Abstract
Antibody engineering must be accompanied by mapping strategies focused on identifying the epitope recognized by each antibody to define its unique functional identity. High throughput fine specificity determination remains technically challenging. We review recent experiences aimed at revisiting the oldest and most extended display technology to develop a robust epitope mapping platform, based on the ability to manipulate target-derived molecules (ranging from the whole native antigen to antigen domains and smaller fragments) on filamentous phages. Single, multiple and combinatorial mutagenesis allowed comprehensive scanning of phage-displayed antigen surface that resulted in the identification of clusters of residues contributing to epitope formation. Functional pictures of the epitope(s) were thus delineated in the natural context. Successful mapping of antibodies against interleukin-2, epidermal growth factor and its receptor, and vascular endothelial growth factor showed the versatility of these procedures, which combine the accuracy of site-directed mutagenesis with the high throughput potential of phage display.
Keywords: EGF, EGF receptor, epitope mapping, IL-2, library, phage display, site-directed mutagenesis, VEGF
Abbreviations
- aa
amino acid
- Abs
antibodies
- Ag
antigen
- EGF
epidermal growth factor
- EGFR
EGF receptor
- ELISA
enzyme-linked immunosorbent assay
- IL-2
interleukin-2
- mAb
monoclonal Ab
- PCR
polymerase chain reaction
- VEGF
vascular endothelial growth factor
Introduction
The ability to generate multiple novel antibodies (Abs) against a given target antigen (Ag), largely enhanced by the availability of huge combinatorial libraries,1 imposes the substantial challenge of choosing the most promising ones. The 2 key elements defining binding properties (affinity and fine specificity) dictate the usefulness of each antibody. Affinity of multiple Abs can be simultaneously measured in a relatively simple way2 and further engineered to reach the desired levels.3 Epitope specificity is a unique intrinsic characteristic distinguishing each monoclonal antibody (mAb). Mapping methods aimed at identifying the recognized epitope(s) are thus becoming an essential part of any Ab discovery pipeline.4 An optimal epitope mapping procedure should combine speed and simplicity (allowing simultaneous characterization of multiple Abs) with high reliability (resulting in a precise epitope determination). The current article summarizes mapping experiences accumulated during the last 2 y on the bases of phage display of native Ags and a comprehensive mutagenesis exploration of their surfaces to locate the target epitopes.5-9 The usefulness of such an approach in the context of a plethora of available epitope discovery techniques is discussed. Despite the wide use of phage display to study protein interactions, including epitope mapping,10 the particular strategy we review here had not been fully exploited until recently. Successful results with several Abs against different target antigens show its suitability and open the possibility of expanding its use to other antigenic systems, improving our ability to interrogate antibody paratopes.
The Importance of Defining Functional Epitopes on a Target
Epitope mapping can be accomplished through either structural or functional methods.11 Even though structural studies (i.e., X-ray crystallography of the Ag/Ab complexes) render detailed pictures of the interaction interfaces at atomic resolution,12 they are laborious and time-consuming, not properly suited for the initial research stages, when multiple candidates exist. Functional methods, based on identifying mutational hot spots abrogating Ag recognition, define the epitope location in a quick way.13 Beyond the speed advantage, functional mapping allows determination of the energetic contribution of each side chain to antigenicity, resulting in a functional picture of the interaction focused in the small subset of interface residues that determine binding.14 Unlike structural studies, functional mapping provides direct information about the effects of mutations in and around the epitope. The relevance of such knowledge is illustrated by the report of therapeutic antibody failure due to the emergence of target mutations abrogating recognition.15
Site-Directed Mutagenesis: Where and How Should Mutations be Introduced?
Despite the wide usefulness of site-directed mutagenesis to identify functional epitopes, the number of mutated variants that can be screened depends upon the ability to construct, express and purify them. Sometimes epitope identification is based on a few mutations.16 The role of non-targeted critical residues can thus be simply ignored. On the other hand, detrimental effects of a mutation on antigenicity can be due to a direct involvement of the targeted residue in paratope binding, but also to global or local folding defects resulting in the indirect disruption of epitopes located in other regions. The isolated effects of one or a few replacements could thus be misinterpreted leading to a wrong identification of the location of the antigenic determinant.
Display technologies have been exploited to test a higher number of mutated variants in the search for clusters of neighbor residues contributing to epitope formation. Mammalian cell display allows dissection of the antigenicity of membrane-anchored eukaryotic Ags in a realistic context, but its technical complexity still limits the number of molecules to be screened (often a few dozens).17 Massive screening of transfected cells harboring hundreds of mutated Ag variants has been recently reported.18 Yeast display renders large libraries containing millions of mutated Ag variants, usually derived from error-prone polymerase chain reaction (PCR) amplification of the target Ag gene, which can be characterized by high throughput flow cytometry. Although this mapping approach has allowed the isolation of hundreds of mutated variants of diverse antigens, 19-24 the completeness and accuracy of the resulting functional maps are hampered by 3 major drawbacks: a) random mutagenesis often results in a few mutations targeting the epitope neighborhood, b) the presence of more than one mutation in the same variant makes it difficult to assign a functional role to any of them, and c) many variants are useless because of mutations affecting key amino acids (aa) that determine folding like Cys or protein core residues.19 Additional experiments with designed collections of mutated variants allow the refinement of the resulting functional maps.24
The Combination of Phage Display and Site-Directed Mutagenesis Provides Very Detailed Functional Maps
The huge high-throughput potential of phage display has been exploited to scan the Ag surface in an efficient way. While low-level random diversification of phagemid-cloned Ag gene by error-prone PCR was initially performed,25,26 resembling the usual yeast display-based mapping procedures, detailed information about the residues contributing to epitope formation has been recently obtained through comprehensive mutagenesis scanning of the role of solvent-exposed side chains within a discrete surface patch.5-8 Once the target Ag is phage-displayed and a putative relevant antigenic region within it is identified through any available method, focused exploration of such region can result in a very high local sequence space coverage (Fig. 1). Functional maps thus generated integrate useful information from multiple individual replacements at several neighbor positions, leading to a robust identification of the target epitopes (Table 1).
