Abstract
We report 3 cases of durable complete response (CR) in patients with BRAF-mutated metastatic melanoma who were initially treated unsuccessfully with sequential immunotherapies (high dose interleukin 2 followed by ipilimumab with or without concurrent radiation therapy). After progression during or post immunotherapy, these patients were given BRAF inhibitor therapy and developed rapid CRs. Based on the concomitant presence of autoimmune manifestations (including vitiligo and hypophysitis), we postulated that there was a synergistic effect between the prior immune therapy and the BRAF targeting agents. Accordingly, the inhibitors were gradually weaned off beginning at 3 months and were stopped completely at 9–12 months. The three patients remain well and in CR off of all therapy at up to 15 months radiographic follow-up. The institution of the BRAF therapy was associated with development of severe rheumatoid-like arthritis in 2 patients which persisted for months after discontinuation of therapy, suggesting it was not merely a known toxicity of BRAF inhibitors (arthralgias). On immunologic analysis, these patients had high levels of non-T-regulatory, CD4 positive effector phenotype T-cells, which persisted after completion of therapy. Of note, we had previously reported a similar phenomenon in patients with metastatic melanoma who failed high dose interleukin-2 and were then placed on a finite course of temozolomide with rapid complete responses that have remained durable for many years after discontinuation of temozolomide. We postulate that a finite course of cytotoxic or targeted therapy specific for melanoma given after apparent failure of prior immunotherapy can result in complete and durable remissions that may persist long after the specific cytotoxic or targeted agents have been discontinued suggesting the existence of sequence specific synergism between immunotherapy and these agents. Here, we discuss these cases in the context of the literature on synergy between conventional or targeted cytotoxic therapy and immunotherapy in cancer treatment.
Keywords: metastatic melanoma, BRAF inhibitor, interleukin-2, ipilimumab, cytotoxic therapy, immunotherapy, treatment of melanoma
Abbreviations
- CBC
complete blood count
- CR
complete response
- CRP
c-reactive protein
- CT
computed tomography
- CTL
cytotoxic lymphocyte
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- GrzB
granzyme B
- HD
high dose
- IFN
interferon
- IL-2
interleukin 2
- LDH
lactate dehydrogenase
- M6P
manose 6 phosphate
- MAPK
mitogen-activated protein kinase pathway
- PD-1
programmed death 1
- PDL-1
programmed death ligand 1
- PDL-2
programmed death ligand 2
- PET
positron emission tomography
- PR
partial response
- RT
radiation therapy
- SLE
systemic lupus erythematosus
- WBC
white blood cell count
Introduction
Treatment of metastatic malignant melanoma is rapidly advancing. Several novel agents have shown superior efficacy and toxicity profiles with improved outcomes in terms of both response and survival compared to conventional chemotherapy. Currently approved agents include: immunotherapeutic agents such as interleukin 2;1,2 ipilimumab, a monoclonal antibody against cytotoxic T lymphocyte antigen-4 (CTLA-4);3 and pembrolizumab and nivolumimab, monoclonal antibodies that bind to the PD-1 receptor and block its interaction with PD-L1 and PD-L2, thereby releasing PD-1 pathway-mediated inhibition of the immune response, including anti-tumor immune response 4 as well as vemurafenib, dabrafenib, and trametinib, which are targeted agents for tumors with BRAF mutations;5,6 and conventional chemotherapy such as dacarbazine, temozolomide, nab-paclitaxel and similar agents, which have a limited palliative role.7,8 Although some of these agents have made a major impact on this disease in terms of survival, most patients are still not cured; and rates of durable complete response (CR) as a prelude to cure remain low in both first-line and second-line settings.9
The combination of BRAF inhibitors with immune therapy is of great interest, since it combines the high response rate of these targeted drugs with the durability of the few major responses associated with immunotherapeutic agents such as ipilimumab.3,10 However, a phase I trial of concurrent vemurafenib and ipilimumab was halted early due to toxicities, mainly severe autoimmune hepatitis.11 After this observation, concurrent therapy with vemurafenib and ipilimumab was not advised. We previously reported our experience with patients who progressed after high-dose interleukin-2 and then received temozolomide soon after completion of the IL-2.12 Some of these patients sustained rapid and durable CR to the temozolomide which was subsequently stopped after a finite course of therapy. Some of these responses have remained durable to date (>5 years) off of all treatment, raising the possibility of definitive cure. We postulated that there might be a sequence-specific synergistic effect between the immunotherapy with high dose IL-2 and the subsequent early employment of cytotoxic therapy with temozolomide. Here we present cases of several patients who achieved CR with BRAF inhibitor therapy administered for progressive disease after sequential immunotherapy with high dose IL-2 followed by ipilumumab. We weaned off and eventually stopped the BRAF inhibitor therapy and found that these patients remain in a durable CR. Two of our patients developed marked inflammatory arthritis which persisted long after the BRAF inhibitor therapy was discontinued. Since this was also noted in some of the patients who received a finite course of temozolomide after IL-2, we believe that this may be a manifestation of autoimmunity associated with the antitumor activity.
