Abstract
Choice of an appropriate promoter is critical to express target genes in intended tissues and developmental stages. However, promoters capable of directing gene expression in specific tissues and stages are not well characterized in monocot species. To identify such a promoter in wheat, this study isolated a partial sequence of the wheat small subunit of RuBisCO (TarbcS) promoter. In silico analysis revealed the presence of elements that are characteristic to rbcS promoters of other, mainly dicot, species. Transient expression of the TarbcS:GUS in immature wheat embryos and tobacco leaves but not in the wheat roots indicate the functionality of the TarbcS promoter fragment in directing the expression of target genes in green plant tissues.
Keywords: promoter, rbcS, reporter gene, tissue specificity, transient expression, wheat
A promoter is a DNA sequence located upstream of the coding region of a given gene. It contains specific sequences and response elements that function as binding sites for RNA polymerase and transcription factors that regulate gene expression. Several promoters have been identified from different organisms, for example from virus and plants, and are used for different purposes in plant molecular biology studies such as to express the gene of interest either constitutively or in a tissue- or stage- specific manner. In plant biotechnology, it is very critical to make the right choice of promoters for successful transfer of the gene of interest into plants and its subsequent expression in the targeted tissue and developmental stage to avoid undesirable consequences.1
Constitutive promoters are often used to drive high levels of gene expression in nearly all tissues across all developmental stages and over a wide range of environmental conditions. The maize ubiquitin 1 (Ubi1)2 and the rice actin 1 (Act1)3 promoters, and the 35S promoter from Cauliflower Mosaic Virus (CaMV)4 are typical examples of such kind of promoters and are frequently used in plant biotechnological applications. The Ubi1 and Act1 promoters are often used with their first untranslated exon and intron, as inclusion of these elements results in strong expression of the transgene.5 The other kinds of promoters used in transgene expression are the tissue/stage specific promoters that restrict the expression of transgenes in targeted tissues or developmental stages, and thereby reduce unintended effects in non-target tissues/stages. Some examples of such promoters include the seed-specific high molecular weight glutenin subunit (HMW-GS) promoter isolated from monocot species,6 and the promoter derived from the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) of dicot species,7 which is activated specifically in the photosynthetic tissues. There are also promoters originated from genes of monocot species that can be induced under certain conditions. For example, the promoter of a wheat gene encoding glutathione-S-transferase (GstA1) is known to direct strong gene expression preferentially in the epidermis. As a result, the promoter of GstA1 has been used as a tool to express defense-related genes in the inner leaf tissues to confer plants with resistance against pathogens that enter through the epidermis.8 The barley abundant protein (HVA1) gene encoding the late embryogenesis abundant 3 (LEA3), one of the proteins involved in controlling the closure of leaf stomata to prevent evaporation and thereby cellular dehydration, has been shown to be induced by ABA, and stress conditions including cold, heat, drought and salinity.9 This result indicates the presence of ABA and stress responsive elements in the promoter of HVA1.