Figure 1.
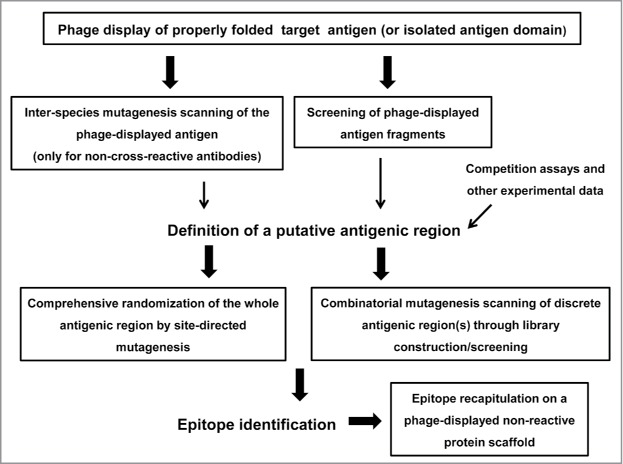
Schematic representation of the phage display-based antigen surface scanning platform for epitope mapping. The first step is successful phage display of the target antigen. A second challenge is the identification of a putative recognized antigenic region, which can be defined through several approaches. Once the candidate region is identified, it should be deeply explored, through extensive site-directed or combinatorial mutagenesis, in order to decipher the functional map of the epitope. Subsequent grafting experiments can confirm the identity of the functional epitope. All the procedures that could take advantage of the high throughput potential of phage display are enclosed in boxes.
Table 1.
Examples of functional epitope mapping through phage display-based antigen surface scanning
| Target antigen | mAb | Method to identify the antigenic region | Comprehensive scanning of the antigenic region | Residues identified as contributors to functional epitope formation | Additional mapping approaches | Reference |
|---|---|---|---|---|---|---|
| Human IL-2 | IL-2.1 | Single site-directed inter-species mutagenesis* (targeting 34 positions) | Site-directed randomization* (targeting 3 positions) | K64, E67, E68 | Epitope recapitulation on mouse IL-2* | Rojas et al., 20135 |
| IL-2.2 | Single site-directed inter-species mutagenesis* (targeting 34 positions) | Site-directed randomization* (targeting 5 positions) | E100, T101, T102, F103, M104 | Epitope recapitulation on mouse IL-2* | Rojas et al., 20135 | |
| Mouse IL-2 | JES6–5H4 | Single site-directed inter-species mutagenesis* (targeting 38 positions) | Site-directed randomization* (targeting 8 positions) | Y59, Q71, E74, D75, E76, E119, F122 | — | Rojas et al., 20135 |
| S4B6 | Competition data with an already mapped antibody | Site-directed randomization* (targeting 8 positions) | Y59, E74, D75, E76, F122 | — | Rojas et al., 20135 | |
| JES6–1A12 | Multiple site-directed inter-species mutagenesis* (targeting 45 positions) | Site-directed randomization* (targeting 14 positions) | Q43, N44, Y45, R46 | Epitope recapitulation on human IL-2* | Rojas et al., 20147 | |
| Peptide mimotope selection* | ||||||
| Human VEGF | L3H6 | Single site-directed inter-species mutagenesis* (targeting 8 positions) | Alanine scanning* (targeting 6 positions) | Y25, T71, E72, N100, K101, E103, R105 | Epitope recapitulation on mouse VEGF* | Lamdan et al., 20136 |
| Human EGF | CB-EGF.1 | Ag fragmentation* | Combinatorial randomization* (targeting 12 positions) | P7, L8, S9, D11, G12, Y13, G18 | — | Infante et al., 20149 |
| CB-EGF.2 | Ag fragmentation* | Combinatorial randomization* (targeting 16 positions) | Y22, I23, L26, D27, K28 | — | Infante et al., 20149 | |
| Human EGFR domain III | nimotuzumab | Single site-directed inter-species mutagenesis* (targeting 23 positions) | Site-directed randomization* (targeting 11 positions) | R353, S356, F357, T358, H359 | — | Tundidor et al., 20148 |
Functional epitopes have been identified on phage-displayed interleukin-2 (IL-2), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF) and EGF receptor (EGFR) domain III. The initial antigenic regions to be characterized were defined through different approaches. These regions were subsequently explored by several mutagenesis procedures, resulting in the identification of sets of residues contributing to the formation of each epitope. In some cases, additional mapping approaches were performed to confirm the identity of the identified epitope(s). The symbol * indicates those procedures that were performed on phage-displayed antigen-related molecules (the whole native antigen, its mutated variants, antigen fragments or mimotopes).