Case Reports
Patient 1
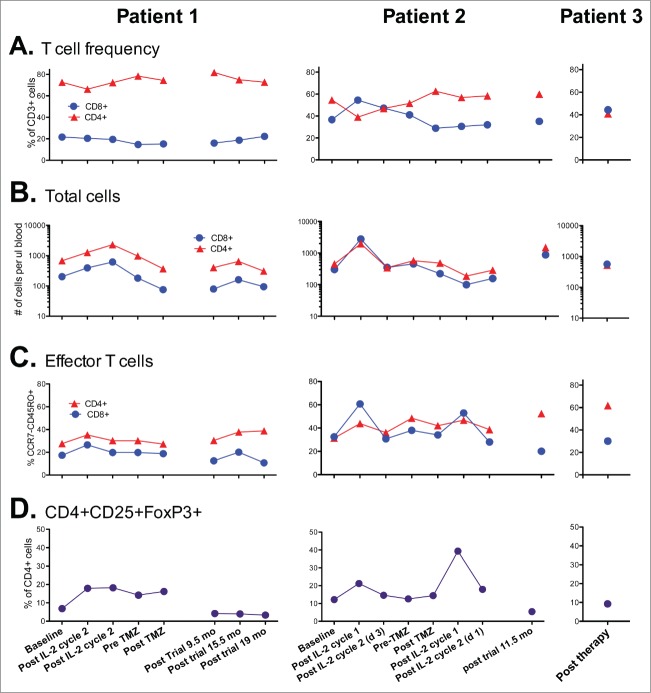
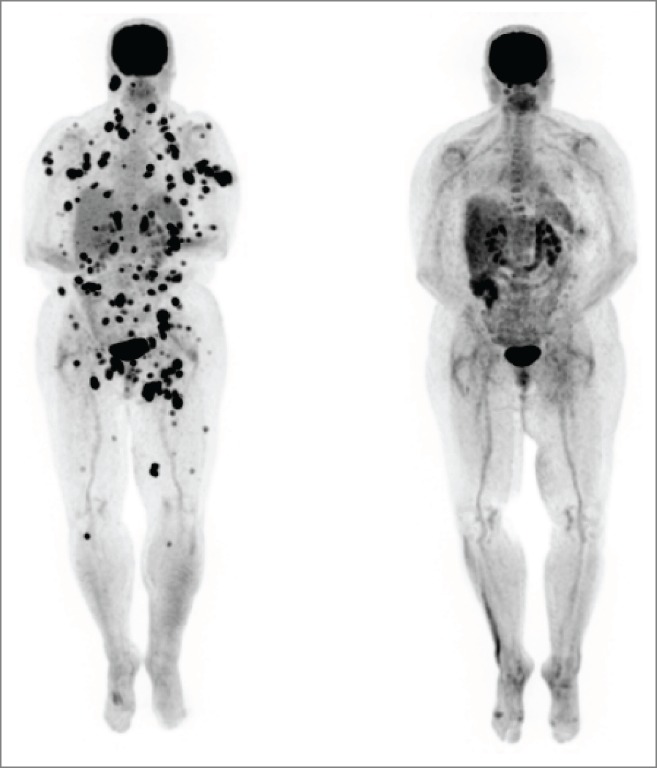
A 53-year-old female patient was diagnosed with stage IIIC (pTxpN3M0) melanoma involving left inguinal lymph nodes after biopsy in March of 2012. Work-up did not reveal a primary site. She was treated with lymph node dissection with a plan for adjuvant immunotherapy, but she rapidly developed metastatic adenopathy in multiple sites, including her left iliac and right axillary chains (biopsy confirmed in latter site). She participated in a phase II clinical trial with high dose IL-2 followed by intermittent low dose temozolomide.12,13 The patient was noted to have BRAF V600E mutation and an M1a pattern of spread; with marked LDH elevation. She received one course of therapy but had marked progression with new sites of disease in multiple lymph nodes, subcutaneous and mesenteric nodules by October 2012. She did develop vitiligo post IL-2 treatment. The patient was then started on ipilimumab, but had marked progressive disease after 2 cycles, as shown by PET/CT in December 2012 (Fig. 1 left). She developed bone, adrenal gland and splenic metastases, as well as worsening of existing lymph node metastases (M1c pattern). We continued the last 2 cycles of ipilimumab and began concurrent vemurafenib at 960 mg twice daily, given the massive symptomatic disease progression, along with radiation therapy (RT) to the larger masses in the cervical and inguinal regions (30 Gy in 10 fractions). The RT was done both for palliation and for synergism with ipilimumab, as it could theoretically render the tumor more immunogenic.14,15 She rapidly achieved a clinical response to vemurafenib (along with a pruritic rash requiring a dose reduction and a brief course of steroids). The patient developed fevers, chills and marked synovitis in a symmetric fashion, particularly in her wrists, hands and feet. She completed a course of ipilimumab, with 4 doses at 3mg/kg every 3 weeks. The patient did not develop autoimmune hepatitis but her vitiligo became more prominent (Fig. 2) and she also developed autoimmune hypophysitis, which required hydrocortisone replacement. She had a transient positive antinuclear antibody test. PET/CT scan in February 2013, 3 months after the vemurafenib therapy, showed CR (Fig. 1, right). We gradually weaned the vemurafenib for 3 reasons: 1) the arthritis was nearly incapacitating despite dose reduction of the vemurafenib and initiation of celecoxib, 2) the patient had signs of marked autoimmunity, suggesting that there could be a beneficial synergy with the prior immune therapy and 3) in theory, to forestall development of resistance to the vemurafenib.16 At 3 months, we began treatment alternating one week on then one week off of the agent. Subsequent PET scans revealed persistent CR. She received a total of 12 months of intermittent vemurafenib treatment. Her arthritis did not improve on the weeks she was off therapy and has persisted to date but is gradually reducing in severity over time. She remains in CR at 15 months from the completion of the vemurafenib course.
Figure 1.
Patient 1. PET/CT in 12/12 (left, prior to vemurafenib) showed innumerable intensely FDG avid lymph nodes and soft tissue deposits scattered throughout the body which developed during her course of ipilimumab by 2 cycles. PET/CT in 2/13 (right, after starting vemurafenib and completion of the ipilimumab course) showed the previously described intensely FDG avid metastases had entirely resolved. The vemurafenib was weaned and completely stopped by 12/13. She has remained in complete remission to date off all therapy.
Figure 2.
Vitiligo of right arm in Patient 1. The photo shows patchy depigmentation of skin after the patient was treated with vemurafenib but this process had actually started after completion of high dose IL-2.