RuBisCO, the most abundant protein on earth, catalyzes the first step of carbon fixation in plants. In higher plants, it is composed of 8 large and small subunits arranged in 4 dimers. The large subunits of RuBisCO (rbcL) are encoded in the chloroplast, whereas the small subunits (rbcS) are encoded in the nucleus.10 Although both subunits are important for the functionality of the protein, the transcript levels of rbcS have been considered as a factor determining the level of RuBisCO in plants.11 Because of this, the rbcS promoter has been a popular element of choice in molecular studies. In most plants, rbcS is encoded by a multigene family consisting of 2 to 22 members.11–13 Its promoter has been well characterized mainly in dicot species, and is found to contain elements that confer light inducible and tissue specific gene expressions. It has been shown previously that elements found in gene promoter regions are responsible for mediating light-inducible gene expressions.14 Cis-acting elements that are important for light regulated gene expression such as the I-box and G-box are often present in the promoters of RuBisCo small subunit gene family members.15,16 Promoters containing the I-box and G-box cis-elements are reported to induce gene expression to a level similar to that produced by the constitutive CaMV 35S promoter.17 Another consensus sequence, designated as GT-1, has been found in the promoter of several light regulated genes, including the rbcS of pea and other dicot species,18 and is suggested to have a role in stabilizing the TATA box complex that acts as a binding site for general transcription factors and thereby involve in gene transcription by RNA polymerase. The distance between GT-1 binding sites within the light responsive element of the rbcS promoter plays an important role in determining the level of gene transcription.19
Several studies in dicot species have shown that the rbcS promoter can be used as tool to restrict the expression of target genes in photosynthetic tissues. For example, the rbcS promoter of tobacco has been used to express a nitrilase gene (bxn) from a soil bacterium, Klebsiella ozaenae, in tobacco leaves to provide resistance against the herbicide bromoxynil.20 The rice rbcS promoter has also been successfully used to express a truncated delta-endotoxin gene, cryIA (b), of Bacillus thuringiensis in rice leaves to provide resistance against injury by insects. In cotton, fusion of the rbcS promoter with β-glucuronidase (GUS) reporter gene was used to characterize the expression patterns of rbcS in the leaf tissue.17 Furthermore, the rbcS promoter derived from Coffea arabica has been shown to be light regulated and function as leaf-specific promoter in tobacco plants.21 However, identification and functional characterization of this molecular element in monocot species is still lagging behind. Previous study has shown that the wheat rbcS (TarbcS) promoter is active in the maize leaf mesophyll protoplasts.22 However; the activity of this promoter in other green tissues such as immature embryos and non-green tissues such as roots has not been examined. This study investigated the functionality of TarbcS promoter in directing the expression of target genes in these tissues, and also compared its activity with that of CaMV 35S and Ubi1 promoters.
In order to isolate the DNA fragment of TarbcS promoter, forward (5′-TCAATAGTTGCCTTGCGAG-3′) and reverse (5′-ATCGGAGGAGAGGAGGA-3′) primers designed from a region upstream of the start codon of TarbcS (GenBank ID: AB042069) were used to amplify the genomic DNA samples prepared from leaf tissues of hexaploid wheat, cultivar Chinese Spring. After separation on a 1% agarose gel, the amplification product was gel-purified, cloned into pGEM-T Easy vector (Promega, Fitchburg, WI, USA) using HindIII and BamHI restriction sites, and then sequenced. The binary vector pBI121 was constructed to carry the newly isolated TarbcS promoter fragment or the constitutive CaMV 35S promoter (used as a positive control) driving the GUS reporter gene. Alternative construct carrying the GUS driven by the Ubi1 promoter was also prepared with vector pBRACT214. The promoterless pCAMBIA1391Z vector was used as a negative control. Transformation of the different tissues with the constructs was carried out through bombardment with gold particles using the particle delivery system PDS-1000/He (BioRad, Hercules, CA, USA). To this effect, gold particles (1.0 μm) coated with 5 μg plasmid DNA of each construct were bombarded into tobacco leaves harvested from 3- to 4-week-old plants, wheat embryos (14 d after anthesis [DAA]), and wheat roots harvested from 5- to 7-day-old seedlings using 950 psi of He pressure and 27 mmHg of vacuum pressure. Following the particle bombardment, tissues were incubated in darkness at room temperature for 48 h, and then subjected to GUS staining with a solution consisting of 50 mM sodium phosphate buffer (pH 7.0); 10 mM EDTA, 0.5 mM ferrocyanide, 0.5 mM ferricyanide, 0.05% Triton X-100 and 1 mg/mL X-glucuronide at 37°C for 24 h. The resulting blue staining representing the expressions of GUS was observed under a light microscope.