A critical element determining the success of the above described mapping approach is the quality of the phage-displayed Ag. While full-length interleukin-2 (IL-2), epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) have been displayed on filamentous phages for mapping purposes,5-7,9 restricting the scanning to the individual domain containing the epitope(s) under investigation is the alternative of choice for larger multi-domain Ags, as it has been done with phage-displayed EGF receptor (EGFR) domain III.8 In any case, the original folding/antigenicity of the molecule must be preserved. All the mentioned Ags have disulfide bonds (from one in IL-2 to 5 in EGFR domain III). Secretion to the oxidizing periplasmic compartment allowed their formation. Proper antigenicity was shown with the several Abs against each Ag, including some recognizing conformation-sensitive epitopes. Beyond antigenicity, phage-displayed IL-2 and VEGF were shown to bind their natural receptors.5,6 Phage-displayed IL-2 molecules successfully passed an even more stringent test: they were biologically active on IL-2-dependent cells.7,27,28 VEGF deserves particular attention because the native Ag is a homodimer. The presence of an amber stop codon between VEGF and M13 PIII coding genes, and the use of an amber suppressor strain, result in the synthesis of 2 polypeptides (the free VEGF monomer and the phage-linked VEGF-PIII fusion protein), which were able to dimerize on the phage surface.6,29
If standard phage display protocols fail to produce a properly folded antigen, several modifications already proposed to optimize protein display could be explored. Over-expression of periplasmic chaperones, like FkpA, able to assist folding is a suitable way to improve the quality of the displayed protein(s).30 The choice of viral proteins different from the conventionally used PIII and PVIII to fuse the foreign polypeptide can also optimize its display, as it has been shown with PVII and PIX-based systems.31 Some strategies rely on the use of signal peptides directing the polypeptide to be displayed to alternative translocation pathways (different from the widely exploited Sec route) that would be compatible with optimal folding of particular proteins. For instance, cotranslational secretion through the signal recognition particle (SRP) pathway directed by signal sequences like DsbA is optimal for those proteins that must be quickly folded in the periplasmic environment to achieve a proper conformation.32 Phage display of active and fully antigenic IL-2 indeed took advantage of the use of DsbA signal peptide.5,7 On the other hand, targeting to the twin-arginine translocation (TAT) pathway by signal sequences like TorA results in transport to periplasm of fully folded polypeptides, and is ideal for those proteins that need to be folded at cytoplasm.33-35 Even though all the published examples of epitope mapping on phage-displayed Ag deals with extracellular antigens,5-9 the latter display strategy could allow to extend the usefulness of this mapping approach to intracellular targets that are not properly folded at periplasm.
Once the antigen is displayed, comprehensive antigenic surface exploration depends upon an efficient modification of the Ag gene. Kunkel mutagenesis is a very simple procedure allowing controlled diversification through an overnight mutagenesis reaction of the already cloned target gene.36 The technique is high throughput-amenable due to the possibility of targeting the same template with multiple degenerate mutagenic oligonucleotides in 96-well PCR plates,37 resulting in the quick generation of hundreds of mutated variants with a very low frequency of undesired changes. Kunkel mutagenesis is fully compatible with phage display. Commonly used phagemids are easily produced as the required single-strand DNA mutagenesis templates. Other site-directed mutagenesis procedures have been used to obtain Ag-derived variants,6 but the extreme simplicity and speed of Kunkel mutagenesis make it the ideal way to obtain a wide diversity of molecules to be screened. Even though exploration of the phage-displayed Ag can rely on alanine scanning6 or replacement by any other single residue, site-directed randomization provides more information, since some critical positions can accept several (but not all) replacements. The whole profile of tolerated/non-tolerated mutations at each position not only shows the importance of a given original residue for antigenicity, but also underscores the relevant physicochemical features contributing to, or just allowing, binding. Figure 2 illustrates the details of a functional map derived from such an analysis.
Figure 2.
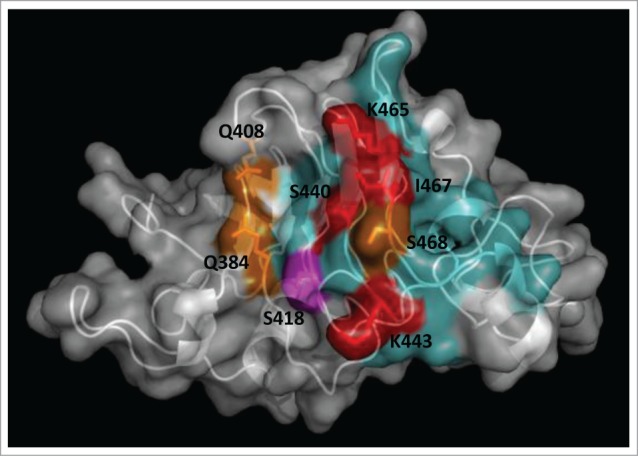
Functional dissection of the structural epitope recognized by cetuximab on EGF receptor. EGFR domain III (PDB code 1YY9) is shown as a cartoon with semi-transparent surface. Side chains of residues that contribute to binding (according to the site-directed mutagenesis scanning on the phage-displayed molecule) are represented with sticks and colored as follows. Critical residues that cannot be replaced (or can only be substituted by residues sharing their physicochemical properties) are highlighted in red. Other residues that can be replaced by some, but not all, amino acids (with no obvious shared features among them) are shown in orange. Magenta indicates that the corresponding residue can only be substituted by amino acids with smaller side chains, implying that its functional contribution is limited to the lack of steric hindrance. The rest of cetuximab structural epitope is represented in cyan. The figure was generated with Pymol.
Antigenicity screening of the mutated variants is usually performed by enzyme-linked immunosorbent assay (ELISA) on Ab-coated plates. Production of phage-containing supernatants at a 96-well plate scale allows high throughput evaluation.37 For some Ags, the sensitivity of supernatants’ ELISA is not high enough, due to either low display levels on the viral particles or to the presence of a low proportion of properly folded molecules (able to be recognized by the antibody) on them, despite normal absolute display levels. In such cases, phage purification/concentration is required and can be performed through a simple precipitation with polyethyleneglycol,38 still allowing the evaluation of hundreds of mutated variants.8 A proper judgment of site-directed mutagenesis results requires careful examination. Since conclusions are supported by loss-of-binding data, it is crucial to rule out gross folding defects affecting protein display or conformation. The simplest tool to do that is an anti-tag antibody (recognizing any tag -like c-myc peptide- fused to phage-displayed proteins). Recognition by this kind of mAb confirms the presence of each mutated variant on the phage surface and allows excluding those not properly displayed from any further analysis. More subtle folding defects can be discarded by testing with specific antibodies other than the one to be mapped.37 If their binding to variants loosing reactivity toward the antibody under investigation is intact, the specificity of mutation effects points to accurate epitope identification.