Patient 2
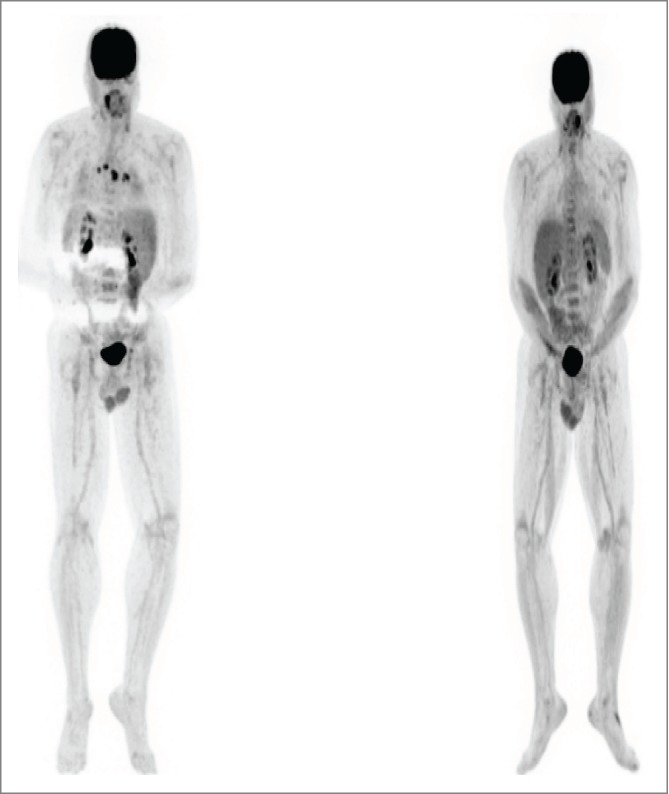
A 56-year-old female patient was diagnosed with metastatic melanoma after a biopsy of an enlarged left axillary lymph node, noticed on breast self-examination. She had no apparent primary. PET/CT scan showed extensive adenopathy in her left neck, axilla and mediastinum, consistent with an M1a pattern (Fig. 3, Left). Her tumor had a BRAF V600E mutation. Of note, she had a history of mild systemic lupus erythematosus (SLE), treated with intermittent course of steroids in the remote past. The patient initiated high dose interleukin-2 followed by intermittent low dose temozolomide in a phase II clinical trial.12,13 She had stable disease after the first course of therapy by RECIST criteria. During the 2nd course of IL-2, she sustained a water-shed cerebrovascular attack associated with right-sided paresis. The patient recovered completely from this complication but went off study due to this event. In view of persistent disease, she completed a course of ipilimumab, with stable disease. We started vemurafenib and almost immediately noted resolution of her palpable lymph nodes, as well as the development of a symmetric polyarthritis, with synovitis of her fingers and wrists. The vemurafenib dose was reduced 50%, without improvement. She reached CR by PET scan and we began alternating therapy one week on, one week off. Vemurafenib therapy was completely discontinued after 12 months total. Her arthritis gradually subsided over time, and she remains in complete remission 12 months from completion of the vemurafenib (Fig. 3, Right).
Figure 4.
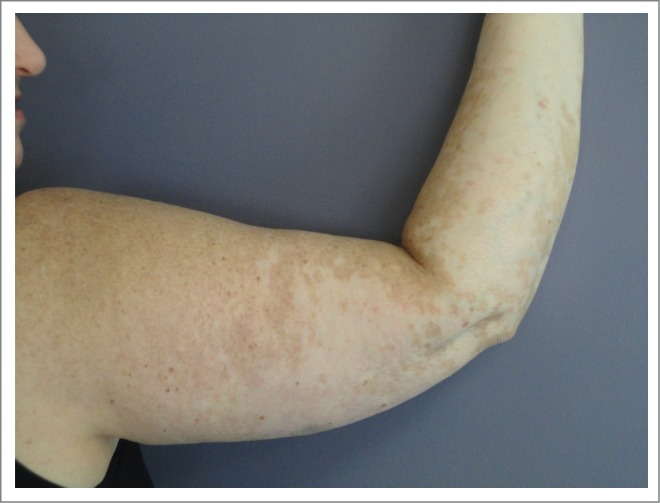
Patient 3. PET/CT in August 2013 (left, prior to BRAF inhibitor therapy) showed intensely FDG avid lymph nodes post ipilimumab which were biopsy confirmed metastatic melanoma. PET/CT in November 2013(right, after trametinib) showed the previously described intensely FDG avid lymph nodes and nodules had entirely resolved. The skin nodules on his scalp also resolved. The trametinib was gradually weaned and stopped by 5/14. He has remained in complete remission to date off therapy.
Figure 5.
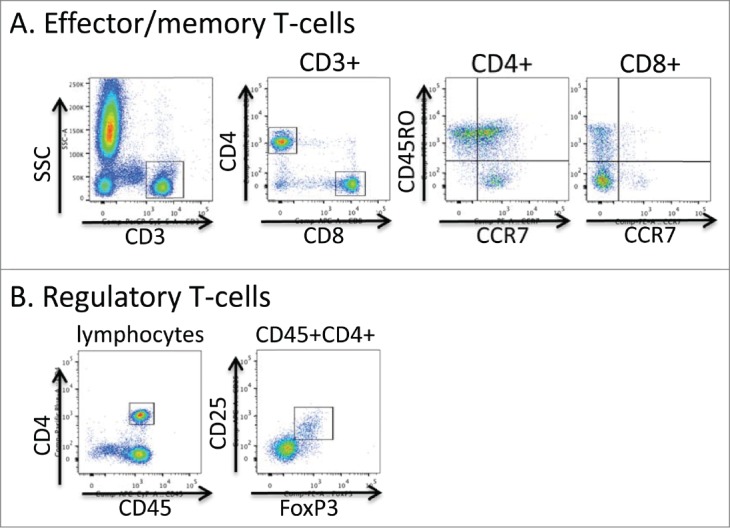
Flow cytometric gating strategy for T-cell phenotyping. Aliquots of freshly collected blood were stained directly with antibodies to the markers shown in A and B. In A, live events were first gated using the FSC × SSC parameters and then the indicated gating strategies were used. In B the lymphocyte fraction was initially gated using the FSC × SSC parameters.