Amplification of the wheat genomic DNA with the specific primers described above produced a promoter fragment of expected size. Sequencing of the DNA fragment revealed a size of 571 bp (Fig. 1). Analysis of the DNA sequence using PLACE (http://www.dna.affrc.go.jp/PLACE/index.html)23 and PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)24 revealed the presence of elements that are characteristic to an rbcS promoter (Fig. 1). These elements include the I-Box (GATAAG) and the GT-1 consensus sequence (GRWAAW; R = A/G, W = A/T). The I-Box core element has been identified in the upstream region of several light regulated genes, including the rbcS of several dicot species such as Arabidopsis and tomato.15,16 The GT-1 consensus sequence, which exist in several light induced genes,18 is proposed to play an important role in stabilizing the TATA box complex. Our analysis also revealed the presence of repetitive GATA sequence (Fig. 1), which is associated with light induced and tissue-specific gene expressions in plants.25,26 Furthermore, the TarbcS promoter fragment consists of the CAAT box, one of the promoter sequence shown to be necessary for gene transcription, for example in pea legumin gene.27 Putative transcriptional start site of the promoter was also predicted using the TSSP/Prediction of PLANT Promoters (Using RegSite Plant DB, Softberry Inc.) program.28
Figure 1.

Nucleotide sequence of the promoter fragment of TarbcS (GenBank accession number AB042069.1). The TATA-box, CAAT-box, I-box, GT1-consensus sequences are boxed in green. The GATA sequences are shown in red fonts. The putative transcriptional start site is indicated by an asterisk while the start of the initiation codon is shown in bold and underlined. Primers used for PCR are shown with arrows. Sequences in bold italic fonts represent the first intron.
Transient expression of the TarbcS:GUS construct in immature wheat embryos under light but not in darkness resulted in stronger blue staining (Fig. 2A and B), indicating the functionality of the TarbcS promoter fragment in directing gene expression in this tissue and also its regulation by light. However, the degree of blue staining observed was much less than that produced by the CaMV35S:GUS construct (Fig. 2C). The detection of the leaf specific TarbcS promoter activity in the immature embryos might be due to the fact that these tissues are totipotent at that stage.29 It has been reported that prominent differentiation of the wheat embryo into the axial organs including coleoptile, shoot and root primordia and coleorhiza is initiated by 20 DAA.30
Figure 2.
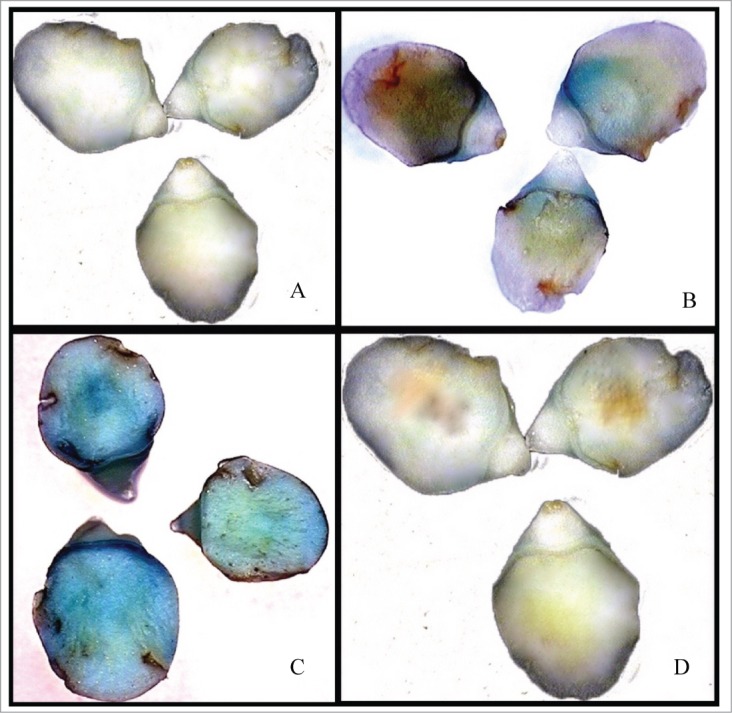
Transient expression of GUS in immature wheat embryos. Embryos incubated under darkness before and after transformation with TarbcS:GUS construct (A), embryos incubated in light before and after transformation with TarbcS:GUS construct (B), embryos transformed with a CaMV 35S:GUS construct (C), and embryos transformed with a vector carrying the GUS with no promoter as a negative control (D).