The above described mapping approach was challenged using 2 well-known therapeutic mAbs. Functional data generated for cetuximab and bevacizumab through phage display and mutagenesis of their target Ags6,8 matched with previous information obtained through both structural and functional methods,17,19,39,40 validating the accuracy of the phage-based procedure. A comparison between this method and previous epitope identification through mimotope selection from a random phage-displayed peptide library (for anti-IL-2 mAbs) rendered mixed results. The IL-2.2 mAb epitope identity was confirmed (only expanded by the identification of an additional relevant residue), but the antigenic determinant identified for IL-2.1 mAb was completely different from the one predicted on the basis of mimotope characterization.5 The latter was probably determined by the weak similarities within the selected peptide pool and between peptides and the nominal Ag.41 Site-directed mutagenesis in the original antigenic context represents a more reliable alternative.
Deciphering Epitopes with a Diffuse Energetic Landscape Through Combinatorial Mutagenesis
Despite the accuracy of the above described approach, additive or cooperative effects of amino acids that are not individually critical tend to be neglected or underestimated because they could be replaced (one by one) without disturbing antigenicity. This limitation decreases the completeness of the resulting functional maps. In scenarios where no critical residues can be defined at all, because recognition is the net result of many weak simultaneous interactions, single-position site-directed mutagenesis could totally fail to locate the antigenic determinant.
A combinatorial mutagenesis approach, aimed at circumventing these drawbacks, was recently described.9 Simultaneous mutagenesis of residues within discrete antigenic regions allowed evaluation of the contribution of each amino acid cluster as a whole to antigenicity. This procedure provides both loss-of-function and gain-of-function data. The first screening detects loss-of-binding in a randomization library targeting a given antigenic region, showing its involvement in epitope formation. The second step is selection of library phages on the antibody under investigation. Regular sequence patterns within selected Ab-binding positive variants highlight those original residues that are recurrently selected and are thus likely to contribute to antigenicity. Remarkably, Ag binding recovery is frequently associated with conserved physicochemical properties resembling those of the original residues rather than with strict aa conservation. The use of stop templates (containing several consecutive stop codons in the region(s) of the gene to be targeted) for library construction guarantees that only diversified variants are produced at the protein level, avoiding an overwhelming selection-driven accumulation of useless non-mutated Ag that could hide the mutated variants.
The features of functional maps depicted through combinatorial mutagenesis scanning of a model Ag (EGF) are illustrated in Figure 3. The method is very powerful to detect the combined influence of clusters of residues on epitope formation (Table 1), but is not properly suited to define their individual roles in an unequivocal way. Despite this limitation, residues can be classified with a high probability as functional contributors and major functional contributors based on their prevalence among Ab-binding positive variants. This output is in contrast with the above described single-position site-directed mutagenesis scanning, which reveals individual interactions within a binding interface with high accuracy (Fig. 2). An ideal mapping strategy would thus combine combinatorial mutagenesis scanning of discrete regions with single-position mutagenesis, in order to delineate a complete functional picture of the epitope, including all (or at least most) weak contributors, and highlighting the critical roles of major individual contributors.
Figure 3.
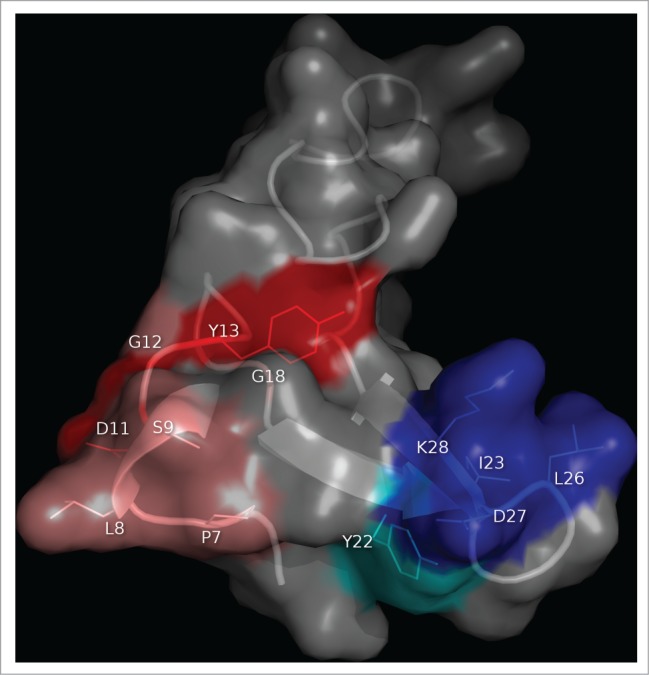
Clusters of residues identified by combinatorial mutagenesis as the epitopes recognized by 2 monoclonal antibodies against EGF. The antigen (PDB code 1IVO_C) is represented as a cartoon with semi-transparent surface. Residues recurrently found among EGF mutated variants selected from libraries on CB-EGF.1 or CB-EGF.2 mAbs were considered to contribute to the formation of each epitope. The corresponding side chains are represented with lines and colored as described below. A closer examination of their relative abundances allowed the subsequent classification of relevant residues as functional contributors/major functional contributors. Residues belonging to each of these categories are highlighted in salmon/red (for CB-EGF.1 mAb) and cyan/blue (in the case of CB-EGF.2). The figure was generated with Pymol.
Combinatorial mutagenesis had been used before to scan protein interaction interfaces.42-44 The rationale behind these studies had been to introduce diversity (through either replacements by Ala or randomization) simultaneously at several positions in order to identify multiple detrimental and tolerated mutations with a reduced mutagenesis effort (constructing a few libraries). Even though the use of large libraries having combinations of mutations allows these studies to be formally classified as combinatorial, their goals have been focused on identifying individual aa contributions.42 Library design itself (targeting aa that are as far apart as possible and no more than one already known critical residue in each library) was aimed at minimizing the combined effects of several replacements.44 The recently described combinatorial epitope mapping approach9 relies on modifying clusters of residues that are in close proximity to each other and have a high likelihood of establishing additive or cooperative interactions with the paratope. Recording the whole picture of these combined effects is thus the goal of this new approach.9
Mutated Antigen vs. Random Peptide Libraries: Accuracy or Universality?