Figure 3.
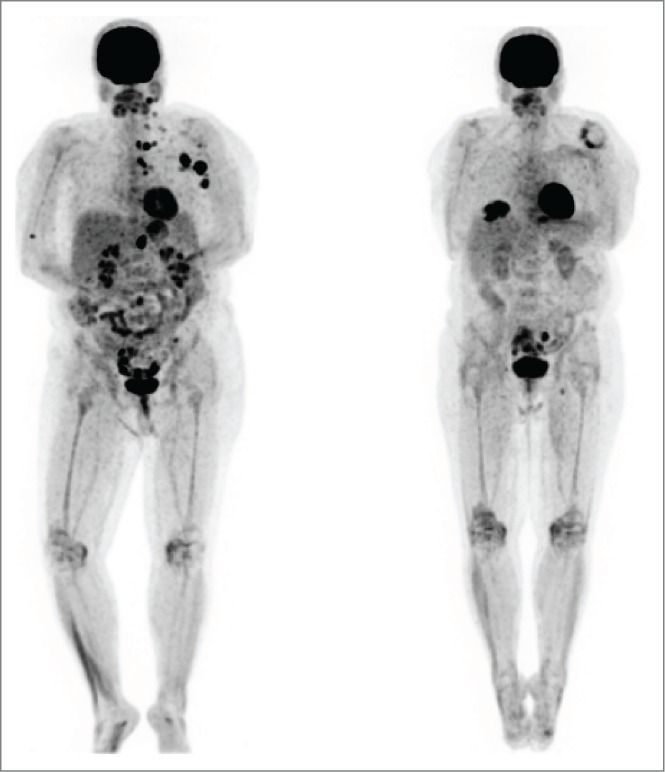
Patient 2. PET/CT on 3/13 (left, prior to vemurafenib) showed intensely FDG avid lymph nodes and soft tissue deposits post ipilimumab. PET/CT on 6/13 (right, after vemurafenib) showed the previously described intensely FDG avid lymph nodes and nodules had entirely resolved. The vemurafenib was gradually weaned and stopped by 3/14. She has remained in complete remission to date off therapy.
Patient 3
A 56-year-old male was diagnosed with staged T1b melanoma of the right scalp in August 2011. He underwent a wide local excision with negative margins. Three months later, he developed multiple satellite and transit metastases (biopsy-confirmed) over the vertex, in an area about 8 cm in diameter. He had no evidence of distant metastatic disease on imaging and underwent another wide local excision with skin graft placement. Sentinel node sampling in 3 lymph nodes revealed a microscopic implant in one occipital node. After a complete right sided posterior neck dissection, he developed additional in-transit metastases, which were resected. Given this propensity for locoregional epidermotropic metastases, we gave adjuvant RT to the affected area but developed new in-transit metastases. The tumor was found to express a V600E mutation. We started systemic immunotherapy with high dose IL-2 but was aborted due to disease progression. The patient was then started on ipilimumab, which was complicated by the development of symptomatic hypophysitis, requiring replacement therapy with hydrocortisone and levothyroxine. PET/CT scan after completion of the ipilumumab course showed progressive disease with new mediastinal lymphadenopathy (biopsy-confirmed). We then began vemurafenib but he developed immediate severe desquamative eruption and therapy was changed to trametinib. The patient reached CR by PET scan 3 months later (Fig. 4). He developed transient mild arthralgias in his finger and wrist joints. We changed the schedule of trametinib to one week on one off for 6 additional months (9 total), then was discontinued and the patient remains in CR 9 months after the completion of trametinib.
Methods and Materials
Evaluation of peripheral blood T lymphocytes by flow cytometry
Peripheral blood was collected into sodium heparin blood tubes. A complete blood count (CBC) was acquired using a Sysmex 1000i™automated hematology analyzer and blood samples were processed and stained in the Clinical Correlative Immunology Laboratory of the Penn State Hershey Cancer Institute. 100 μl aliquots of whole blood were stained with one of 2 antibody panels; Panel A: CD4-e450 (OKT4, eBioscience), CD8-APC (RPA-T8, eBioscience), CD3-PerCP-Cy5.5 (OKT3, eBioscience), CD45RO-FITC (UCHL1, eBioscience) and CCR7-PE (3D12, eBioscience), Panel B: CD25-APC (BC96, eBioscience), CD127-FITC (eBioRDR5, eBioscience), CD45-APC-Cy7 (HI30, eBioscience) and anti-FoxP3-PE (PCH101, eBioscience). Cells were incubated with antibodies to surface proteins for 15 minutes at room temperature followed by lysis of red blood cells using 10x RBC Lysis Buffer (eBioscience, 00-4300-54) for 20 minutes at room temperature. Cells stained with panel B were further washed, fixed and permeabilized with 1X FoxP3/Transcription Factor Fixation/Permeabilization buffer (eBioscience) for 20 minutes at room temperature and stained for FoxP3 according to the manufacturer's instructions. Cells were fixed with 2% paraformaldehyde in PBS and data collected within the Penn State Hershey Flow Cytometry Core Facility using a BD FACSCanto II or BD LSR Fortessa flow cytometer. Data were analyzed using FlowJo software. Total cell counts were calculated by multiplying the cell frequency determined by flow cytometry with the total WBC count/μl of blood determined by CBC.