In the case of transient expression of the same constructs in tobacco leaves, we expressed the degree of blue staining produced in the leaves as Transient Expression Unit (TEU),31 which refers to the average number of blue spots per GUS expressing leaves. The TEU exhibited by leaves expressing the TarbcS:GUS construct was over 3-fold less than those expressing CaMV35S:GUS (Figs. 3C and D; Table 1). Unlike that observed in the immature wheat embryos, light did not have any effect on the number of blue spots produced in the leaf discs transformed with GUS under the control of either of the promoters. Although it is less efficient than the CaMV 35S promoter, our result shows that the newly isolated TarbcS promoter fragment is active in controlling gene expression in leaf tissues. Consistent with this result, activity of the TarbcS promoter has been reported in maize leaf mesophyll protoplasts.22 Transient expression of the GUS reporter gene is influenced by several factors including, but not limited to, incubation period for GUS staining and physiological state of the cells. Therefore, further optimization of these conditions might improve the efficiency of the TarbcS promoter fragment in directing the expression of the GUS. Alternatively, adding other regions of the gene such as the first intron to the promoter might enhance its efficiency of directing gene expression, as observed for some other promoters such as Ubi1 and Act1.5 Since the TarbcS promoter identified in this study represents only a partial fragment, we cannot exclude the possibility that other regulatory elements required for a strong expression are missing.
Figure 3.
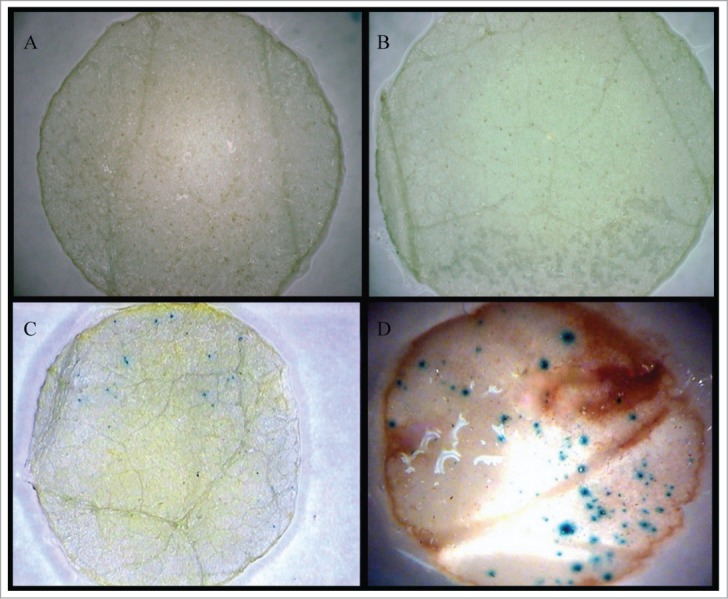
Transient expression of GUS in young leaves of tobacco. Untransformed leaves subjected to X-glucuronidase staining (A), leaves transformed with a vector carrying GUS but with no promoter as negative control (B), leaves transformed with TarbcS:GUS construct (C), and leaves transformed with a CaMV35S:GUS construct (D).
Table 1.
Comparative analysis of GUS reporter gene expression directed by the TarbcS and CaMV35S promoters
| Promoter | Total number of leaf discs bombarded | Total number of leaf discs showing GUS expression | Average TEUa/GUS expressing leaf discs |
|---|---|---|---|
| TarbcS | 116 | 23 | 21 |
| CaMV35S | 74 | 57 | 70 |
TEU = Transient Expression Unit; the number of blue spots showing GUS expression.