Combinatorial Ag mutagenesis followed by selection on the antibody under investigation has some shared features with one of the most extended epitope mapping procedures: selection of ligands recognized by mAbs from phage-displayed peptide libraries.45 Both approaches lead to the isolation of small amino acid arrays (from a large collection of random molecules) interacting with the paratope. While the second procedure relies on the availability a single-pot universal peptide library (useful to map multiple Abs against virtually any target), combinatorial Ag surface scanning requires the construction of one or more libraries for each Ag. Large-scale Kunkel mutagenesis has greatly simplified library construction,46 and the additional effort is certainly compensated by the higher reliability of mapping results.
Peptide mimotopes isolated from general purpose libraries can resemble to different extents the original interactions established by the nominal Ag. Some of them reproduce accurately short Ag sequence motifs determining antigenicity.47 Others mimic the tridimensional array of physicochemical properties behind recognition.48 Making an unequivocal connection between peptide sequences and the original antigenic determinants is not always simple,49 despite the availability of computational tools designed to look for such correspondence.50 The emergence of multiple theoretical solutions pointing to diverse possible epitopes complicates the task. Sometimes peptides establish totally different interactions with the paratope compared to the nominal Ags, resulting in the absence of structural and even functional, antigen mimicry.51 The existence of paratopes with dual (and probably multiple) specificities,52 added further support to the notion that a selector antibody can pick mimotopes unrelated to the original Ag from random libraries.
On the other hand, when library diversity is constrained into the scaffold of the Ag itself (and concentrated into those antigenic regions known to contribute to binding), there is a high likelihood that the selected variants keep the essential antigen features resulting in the original recognition event. Screening this kind of library is like zooming-in on the Ag/Ab reaction under study, dismissing the plethora of potential interactions that the same paratope could in principle establish within an unlimited antigenic universe.
The Starting Point: Locating a Broad Antigenic Region to be Scanned
A pre-requisite for a deep exploration of a relevant Ag region through either single-position or combinatorial mutagenesis is the definition of such a candidate antigenic region. This can be accomplished in several ways (Table 1). One of the most useful approaches is based on cross-reactivity data. If the antibody does not cross-react with the target antigen counterpart from a different origin, a first mutagenesis round can explore the effects of replacing every individual residue that differs between the 2 species.37 The resulting small collections of phage-displayed mutated variants are ready to be screened with any non-cross-reactive mAb. Such analysis has resulted in the identification of one or more key residues useful to define a surrounding region, including solvent-exposed residues located in a radius of 8–12 Å, to be explored on IL-2, VEGF and EGFR domain III.5,6,8 When single inter-species replacements are not enough to disrupt binding, presumably due to the combined effects of several sequence variations resulting in the observed lack of cross-reactivity, multiple mutagenesis (i.e., targeting several residues located in close proximity and different between the 2 species) is a valuable alternative. This approach was used successfully to identify a segment within mouse IL-2 responsible for the lack of cross-reactivity of JES6–1A12 mAb with the human molecule, and to define a broad antigenic area where the actual epitope was subsequently located.7
Another approach to find the antigenic region to be scanned is the screening of Ag fragments, either synthetic or recombinant. Ag fragmentation to identify the recognized segments is a widely used epitope mapping method per se.53-58 The major drawbacks of this approach are that short segments of Ag sequence not always contain all the information to give rise to the native epitopes, particularly if they are discontinuous or conformation-sensitive, and the use of long segments results in low resolution maps.55 Despite these limitations, the epitopes for some mAbs and for a substantial fraction of the antibodies contained in polyclonal antisera can be readily elucidated through Ag fragmentation. High-throughput screening of peptide microarrays covering the primary sequence of a given Ag, of a set of related Ags,56 and even of the whole proteome58 has been successfully used. These are ideal tools to dissect the complex specificity mixtures in polyclonal Abs. In the case of mAbs, screening the recognition of antigen fragments can be used as a general mapping procedure, but also as a quick way to locate an initial antigenic region to be further explored by extensive mutagenesis to delineate the detailed functional picture of each epitope.9,26 Remarkably, the whole process can take advantage of the phage display platform, because Ag fragments of different lengths can be displayed and eventually give rise to large libraries. 53-55,57 Sometimes the displayed fragments keep essential structure-determining elements like internal disulfide bonds, allowing the study of conformation-sensitive epitopes.9
If no starting point can be defined for mapping a given mAb, competition with an already mapped antibody can still point to the antigenic region recognized by the latter as the area to search for the epitopes of competitor Abs. Since mAbs can be grouped by high throughput epitope binning,4 the identification of starting points to map a few mAbs could be enough to characterize a plethora of competitor antibodies. The case of S4B6 anti-IL-2 mAb illustrates epitope discovery based on exploring an antigenic region originally defined for another antibody.5
The Power of Epitope Recapitulation as a Confirmation Tool
The optimal complement to loss-of-function epitope mutagenesis data is a gain-of-function epitope reconstruction approach. If grafting the putative residues contributing to epitope formation is enough to achieve recognition of a previously non-reactive protein scaffold, this is an unequivocal proof of epitope identity. In the case of non-cross-reactive antibodies, the scaffold can be the Ag counterpart from a different species (Table 1). Both human and mouse species-specific IL-2 epitopes have been reconstructed on the other molecule.5,7. Human VEGF epitope recapitulation on mouse VEGF was a more significant achievement, due to the conformation-sensitivity of the original epitope.6 Reconstruction of other conformational epitopes has not been possible.5
What's Next?
The above described phage display-based mapping platform (Fig. 1) is robust, accurate and versatile enough to allow display of the whole antigens and their domains, Ag fragmentation, single, multiple and combinatorial mutagenesis scanning of the antigenic surfaces, as well as epitope grafting. The appropriate combination of these procedures, together with the information retrieved from other experimental systems, has been useful to underscore the identity of multiple epitopes on different antigens and to reveal details about their interactions (Table 1). Clusters of residues comprising 3–7 aa, being either continuous or discontinuous within the Ag sequence, have been shown to contribute to epitope formation. The usefulness of the above described mapping platform to dissect conformational epitopes is particularly remarkable. While a previous attempt to recapitulate conformational antigenic determinants, formed by discontinuous sequence segments of the Ag, was based on computational design and synthesis of peptides resembling the corresponding surface patches,59 targeting the epitopes in their natural context (the whole Ag itself) provides an optimal scenario to disrupt and reconstruct them.
