH, Freeman D, Dunn G, et al. Jumping to conclusions, a lack of belief flexibility and delusional conviction in psychosis: a longitudinal investigation of the structure, frequency, and relatedness of reasoning biases. J Abnorm Psychol. 2012;121:129–39. doi: 10.1037/a0025297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garety PA, Freeman D, Jolley S, et al. Reasoning, emotions, and delusional conviction in psychosis. J Abnorm Psychol. 2005;114:373–84. doi: 10.1037/0021-843X.114.3.373. [DOI] [PubMed] [Google Scholar]
- 18.Menon M, Mizrahi R, Kapur S. ‘Jumping to conclusions’ and delusions in psychosis: relationship and response to treatment. Schizophr Res. 2008;98:225–31. doi: 10.1016/j.schres.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 19.So SH, Garety PA, Peters ER, et al. Do antipsychotics improve reasoning biases? A review. Psychosom Med. 2010;72:681–93. doi: 10.1097/PSY.0b013e3181e7cca6. [DOI] [PubMed] [Google Scholar]
- 20.So SH, Peters ER, Swendsen J, et al. Changes in delusions in the early phase of antipsychotic treatment — an experience sampling study. Psychiatry Res. 2014;215:568–73. doi: 10.1016/j.psychres.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 21.Moritz S, Woodward TS, Ruff CC. Source monitoring and memory confidence in schizophrenia. Psychol Med. 2003;33:131–9. doi: 10.1017/s0033291702006852. [DOI] [PubMed] [Google Scholar]
- 22.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–62. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moritz S, Andreou C, Schneider BC, et al. Sowing the seeds of doubt: a narrative review on metacognitive training in schizophrenia. Clin Psychol Rev. 2014;34:358–66. doi: 10.1016/j.cpr.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Garety P, Waller H, Emsley R, et al. Cognitive mechanisms of change in delusions: an experimental investigation targeting reasoning to effect change in paranoia. Schizophr Bull. 2015;41:400–10. doi: 10.1093/schbul/sbu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–45. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 26.Eifler S, Rausch F, Schirmbeck F, et al. Neurocognitive capabilities modulate the integration of evidence in schizophrenia. Psychiatry Res. 2014;219:72–8. doi: 10.1016/j.psychres.2014.04.056. [DOI] [PubMed] [Google Scholar]
- 27.Garety P, Joyce E, Jolley S, et al. Neuropsychological functioning and jumping to conclusions in delusions. Schizophr Res. 2013;150:570–4. doi: 10.1016/j.schres.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andreou C, Moritz S, Veith K, et al. Dopaminergic modulation of probabilistic reasoning and overconfidence in errors: a double-blind study. Schizophr Bull. 2014;40:558–65. doi: 10.1093/schbul/sbt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huq SF, Garety PA, Hemsley DR. Probabilistic judgements in deluded and non-deluded subjects. Q J Exp Psychol A. 1988;40:801–12. doi: 10.1080/14640748808402300. [DOI] [PubMed] [Google Scholar]
- 30.Peters E, Garety P. Cognitive functioning in delusions: a longitudinal analysis. Behav Res Ther. 2006;44:481–514. doi: 10.1016/j.brat.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Woodward TS, Munz M, LeClerc C, et al. Change in delusions is associated with change in “jumping to conclusions”. Psychiatry Res. 2009;170:124–7. doi: 10.1016/j.psychres.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 32.Andreou C, Veith K, Bozikas VP, et al. Effects of dopaminergic modulation on automatic semantic priming: a double-blind study. J Psychiatry Neurosci. 2014;39:110–7. doi: 10.1503/jpn.130035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardman JG, Limbird LE, Gilman AG, editors. Goodman & Gilmans The pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill; 2001. [Google Scholar]
- 34.Clark L, Robbins TW, Ersche KD, et al. Reflection impulsivity in current and former substance users. Biol Psychiatry. 2006;60:515–22. doi: 10.1016/j.biopsych.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Moritz S, Van Quaquebeke N, Lincoln TM. Jumping to conclusions is associated with paranoia but not general suspiciousness: a comparison of two versions of the probabilistic reasoning paradigm. Schizophr Res Treatment. 2012;2012:384039. doi: 10.1155/2012/384039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woodward TS, Moritz S, Menon M, et al. Belief inflexibility in schizophrenia. Cogn Neuropsychiatry. 2008;13:267–77. doi: 10.1080/13546800802099033. [DOI] [PubMed] [Google Scholar]
- 37.Brickenkamp R. Test d2 Aufmerksamkeits-Belastungs-Test. 7th ed. Göttingen, Toronto, and Zürich: Verlag für Psychologie (Hogrefe); 1981. [Google Scholar]
- 38.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part 2 — correlation between subjects. BMJ. 1995;310:633. doi: 10.1136/bmj.310.6980.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part 1 — correlation within subjects. BMJ. 1995;310:446. doi: 10.1136/bmj.310.6977.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Field A. Discovering statistics using IBM SPSS statistics. London: SAGE Publications Ltd; 2013. [Google Scholar]
- 41.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–7. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- 42.Evans S, Almahdi B, Sultan P, et al. Performance on a probabilistic inference task in healthy subjects receiving ketamine compared with patients with schizophrenia. J Psychopharmacol. 2012;26:1211–7. doi: 10.1177/0269881111435252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ermakova AO, Ramachandra P, Corlett PR, et al. Effects of methamphetamine administration on information gathering during probabilistic reasoning in healthy humans. PLoS ONE. 2014;9:e102683. doi: 10.1371/journal.pone.0102683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ersche KD, Roiser JP, Lucas M, et al. Peripheral biomarkers of cognitive response to dopamine receptor agonist treatment. Psychopharmacology (Berl) 2011;214:779–89. doi: 10.1007/s00213-010-2087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 46.Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis — linking biology, pharmacology and phenomenology of psychosis. Schizophr Res. 2005;79:59–68. doi: 10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10:48–58. doi: 10.1038/nrn2536. [DOI] [PubMed] [Google Scholar]
- 48.Schwarze U, Bingel U, Badre D, et al. Ventral striatal activity correlates with memory confidence for old- and new-responses in a difficult recognition test. PLoS ONE. 2013;8:e54324. doi: 10.1371/journal.pone.0054324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daniel R, Pollmann S. Striatal activations signal prediction errors on confidence in the absence of external feedback. Neuroimage. 2012;59:3457–67. doi: 10.1016/j.neuroimage.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 50.Furl N, Averbeck BB. Parietal cortex and insula relate to evidence seeking relevant to reward-related decisions. J Neurosci. 2011;31:17572–82. doi: 10.1523/JNEUROSCI.4236-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Averbeck BB, Evans S, Chouhan V, et al. Probabilistic learning and inference in schizophrenia. Schizophr Res. 2011;127:115–22. doi: 10.1016/j.schres.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bunzeck N, Doeller CF, Dolan RJ, et al. Contextual interaction between novelty and reward processing within the mesolimbic system. Hum Brain Mapp. 2012;33:1309–24. doi: 10.1002/hbm.21288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Copland DA, McMahon KL, Silburn PA, et al. Dopaminergic neuromodulation of semantic processing: a 4-T FMRI study with levodopa. Cereb Cortex. 2009;19:2651–8. doi: 10.1093/cercor/bhp017. [DOI] [PubMed] [Google Scholar]
- 54.Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–86. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]