The TarbcS:GUS construct was also used to examine the functionality of the newly isolated promoter fragment in wheat root, a tissue that normally grows with no exposure to light. Our analysis indicated no sign of blue staining in roots transformed with the TarbcS:GUS (Fig. 4C) or CaMV35S:GUS (data not shown) and incubated in darkness before staining. Although the underlying reason for the inability of the constitutive CaMV 35S promoter to drive the expression of GUS in the roots is unknown, a similar result has been reported in cassava in which the same promoter successfully directed GUS expression in other tissues but not in roots.32 Transformation of the young wheat roots with Ubi1:GUS construct, however, produced blue staining (Fig. 4D), suggesting the significance of the origin of a given promoter in regulating gene expression in plant species. Comparative analysis of the expression of GUS driven by 4 different promoters in creeping bentgrass has also shown that the rice Act1 or maize Ubi1 promoters performed better in directing GUS expression in root tissues than the CaMV 35S and potato Ubi promoters.33
Figure 4.
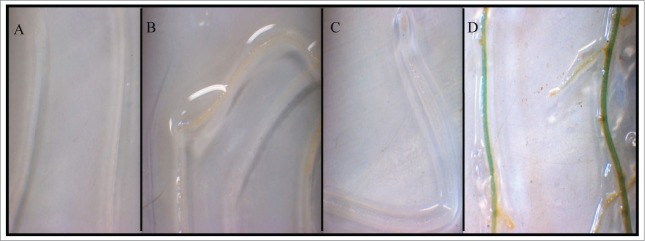
Transient expression of GUS in young roots of wheat. Untransformed roots subjected to X-glucuronidase staining (A), roots transformed with a vector carrying GUS but with no promoter as a negative control (B), roots transformed with TarbcS:GUS construct (C), and roots transformed with Ubi1:GUS construct (D).
In summary, this study showed that the TarbcS promoter is functional in regulating gene expression in wheat immature (not completely differentiated) embryos but not in roots. It also confirmed that the TarbcS promoter is active in leaf tissues.22 However; further study is required to elucidate the role of each regulatory element of the promoter in directing gene expression.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada to BTA.
References
- 1. Dale PJ, Clarke B, Fontes EMG. Potential for the environmental impact of transgenic crops. Nat Biotechnol 2002; 20:567-74; PMID:12042859; http://dx.doi.org/ 10.1038/nbt0802-843b [DOI] [PubMed] [Google Scholar]
- 2. Christensen AH, Sharrock RA, Quail PH. Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 1992; 18:675-89; PMID:1313711; http://dx.doi.org/ 10.1007/BF00020010 [DOI] [PubMed] [Google Scholar]
- 3. McElroy D, Zhang W, Cao J, Wu R. Isolation of an efficient actin promoter for use in rice transformation. Plant Cell 1990; 2:163-71; PMID:2136633; http://dx.doi.org/ 10.1105/tpc.2.2.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Odell JT, Nagy F, Chua NH. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 1985; 313:810-2; PMID:3974711; http://dx.doi.org/ 10.1038/313810a0 [DOI] [PubMed] [Google Scholar]
- 5. Hensel G, Himmelbach A, Chen D, Douchkov K, Kumlehn J. Transgene expression systems in the Triticeae cereals. J Plant Physiol 2011; 168:30-44; PMID:20739094; http://dx.doi.org/ 10.1016/j.jplph.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 6. Lamacchia C, Shewry PR, Di Fonzo N, Forsyth JL, Harris N, Lazzeri PA, Napier JA, Halford NG, Barcelo P. Endosperm-specific activity of a storage protein gene promoter in transgenic wheat seed. J Exp Bot 2001; 52:243-50; PMID:11283168; http://dx.doi.org/ 10.1093/jexbot/52.355.243 [DOI] [PubMed] [Google Scholar]