Evaluation of Peripheral Blood T-cells
For patients 1 and 2 enrolled in a phase II clinical trial of high dose interleukin-2 followed by intermittent low dose temozolomide, T-cell phenotype in peripheral blood was evaluated at baseline and after each treatment cycle and then at distant time points post-therapy. CD4+ and CD8+ T-cell frequencies and surface phenotype were evaluated directly ex-vivo using flow cytometry (Fig. 5). Patient 1 notably and persistently had a high CD4/CD8 ratio of >3, most likely due to below average CD8 T-cell numbers at most time points (Fig. 6A and B). Patient 2 showed a normal CD4/CD8 ratio of between 1 and 2 at baseline, during the second course of treatment, and after the clinical trial (Fig. 6A and B). Intriguingly, we found that the frequency of effector phenotype CCR7negCD45RO+ T-cells were similar at baseline in both patients, but was skewed toward effector CD4+ T-cells at post-trial time points (Fig. 6C). Patient 3, who was not on the clinical trial in which the other patients were enrolled, demonstrated a similar phenotype, with very high levels of effector phenotype CD4+ T-cells at the one post-therapy time point assessed. In general, we found that T-cell numbers were increased with IL-2 therapy and returned to baseline levels by the end of therapy (Fig. 6B), although total T-cell numbers appeared elevated in patient 2 at approximately 1 y post trial. CD4+CD25+FoxP3+ T-cells increased with IL-2 therapy, as has been previously reported.17–19 Patient 2 showed an extremely high frequency of CD4+CD25+FoxP3+ T-cells post IL-2 cycle 1 of the second course of therapy (Fig. 6D), although this frequency correlated with an overall drop in CD4+ T-cell numbers in the blood at this time point (Fig. 6B). Intriguingly, the CD4+CD25+FoxP3+ T-cell frequencies were reduced in both patient 1 and patient 2 at time points collected post trial relative to initial baseline levels. These results suggest that clinical response may be associated with skewing of CD4+ T-cells toward an effector phenotype and away from a regulatory phenotype.
Figure 6.
Kinetics of T-cells in the peripheral blood of patients during and following therapy. (A) Ratio of CD4+ and CD8+ T-cells within the CD45+CD3+ cell fraction of fresh blood. (B) Total CD3+CD4+ and CD3+CD8+ T-cells per ul of peripheral blood. (C) Fraction of CD4+ and CD8+ T-cells with the CCR7- CD45RO+ effector phenotype. (D) Frequency of CD4+ cells co-expressing CD25 and FoxP3. Note Patient 3 was not on a prior clinical trial so had no prior baseline determinations for comparison.
Discussion
The incidence of melanoma is increasing worldwide so it is likely to remain a persistent problem. Melanoma comprises approximately 5% of all skin cancers and is responsible for 80% of all skin cancer related deaths according to WHO.20 Overall prognosis of advanced metastatic melanoma had been dismal until the recent addition of the checkpoint inhibitors and targeted agents. Despite, the overall poor prognosis for metastatic disease, immunotherapy can result in durable CRs consistent with cure, an amazing feat for a metastatic solid tumor. Interleukin 2 showed a CR rate of 6% and PR rate of 10%, although many of these responses were durable consistent with cure.21,22 Because of the quality of the responses associated with cure, HD IL-2 was approved by the FDA in 1998 despite the overall very low response rate.22 Overall survival was shown to be significantly increased with ipilimumab in 2 randomized, phase III clinical trials.3,10 However, the actual tumor response rates were modest with rates between 10 and 17%. As with IL-2, some of the responses were durable, consistent with cure.3 Pembrolizumab and nivolimumb, which target the PD-1 checkpoint, have shown higher response rates with an ORR of 26%.23 Of these patients, 88% have had durable responses at a noted median follow-up of 8 months.23 The combination of ipililimumab and the PD-1 inhibitor, nivolumab, showed better response rates than either agent alone.3,24,25 Although the checkpoint inhibitors alone and in combination have improved durable CR rates, the majority of patients do not experience cure.
BRAF mutations are seen in metastatic melanoma at a relatively high rate (BRAF-V600E is seen in 50% of these patients) and lead to constitutive up-regulation of the mitogen-activated protein kinase pathway (MAPK), potentiating tumor cell growth.26,27 Vemurafenib is an FDA approved BRAF inhibitor with high antitumor activity in patients with BRAF mutations based on a phase III trial.6,28,29 Dabrafenib, a fellow BRAF inhibitor has also shown efficacy in treatment of advanced melanoma.6,30 Trametinib, which targets MEK downstream of BRAF pathway also has been approved as has the combination of drabrafenib/trametinib.31-33 Unfortunately nearly all patients will develop acquired resistance.28 The combination of BRAF and MEK inhibitors seems to delay this effect but resistance appears inevitable. Combining the high response rate of these targeted inhibitors with the durability of responses to immunotherapy was a logical approach that led to further testing of combined regimens but as mentioned previously was associated with significant toxicities.11
Our results suggest a possible solution to this problem that involves administration of agents in sequence rather than combining them. We report here that 3 patients with BRAF mutated metastatic melanoma treated with sequential immunotherapy starting with high dose IL-2 followed by ipilimumab had symptomatic progressive disease during or soon after immunotherapy. These patients then received targeted therapy with BRAF or MEK inhibitor therapy and enjoyed rapid clinical responses which remained durable long after the discontinuation of these agents. It is well known that with immune therapy, as described particularly for ipilimumab, patients may demonstrate disease progression and then late responses.3,10 This observation has led to the use of an alternative to the RECIST system specific for immunotherapy to describe responses.34 Although the responses observed in our patients may have eventually occurred due to the immunotherapy alone, the rapid clinical responses observed immediately after the start of BRAF inhibitor therapy suggested the latter clearly played a role in initiating a more rapid response. Our patients would have had progressive disease by the immunotherapy criteria.34 The BRAF inhibitor agents are given until disease progression is observed, which is typically after a finite period of response. Although reported, CRs to these agents alone are fairly uncommon and the weaning and discontinuation of these agents after achieving a CR resulting in continued CR off all treatment is a novel observation to our knowledge. The outcomes suggest that sequence specific synergy occurred.