Despite its simplicity, wide applicability, and successful validation with well-known antigenic determinants, some technical improvements of the already described work flow will likely increase the efficiency and reliability of epitope discovery. Single-position and combinatorial mutagenesis scanning are still far from exploring the whole sequence space in a homogeneous fashion. For instance, none of the published examples includes the evaluation of the effects of the 19 possible replacements at each position. Although local sequence space coverage is much higher than the ones achieved through alanine/homolog scanning or through random low-level mutagenesis, the number of tested mutations ranges between 3–12 replacements per position. This number is limited by the generation of mutated variants that cannot be displayed on phages, by the fortuitous emergence of undesired mutations in some of them, which should then be excluded from the analysis, but mainly by the intrinsic bias determined by randomizing with NNK codons. The degenerate triplet NNK includes 32 specific codons. While some aa are coded by a single codon in that mixture, others are coded by several of them and have a higher probability of being introduced during mutagenesis. This unequal distribution of mutations is even more significant in libraries, where combinatorial effects amplify any sequence bias. Unbiased mutagenic oligonucleotides having a single triplet coding for each residue would be the solution to achieve optimal sequence space coverage.60 Beyond the mutagenesis step, library diversity sampling (either before selection or after enrichment on a selector Ab) would greatly benefit from deep sequencing. This procedure is predicted to reveal not only the major sequence features distinguishing groups of Ag variants (recognized or not by the selector mAb), but also minor details contributing to antigenicity that are currently dismissed through sequencing of small sets of clones.61
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Schofield DJ, Pope AR, Clementel V, Buckell J, Chapple SDJ, Clarke KF, Conquer JS, Crofts AS, Crowther SRE, Dyson MR, et al. Application of phage display to high throughput antibody generation and characterization. Genome Biol 2007; 8:R254; PMID:18047641; http://dx.doi.org/ 10.1186/gb-2007-8-11-r254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wassaf D, Kuang G, Kopacz K, Wu QL, Nguyen Q, Toews M, Cosic G, Jacques J, Wiltshire S, Lambert J, et al. High-throughput affinity ranking of antibodies using surface plasmon resonance microarrays. Anal Biochem 2006; 351:241-53; PMID:16510109; http://dx.doi.org/ 10.1016/j.ab.2006.01.043 [DOI] [PubMed] [Google Scholar]
- 3.Dyson MR, Zheng Y, Zhang C, Colwill K, Pershad K, Kay BK, Pawson T, McCafferty J. Mapping protein interactions by combining antibody affinity maturation and mass spectrometry. Anal Biochem 2011; 417:25-35; PMID:21704603; http://dx.doi.org/ 10.1016/j.ab.2011.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdiche YN, Miles M, Eckman J, Foletti D, Van Blarcom TJ, Yeung YA, Pons J, Rajpal A. high-throughput epitope binning assays on label-free array-based biosensors can yield exquisite epitope discrimination that facilitates the selection of monoclonal antibodies with functional activity. PLoS One 2014; 9:e92451; PMID:24651868; http://dx.doi.org/ 10.1371/journal.pone.0092451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rojas G, Pupo A, Leon K, Avellanet J, Carmenate T, Sidhu S. Deciphering the molecular bases of the biological effects of antibodies against Interleukin-2: a versatile platform for fine epitope mapping. Immunobiology 2013; 218:105-13; PMID:22459271; http://dx.doi.org/ 10.1016/j.imbio.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 6.Lamdan H, Gavilondo JV, Muñoz Y, Pupo A, Huerta V, Musacchio A, Perez L, Ayala M, Rojas G, Balint RF, et al. Affinity maturation and fine functional mapping of an antibody fragment against a novel neutralizing epitope on human vascular endothelial growth factor. Mol Biosyst 2013; 9:2097-106; PMID:23702826; http://dx.doi.org/ 10.1039/c3mb70136k [DOI] [PubMed] [Google Scholar]
- 7.Rojas G, Infante YC, Pupo A, Carmenate T. Fine specificity of antibodies against Interleukin-2 explains their paradoxical immunomodulatory effects. mAbs 2014; 6:273-85; PMID:24253188; http://dx.doi.org/ 10.4161/mabs.27224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tundidor Y, Garcia-Hernandez CP, Pupo A, Infante YC, Rojas G. Delineating the functional map of the interaction between nimotuzumab and the epidermal growth factor receptor. mAbs 2014; 6:1013-25; PMID:24759767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Infante YC, Pupo A, Rojas G. A combinatorial mutagenesis approach for functional epitope mapping on phage-displayed target antigen: application to antibodies against epidermal growth factor. mAbs 2014; 6:637-48; PMID:24589624; http://dx.doi.org/ 10.4161/mabs.28395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith GP, Petrenko VA. Phage display. Chem Rev 1997; 97:391-410; PMID:11848876; http://dx.doi.org/ 10.1021/cr960065d [DOI] [PubMed] [Google Scholar]
- 11.Greenspan NS, DiCera E. Defining epitopes: it's not as easy as it seems. Nat Biotechnol 1999; 17:936-7; PMID:10504677; http://dx.doi.org/ 10.1038/13590 [DOI] [PubMed] [Google Scholar]