- 7. Wang Y, Fu B, Pan L, Chen L, Fu X, Li K. Overexpression of Arabidopsis Dof1, GS1 and GS2 enhanced nitrogen assimilation in transgenic tobacco grown under low-nitrogen conditions. Plant Mol Biol Report 2013; 31:886-900; http://dx.doi.org/ 10.1007/s11105-013-0561-8 [DOI] [Google Scholar]
- 8. Altpeter F, Varshney A, Abderhalden O, Douchkov D, Sautter C, Kumlehn J, Dudler R, Schweizer P. Stable expression of a defense-related gene in wheat epidermis under transcriptional control of a novel promoter confers pathogen resistance. Plant Mol Biol 2005; 57:271-83; PMID:15821882; http://dx.doi.org/ 10.1007/s11103-004-7564-7 [DOI] [PubMed] [Google Scholar]
- 9. Hong B, Barg R, Ho TH. Developmental and organ-specific expression of an ABA- and stress-induced protein in barley. Plant Mol Biol 1992; 18:663-74; PMID:1532749; http://dx.doi.org/ 10.1007/BF00020009 [DOI] [PubMed] [Google Scholar]
- 10. Ellis RJ. The most abundant protein in the world. Trends Biochem Sci 1979; 4:241-4; http://dx.doi.org/ 10.1016/0968-0004(79)90212-3 [DOI] [Google Scholar]
- 11. Suzuki Y, Nakabayashi K, Yoshizawa R, Mae T, Makino A. Differences in expression of the RBCS multigene family and rubisco protein content in various rice plant tissues at different growth stages. Plant Cell Physiol 2009; 50:1851-5; PMID:19720627; http://dx.doi.org/ 10.1093/pcp/pcp120 [DOI] [PubMed] [Google Scholar]
- 12. Rodermel S. Subunit control of Rubisco biosynthesis – a relic of an endosymbiotic past? Photosynth Res 1999; 59:105-23; http://dx.doi.org/ 10.1023/A:1006122619851 [DOI] [Google Scholar]
- 13. Sasanuma T. Characterization of the rbcS multigene family in wheat: subfamily classification, determination of chromosomal location and evolutionary analysis. Mol Genet Genomics 2001; 265:161-71; PMID:11370863; http://dx.doi.org/ 10.1007/s004380000404 [DOI] [PubMed] [Google Scholar]
- 14. Fluhr R, Kuhlemeier C, Nagy F, Chua NH. Organ-specific and light-induced expression of plant genes. Science 1986; 232:1106-12; PMID:17754498; http://dx.doi.org/ 10.1126/science.232.4754.1106 [DOI] [PubMed] [Google Scholar]
- 15. Donald RG, Cashmore AR. Mutation of either G box or I box sequences profoundly affects expression from the Arabidopsis rbcS-1A promoter. EMBO J 1990; 9:1717-26; PMID:2347304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giuliano G, Pichersky E, Malik VS, Timko MP, Scolnik PA, Cashmore AR. An evolutionarily conserved protein binding sequence upstream of a plant light-regulated gene. Proc Natl Acad Sci U S A 1988; 85:7089-93; PMID:2902624; http://dx.doi.org/ 10.1073/pnas.85.19.7089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Song P, Heinen JL, Burns TH, Allen RD. Expression of two tissue-specific promoters in transgenic cotton plants. J Cotton Sci 2000; 4:217-23 [Google Scholar]
- 18. Terzaghi WB, Cashmore AR. Light-regulated transcription. Annu Rev Plant Physiol Plant Mol Biol 1995; 46:445-74; http://dx.doi.org/ 10.1146/annurev.pp.46.060195.002305 [DOI] [Google Scholar]
- 19. Gilmartin PM, Chua NH. Spacing between GT-1 binding sites within a light-responsive element is critical for transcriptional activity. Plant Cell 1990; 2:447-55; PMID:2152170; http://dx.doi.org/ 10.1105/tpc.2.5.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stalker DM, Mcbride KE, Malyj LD. Herbicide resistance in transgenic plants expressing a bacterial detoxification gene. Science 1988; 242:419-23; PMID:17789813; http://dx.doi.org/ 10.1126/science.242.4877.419 [DOI] [PubMed] [Google Scholar]