We postulate that the immune therapy resulted in immune activation against the tumor and was manifested by autoimmunity in the patients but this immune activation was unable to result in complete tumor destruction alone until the addition of the BRAF inhibitor. We had already reported a similar situation in patients who failed high dose IL-2 but who then received temozolomide shortly after progression and who likewise had rapid responses that persisted after discontinuation of the cytotoxic drug lasting for many years to date.12 Chemotherapy has been noted to alter immune reactions to tumors and may even play a synergistic role at times.35 This outcome is contrary to the idea that cytotoxic agents would be immunosuppressive, and evidence has accumulated that they can promote antitumor immunity.12,36 This may occur by various mechanisms including: release of “danger” molecules36 or the induction of immunogenic cell death,37 inhibition of T-regulatory T-cells38 or myeloid-derived suppressor cells.39,40 Perhaps, simple cytoreduction with an effective cytotoxic agent may likewise allow the immune system to finish the job so to speak. Cytotoxic agents may also help defeat some of the defense mechanisms of the tumor against the host immune response such as down-regulation of PD-L1.36
Targeted agents such as the BRAF inhibitor vemurafenib have also been associated with various immune effects.26,41 One study suggested that vemurafenib promoted the development of an immune stimulatory microenvironment with increases in CD40L and IFN gamma and that this antitumor effect could be abrogated with CD40L and IFN gamma blockade.42 Likewise, overall viability of human lymphocytes does not appear to be compromised by BRAF inhibitors.41 In fact, vemurafenib has since been found to cause an increase in expression of melanoma antigens such as gp100 and MART1 accompanied with a noted increased response in CTL's specific for these antigens.27,43 These results suggest that BRAF inhibitors should indeed be synergistic with immunotherapy. Our data supports this contention and suggests that they should be given after immune activation has already occurred rather than before or concurrently as it was done in the Phase I trial of ipilimumab and vemurafenib,11 where toxicity was limiting.
In general, the purpose of immunotherapy is to produce cytotoxic immune effector cells to kill both tumor cells expressing a specific antigen and also tumor cells within close proximity that may not have similar antigen expression.36,44 Ramakrishnan et al found that the chemotherapeutic agents doxorubicin, paclitaxel and cisplatin increased tumor cell permeability to granzyme B (GrzB), leading to their increased sensitization to CTLs. Upregulation of manose 6 phosphate (M6P) led to increased GrzB uptake. Sequential treatment may be important to achieve a favorable response. For example if chemotherapy is given immediately after vaccine administration, CTLs may be more effective in terms of elimination of cancer cells due to antigen presentation, stromal disruption and eventual release of GrzB after CTL and tumor cell antigen interaction leading to adjacenT-cell GrzB permeation.36 In humans, CTLs seem to be more prominent in states of chronic or prolonged infection and may be “antigen-experienced” cells.45 It has been postulated that lysis of tumor cells releases a significant amount of antigenic contents; leading to a “vaccination in situ” allowing immune cells to continue to target certain cancer cell associated antigens.27 Dendritic cells may be able to obtain antigens and display them to more effectively activate or re-stimulate the initial immune response. The BRAF inhibitors or cytotoxic agents may be accomplishing this same feat.
All three of our patients had sequential immunotherapy starting with high dose Interleukin-2-based therapy and followed soon after by ipilimumab due to progressive disease. We postulate that there may be synergistic immune activation facilitated by expansion of effector T-cells induced by the IL-2 and then having ipilimumab take out the CTLA-4 checkpoint brake. Following IL-2 administration, for example, ipilimumab had been shown to further increase the immune response toward melanoma cells.46 Whether a sequence specific synergy between IL-2 and ipilimumab occurs clinically and whether it was needed for the responses we observed remains unclear. It has been postulated by Coventry et al that T regulatory cell suppression fluctuates and that more successful responses to chemotherapy are observed when chemotherapy is given at a point when these cells have increased differentiation. These cell concentrations vary during an approximate 7 day cycle.47 If one could determine when regulatory cells would be most susceptible to therapy by monitoring CRP levels, chemotherapy could be administered at this time with a hopeful significant response. If this hypothesis is valid, then sequencing therapy may allow one to “catch the wave” so to speak resulting in a more favorable response.
We noticed that 2 of our patients developed severe symmetric arthritis with clinical synovitis after initiating BRAF inhibitor therapy, while the third patient had these symptoms to a lesser degree. This clinical manifestation was very suggestive of rheumatoid arthritis. Vemurafenib has been associated with arthralgias48 but we had never observed this toxicity to the degree seen in these patients, and the symptoms persisted for many months after discontinuation of the agents. Of interest, we had observed the same arthritic phenomenon in the patients we had previously reported who sustained CRs to temozolomide post IL-2 suggestive of a relationship. As in the patients described here the arthritic symptoms persisted for many months after discontinuation of therapy. Our laboratory studies did note the presence of high proportions of persistent non-T-regulatory effector phenotype CD4+ T-cells, suggesting they were cytotoxic CD4+ T-cells (Figs. 5, 6). Of note, CD4+ T-cells can be associated with potent antitumor activity and are also associated with rheumatic conditions. 45,49 In addition to the arthritis, our patients all exhibited some form of autoimmunity such as hypophysitis and/or vitiligo. Autoimmunity may be an important consequence of antitumor immunity but can be manageable.50
We have shown that patients with metastatic melanoma can enjoy durable complete remissions off of all therapy after a finite course of BRAF inhibitor therapy given after an apparent failure of prior immunotherapy suggestive of sequence specific synergy. We previously observed a similar situation with cytotoxic therapy (temozolomide) given post immunotherapy as well.12 In both situations, patients developed a transient rheumatoid-like arthritis that may persist for some time after discontinuance of the agent. The optimal sequencing and timing of treatment modalities appears to be critical in the treatment of advanced melanoma and our laboratory is conducting ongoing investigations. With the successes of novel immunotherapeutic agents in other forms of cancer, we wonder if this approach may be applicable to other malignancies as well, where cytotoxic therapy specific to the cancer in question delivered after immune activation with immunotherapy may result in better outcomes. For now, it appears that for those patients with metastatic melanoma who develop rapid CR to targeted therapy given after prior immunotherapy, that the targeted agents may be weaned and stopped over time resulting in continued CR off of all therapy.