- 12.Davies DR, Cohen GH. Interactions of protein antigens with antibodies. Proc Natl Acad Sci USA 1996; 93:7-12; http://dx.doi.org/ 10.1073/pnas.93.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamin DC, Perdue SS. Site-directed mutagenesis in epitope mapping. Methods 1996; 9:508-15; PMID:8812706; http://dx.doi.org/ 10.1006/meth.1996.0058 [DOI] [PubMed] [Google Scholar]
- 14.van Regenmortel MH. Structural and functional approaches to study the study of protein antigenicity. Immunol Today 1989; 10:266-72; PMID:2478146 [DOI] [PubMed] [Google Scholar]
- 15.Montagut C, Dalmases A, Belosillo B, Crespo M, Pairet S, Iglesias M, Salido M, Gallen M, Marsters S, Tsai SP, et al. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor receptor conferring cetuximab resistance in colorectal cancer. Nat Med 2012; 18:221-3; PMID:22270724; http://dx.doi.org/ 10.1038/nm.2609 [DOI] [PubMed] [Google Scholar]
- 16.Kamat V, Donaldson JM, Kari C, Quadros MR, Lelkes PI, Chaiken I, Cocklin S, Williams JC, Papazoglou E, Rodeck V. Enhanced EGFR inhibition and distinct epitope recognition by EGFR antagonistic mAbs C225 and 425. Cancer Biol Ther 2008; 7:726-33; PMID:18424917; http://dx.doi.org/ 10.4161/cbt.7.5.6097 [DOI] [PubMed] [Google Scholar]
- 17.Voigt M, Braig F, Gothel M, Schulte A, Lamszus K, Bokemeyer C, Binder M. Functional dissection of the epidermal growth factor receptor epitopes targeted by panitumumab and cetuximab. Neoplasia 2012; 14:1023-31; PMID:23226096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson E, Doranz BJ. A high-throughput shotgun mutagenesis approach to mapping B-cell antibody epitopes. Immunology 2014; 143:13-20; PMID:24854488; http://dx.doi.org/ 10.1111/imm.12323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao G, Cochran R, Wittrup KD. Fine epitope mapping of anti-epidermal growth factor receptor antibodies through random mutagenesis and yeast surface display. J Mol Biol 2004; 342:539-50; PMID:15327953; http://dx.doi.org/ 10.1016/j.jmb.2004.07.053 [DOI] [PubMed] [Google Scholar]
- 20.Levy R, Forsyth CM, LaPorte SL, Geren N, Smith LA, Marks JD. Fine and domain-level epitope mapping of botulinum neurotoxin type A neutralizing antibodies by yeast surface display. J Mol Biol 2007; 365:196-210; PMID:17059824; http://dx.doi.org/ 10.1016/j.jmb.2006.09.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spangler JB, Neil JR, Abramovitch S, Yarden Y, White FM, Lauffenburger DA, Wittrup KD. Combination antibody treatment down-regulates epidermal growth factor receptor by inhibiting endosomal recycling. Proc Natl Acad Sci USA 2010; 107:13252-7; PMID:20616078; http://dx.doi.org/ 10.1073/pnas.0913476107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han T, Sui J, Bennett AS, Liddington RC, Donis RO, Zhu Q, Marasco WA. Fine epitope mapping of monoclonal antibodies against hemagglutinin of a highly pathogenic H5N1 influenza virus using yeast surface display. Biochem Biophys Res Commun 2011; 409:253-9; PMID:21569761; http://dx.doi.org/ 10.1016/j.bbrc.2011.04.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giuntini S, Beernink PT, Reason DC, Granoff DM. Monoclonal antibodies to meningococcal factor H binding protein with overlapping epitopes and discordant functional activity. PLoS One 2012; 7:e34272; PMID:22461909; http://dx.doi.org/ 10.1371/journal.pone.0034272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mata-Fink J, Kriegsman B, Yu H-X, Zhu H, Hanson MC, Irvine DJ, Wittrup KD. Rapid conformational epitope mapping of anti-gp120 antibodies with a designed mutant panel displayed on yeast. J Mol Biol 2013; 425:444-56; PMID:23159556; http://dx.doi.org/ 10.1016/j.jmb.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jespers L, Jenne S, Lasters I, Collen D. Epitope mapping by negative selection of randomized antigen libraries displayed on filamentous phages. J Mol Biol 1997; 269:704-18; PMID:9223635; http://dx.doi.org/ 10.1006/jmbi.1997.1077 [DOI] [PubMed] [Google Scholar]
- 26.Yip YL, Novotny J, Edwards M, Ward RL. Structural analysis of the ErbB-2 receptor using monoclonal antibodies: implications for receptor signaling. Int J Cancer 2003; 104:303-9; PMID:12569553; http://dx.doi.org/ 10.1002/ijc.10951 [DOI] [PubMed] [Google Scholar]
- 27.Vispo NS, Callejo M, Ojalvo AG, Santos A, Chinea G, Gavilondo JV, Arana MJ. Displaying human Interleukin-2 on the surface of bacteriophage. Immunotechnology 1997; 3:185-93; PMID:9358271; http://dx.doi.org/ 10.1016/S1380-2933(97)00012-2 [DOI] [PubMed] [Google Scholar]
- 28.Buchli PJ, Wu Z, Ciardelli TL. The functional display of interleukin-2 on filamentous phage. Arch Biochem Biophys 1997; 339; 79-84; PMID:9056236; http://dx.doi.org/ 10.1006/abbi.1996.9853 [DOI] [PubMed] [Google Scholar]