- 21. Marraccini P, Courjault C, Caillet V, Lepage B, Rogers WJ, Tessereau S, Deshayes A. Rubisco small subunit of Coffea arabica: cDNA sequence, gene cloning and promoter analysis in transgenic tobacco plants. Plant Physiol Biochem 2003; 41:17-25; PMID:NOT_FOUND; http://dx.doi.org/ 10.1016/S0981-9428(02)00004-9 [DOI] [Google Scholar]
- 22. Schaeffner AR, Sheen J. Maize rbcS promotor activity depends on sequence elements not found in dicot rbcS promotors. Plant Cell 1991; 3: 997-1012; PMID:1822995; http://dx.doi.org/ 10.1105/tpc.3.9.997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res 1999; 27: 297-300; PMID:9847208; http://dx.doi.org/ 10.1093/nar/27.1.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lescot M, Déhais P, Moreau Y, De Moor B, Rouzé P, Rombauts S. PlantCARE: a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 2002; 30: 325-7; PMID:11752327; http://dx.doi.org/ 10.1093/nar/30.1.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gilmartin PM, Sarokin L, Memelink J, Chua N-H. Molecular light switches for plant genes. Plant Cell 1990; 2: 369-78; PMID:2152164; http://dx.doi.org/ 10.1105/tpc.2.5.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lam E, Chua NH. ASF-2: a factor that binds to the cauliflower mosaic virus 35S promoter and a conserved GATA motif in Cab promoters. Plant Cell 1989; 1:1147-56; PMID:2535536; http://dx.doi.org/ 10.1105/tpc.1.12.1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evans IM, Bown D, Lycett GW, Croy RRD, Boulter D, Gatehouse JA. Transcription of a legumin gene from pea (Pisum sativum L.) in vitro. Planta 1985; 165, 554-60; PMID:24241231; http://dx.doi.org/ 10.1007/BF00398103 [DOI] [PubMed] [Google Scholar]
- 28. Shahmuradov IA, Solovyev VV, Gammerman AJ. Plant promoter prediction with confidence estimation. Nucleic Acids Res 2005; 33: 1069-76; PMID:15722481; http://dx.doi.org/ 10.1093/nar/gki247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frame B, Main M, Schick R, Wang K. Genetic transformation using maize immature zygotic embryos. Plant Embryo Culture Methods and Protocols. Thorpe TA, Yeung EC, editors. New York: Humana Press; 2011. 327-41. [DOI] [PubMed] [Google Scholar]
- 30. Smart MG, O’Brien TP. The development of the wheat embryo in relation to the neighbouring tissues. Protoplasma 1983; 114:1-13; http://dx.doi.org/ 10.1007/BF01279863 [DOI] [Google Scholar]
- 31. Moore PJ, Moore AJ, Collins GB. Genotypic and developmental regulation of transient expression of a reporter gene in soybean zygotic cotyledons. Plant Cell Rep 1994; 13:556-60; PMID:24196220; http://dx.doi.org/ 10.1007/BF00234510 [DOI] [PubMed] [Google Scholar]
- 32. Arias-Garzon DI, Sayre RT. Tissue specific inhibition of transient gene expression in cassava (Manihot esculenta Crantz). Plant Sci 1993; 93:121-30; http://dx.doi.org/ 10.1016/0168-9452(93)90041-W [DOI] [Google Scholar]
- 33. Basu C, Kausch AP, Luo H, Chandlee JM. Promoter analysis in transient assays using a GUS reporter gene construct in creeping bentgrass (Agrostis palustris). J Plant Physiol 2003; 160:1233-9; PMID:14610892; http://dx.doi.org/ 10.1078/0176-1617-01104 [DOI] [PubMed] [Google Scholar]