Disclosure of Potential Conflicts of Interest
Dr. Joseph Drabick discloses a consulting role with Merck.
Acknowledgments
We wish to thank our patients and their families for their courage and grace under pressure.
Funding
The clinical trial for which Patient 1 and 2 were enrolled was supported by Prometheus, Inc.
References
- 1.Smith FO, Downey SG, Klapper JA, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Restifo NP, Levy CL, et al.. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res 2008; 14:5610–8; PMID:18765555; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, et al.. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999; 17:2105–16; PMID:10561265 [DOI] [PubMed] [Google Scholar]
- 3.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711–23; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow S, Cove-Smith L, Schmitt M, Hawkins R. High-dose interleukin 2-induced myocarditis: can myocardial damage reversibility be assessed by cardiac MRI? J Immunother 2014; 37:304–8; PMID:24810642; http://dx.doi.org/ 10.1097/CJI.0000000000000036 [DOI] [PubMed] [Google Scholar]
- 5.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K, et al.. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010; 363:809–19; PMID:20818844; http://dx.doi.org/ 10.1056/NEJMoa1002011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al.. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364:2507–16; PMID:21639808; http://dx.doi.org/ 10.1056/NEJMoa1103782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, Gore M, Aamdal S, Cebon J, Coates A, et al.. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 2000; 18:158–66; PMID:10623706 [DOI] [PubMed] [Google Scholar]
- 8.Kottschade LA, Suman VJ, Amatruda T. 3rd, McWilliams RR, Mattar BI, Nikcevich DA, Behrens R, Fitch TR, Jaslowski AJ, Markovic SN, et al.. A phase II trial of nab-paclitaxel (ABI-007) and carboplatin in patients with unresectable stage IV melanoma : a North Central Cancer Treatment Group Study, N057E(1). Cancer 2011; 117:1704–10; PMID:21472717; http://dx.doi.org/ 10.1002/cncr.25659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network: Melanoma (Version 3.2014). http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. Accessed April19, 2014 [Google Scholar]
- 10.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al.. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364:2517–26; PMID:21639810; http://dx.doi.org/ 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 11.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. New Eng J Med 2013; 368:1365–6; PMID:23550685; http://dx.doi.org/ 10.1056/NEJMc1302338 [DOI] [PubMed] [Google Scholar]
- 12.Fateh S, Schell TD, Gingrich R, Neves RI, Drabick JJ. Unsuccessful high dose IL-2 therapy followed immediately by near continuous low dose temozolomide can result in rapid durable complete and near-complete remissions in metastatic melanoma. Cancer Biol Ther 2010; 10:1091–7; PMID:20930514; http://dx.doi.org/ 10.4161/cbt.10.11.13452 [DOI] [PubMed] [Google Scholar]
- 13.High Dose Interleukin-2 Followed by Intermittent Low Dose Temozolomide in Patients With Melanoma”: NCT 01124734. (Accessed at http://clinicaltrials.gov/ct2/show/NCT01124734.) [Google Scholar]
- 14.Hiniker SM, Chen DS, Reddy S, Chang DT, Jones JC, Mollick JA, Swetter SM, Knox SJ. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol 2012; 5:404–7; PMID:23323154; http://dx.doi.org/ 10.1593/tlo.12280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barker CA, Postow MA. Combinations of radiation therapy and immunotherapy for melanoma: a review of clinical outcomes. Int J Radiat Oncol Biol Phys 2014; 88:986–97; PMID:24661650; http://dx.doi.org/ 10.1016/j.ijrobp.2013.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das Thakur M, Salangsang F, Landman AS, Sellers WR, Pryer NK, Levesque MP, Dummer R, McMahon M, Stuart D. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 2013; 494:251–5; PMID:23302800; http://dx.doi.org/ 10.1038/nature11814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood 2006; 107:2409–14; PMID:16304057; http://dx.doi.org/ 10.1182/blood-2005-06-2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cesana GC, DeRaffele G, Cohen S, Moroziewicz D, Mitcham J, Stoutenburg J, Cheung K, Hesdorffer C, Kim-Schulze S, Kaufman HL. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol 2006; 24:1169–77; PMID:16505437; http://dx.doi.org/ 10.1200/JCO.2005.03.6830 [DOI] [PubMed] [Google Scholar]
- 19.Wei S, Kryczek I, Edwards RP, Zou L, Szeliga W, Banerjee M, Cost M, Cheng P, Chang A, Redman B, et al.. Interleukin-2 administration alters the CD4+FOXP3+ T-cell pool and tumor trafficking in patients with ovarian carcinoma. Cancer Res 2007; 67:7487–94; PMID:17671219; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-0565 [DOI] [PubMed] [Google Scholar]
- 20.Gogas H, Polyzos A, Kirkwood J. Immunotherapy for advanced melanoma: fulfilling the promise. Cancer Treat Rev 2013; 39:879–85; PMID:23725878; http://dx.doi.org/ 10.1016/j.ctrv.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 21.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am 2000; 6 Suppl 1:S11–4; PMID:10685652 [PubMed] [Google Scholar]
- 22.Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology (Williston Park) 2009; 23:488–96; PMID:19544689 [PMC free article] [PubMed] [Google Scholar]
- 23.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, et al.. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014; 384:1109–17; PMID:25034862; http://dx.doi.org/ 10.1016/S0140-6736(14)60958-2 [DOI] [PubMed] [Google Scholar]
- 24.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al.. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122–33; PMID:23724867; http://dx.doi.org/ 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443–54; PMID:22658127; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu-Lieskovan S, Robert L, Homet Moreno B, Ribas A. Combining targeted therapy with immunotherapy in BRAF-mutant melanoma: promise and challenges. J Clin Oncol 2014; 32:2248–54; PMID:24958825; http://dx.doi.org/ 10.1200/JCO.2013.52.1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012; 12:237–51; PMID:22437869; http://dx.doi.org/ 10.1038/nrc3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Homet B, Ribas A. New drug targets in metastatic melanoma. J Pathol 2014; 232:134–41; PMID:24027077; http://dx.doi.org/ 10.1002/path.4259 [DOI] [PubMed] [Google Scholar]
- 29.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT, et al.. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012; 366:707–14; PMID:22356324; http://dx.doi.org/ 10.1056/NEJMoa1112302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH Jr, Kaempgen E, et al.. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012; 380:358–65; PMID:22735384; http://dx.doi.org/ 10.1016/S0140-6736(12)60868-X [DOI] [PubMed] [Google Scholar]
- 31.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, et al.. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014; 371:1877–88; PMID:25265492; http://dx.doi.org/ 10.1056/NEJMoa1406037 [DOI] [PubMed] [Google Scholar]
- 32.Johnson DB, Flaherty KT, Weber JS, Infante JR, Kim KB, Kefford RF, Hamid O, Schuchter L, Cebon J, Sharfman WH, et al.. Combined BRAF (Dabrafenib) and MEK Inhibition (Trametinib) in Patients With BRAFV600-Mutant Melanoma Experiencing Progression With Single-Agent BRAF Inhibitor. J Clin Oncol 2014; 32:3697–704; PMID:25287827; http://dx.doi.org/ 10.1200/JCO.2014.57.3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, Demidov LV, Hassel JC, Rutkowski P, Mohr P, et al.. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012; 367:107–14; PMID:22663011; http://dx.doi.org/ 10.1056/NEJMoa1203421 [DOI] [PubMed] [Google Scholar]
- 34.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al.. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009; 15:7412–20; PMID:19934295; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-1624 [DOI] [PubMed] [Google Scholar]
- 35.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res 2003; 63:4490–6; PMID:12907622 [PubMed] [Google Scholar]
- 36.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, Altiok S, Celis E, Gabrilovich DI. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest 2010; 120:1111–24; PMID:20234093; http://dx.doi.org/ 10.1172/JCI40269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31:51–72; PMID:23157435; http://dx.doi.org/ 10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- 38.Ascierto PA, Marincola FM, Ribas A. Anti-CTLA4 monoclonal antibodies: the past and the future in clinical application. J Transl Med 2011; 9:196; PMID:22077981; http://dx.doi.org/ 10.1186/1479-5876-9-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vieweg J, Su Z, Dahm P, Kusmartsev S. Reversal of tumor-mediated immunosuppression. Clin Cancer Res 2007; 13:727s-32s; PMID:17255301; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-1924 [DOI] [PubMed] [Google Scholar]
- 40.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood 2000; 96:3838–46; PMID:11090068 [PMC free article] [PubMed] [Google Scholar]
- 41.Comin-Anduix B, Chodon T, Sazegar H, Matsunaga D, Mock S, Jalil J, Escuin-Ordinas H, Chmielowski B, Koya RC, Ribas A, et al.. The oncogenic BRAF kinase inhibitor PLX4032/RG7204 does not affect the viability or function of human lymphocytes across a wide range of concentrations. Clin Cancer Res 2010; 16:6040–8; PMID:21169256; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho PC, Meeth KM, Tsui YC, Srivastava B, Bosenberg MW, Kaech SM. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNgamma. Cancer Res 2014; 74:3205–17; PMID:24736544; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, Ferrone CR, Flaherty KT, Lawrence DP, Fisher DE, et al.. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res 2010; 70:5213–9; PMID:20551059; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-0118 [DOI] [PubMed] [Google Scholar]
- 44.Disis ML, Bernhard H, Jaffee EM. Use of tumour-responsive T cells as cancer treatment. Lancet 2009; 373:673–83; PMID:19231634; http://dx.doi.org/ 10.1016/S0140-6736(09)60404-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Appay V. The physiological role of cytotoxic CD4(+) T-cells: the holy grail? Clin Exp Immunol 2004; 138:10–3; PMID:15373899; http://dx.doi.org/ 10.1111/j.1365-2249.2004.02605.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res 2012; 18:2039–47; PMID:22271879; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coventry BJ, Ashdown ML, Quinn MA, Markovic SN, Yatomi-Clarke SL, Robinson AP. CRP identifies homeostatic immune oscillations in cancer patients: a potential treatment targeting tool? J Transl Med 2009; 7:102; PMID:19948067; http://dx.doi.org/ 10.1186/1479-5876-7-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim G, McKee AE, Ning YM, Hazarika M, Theoret M, Johnson JR, Xu QC, Tang S, Sridhara R, Jiang X, et al.. FDA Approval Summary: Vemurafenib for Treatment of Unresectable or Metastatic Melanoma with the BRAFV600E Mutation. Clin Cancer Res 2014; 20:4994–5000; PMID:25096067; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-0776 [DOI] [PubMed] [Google Scholar]
- 49.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, et al.. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 2010; 207:637–50; PMID:20156971; http://dx.doi.org/ 10.1084/jem.20091918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaufman HL, Wolchok JD. Is tumor immunity the same thing as autoimmunity? Implications for cancer immunotherapy. J Clin Oncol 2006; 24:2230–2; PMID:16710020; http://dx.doi.org/ 10.1200/JCO.2006.05.6952 [DOI] [PubMed] [Google Scholar]