- 29.Muller YA, Li B, Christinger HW, Wells JA, Cunningham BS, de Vos AM. Vascular endothelial growth factor: crystal structure and functional mapping of the kinase domain receptor binding site. Proc Natl Acad Sci USA 1997; 94:7192-7; PMID:9207067; http://dx.doi.org/ 10.1073/pnas.94.14.7192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunnarsen KS, Kristinsson SG, Justesen S, Frigstad T, Buus S, Bogen B, Sandlie I, Løset GA. Chaperone-assisted thermostability engineering of a soluble T cell receptor using phage display. Sci Rep 2013; 3:1162; PMID:23362461; http://dx.doi.org/ 10.1038/srep01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Løset GA, Roos N, Bogen B, Sandlie I. Expanding the versatility of phage display II: improved affinity selection of folded domains on protein VII and IX of the filamentous phage. PLoS One 2011; 6:e17433; PMID:21390283; http://dx.doi.org/ 10.1371/journal.pone.0017433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steiner D, Forrer P, Stumpp MT, Plückthun A. Signal sequences directing cotranslational translocation expand the range of proteins amenable to phage display. Nat Biotechnol 2006; 24:823-31; PMID:16823375; http://dx.doi.org/ 10.1038/nbt1218 [DOI] [PubMed] [Google Scholar]
- 33.Paschke M, Höhne W. A twin-arginine translocation (Tat)-mediated phage display system. Gene 2005; 350:79-88; PMID:15794923; http://dx.doi.org/ 10.1016/j.gene.2005.02.005 [DOI] [PubMed] [Google Scholar]
- 34.Thammawong P, Kasinrerk W, Turner RJ, Tayapiwatana C. Twin-arginine signal peptide attributes effective display of CD147 to filamentous phage. Appl Microbiol Biotechnol 2006; 69:697-703; PMID:16320049; http://dx.doi.org/ 10.1007/s00253-005-0242-0 [DOI] [PubMed] [Google Scholar]
- 35.Speck J, Arndt KM, Müller KM. Efficient phage display of intracellularly folded proteins mediated by the TAT pathway, Prot Eng Des Sel 2011; 24:473-84; PMID:21289038; http://dx.doi.org/ 10.1093/protein/gzr001 [DOI] [PubMed] [Google Scholar]
- 36.Kunkel TA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA 1985; 82:488-92; PMID:3881765; http://dx.doi.org/ 10.1073/pnas.82.2.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rojas G. Fine epitope mapping based on phage display and extensive mutagenesis of the target antigen Methods Mol Biol 2014; 1131:447-76; PMID:24515482; http://dx.doi.org/ 10.1007/978-1-62703-992-5_27 [DOI] [PubMed] [Google Scholar]
- 38.Marks JD, Hoogenboom HR, Bonnert TP, McCafferty J, Griffiths AD, Winter G. By-passing immunization- human antibodies from V-gene libraries displayed on phage. J Mol Biol 1991; 222:581-97; PMID:1748994; http://dx.doi.org/ 10.1016/0022-2836(91)90498-U [DOI] [PubMed] [Google Scholar]
- 39.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the Epidermal Growth Factor receptor by cetuximab. Cancer Cell 2005; 7:301-11; PMID:15837620; http://dx.doi.org/ 10.1016/j.ccr.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 40.Muller YA, Chen Y, Christinger W, Li B, Cunnigham BC, Lowman HB, de Vos AM. VEGF and the Fab fragment of a humanized neutralizing antibody: crystal structure of the complex at 2.4 A resolution and mutational analysis of the interface. Structure 1998; 6:1153-67; PMID:9753694; http://dx.doi.org/ 10.1016/S0969-2126(98)00116-6 [DOI] [PubMed] [Google Scholar]
- 41.Vispo NS, Arana MJ, Chinea G, Ojalvo AG, Cesareni G. Characterization of epitopes on human Interleukin-2 using phage-displayed peptide libraries: insights into antibody-peptide interactions. Hybridoma 1999; 18:251-5; PMID:10475239; http://dx.doi.org/ 10.1089/027245799315907 [DOI] [PubMed] [Google Scholar]
- 42.Weiss GA, Watanabe CK, Zhong A. Goddard A, Sidhu SS. Rapid mapping of protein functional epitopes by combinatorial alanine scanning. Proc Natl Acad Sci USA 2000; 97:8950-4; PMID:3881765; http://dx.doi.org/ 10.1073/pnas.160252097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrison KL, Weiss GA. Combinatorial alanine-scanning. Curr Opin Chem Biol 2001; 5:302-7; PMID:11479122; http://dx.doi.org/ 10.1016/S1367-5931(00)00206-4 [DOI] [PubMed] [Google Scholar]
- 44.Pal G, Kouadio J-LK, Artis DR, Kossiakoff AA, Sidhu SS. Comprehensive and quantitative mapping of energy landscapes for protein-protein interactions by rapid combinatorial scanning. J Biol Chem 2006; 281:22378-85; PMID:16762925; http://dx.doi.org/ 10.1074/jbc.M603826200 [DOI] [PubMed] [Google Scholar]
- 45.Felici F, Castagnoli L, Musacchio A, Japelli R, Cesareni G. Selection of antibody ligands from a large library of oligopeptides expressed on a multivalent exposition vector. J Mol Biol 1991; 222:301-10; PMID:1720463; http://dx.doi.org/ 10.1016/0022-2836(91)90213-P [DOI] [PubMed] [Google Scholar]
- 46.Fellouse FA, Sidhu SS. Making Antibodies in Bacteria. In Making and Using Antibodies. A Practical Handbook. Howard GC, Kaser MR, Boca Raton, Florida: CRC Press, 2007, pp 157-77 [Google Scholar]
- 47.Sun E-C, Zhao J, Yang T, Liu N-H, Geng H-W, Qin Y-L, Wang L-F, Bu Z-G, Yang Y-H, Lunt RA, et al. Identification of a conserved JEV serocomplex B-cell epitope by screening a phage-display peptide library with a mAb generated against West Nile virus capsid protein. Virol J 2011; 8:100; PMID:21375771; http://dx.doi.org/ 10.1186/1743-422X-8-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Y, Wang Y, Struble EB, Zhang P, Chowdhury S, Reed JL, Kennedy M, Scott DE, Fisher RW. Epitope mapping by random peptide phage display reveals essential residues for vaccinia extracellular enveloped virion spread. Virol J 2012; 9:217; PMID:23006741; http://dx.doi.org/ 10.1186/1743-422X-9-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perosa F, Favoino E, Vicenti C, Guearnera A, Racanelli V, De Pinto V, Dammacco F. Two structurally different rituximab-specific CD20 mimotope peptides reveal that rituximab recognizes two different CD20-associated epitopes. J Immunol 2009; 182:416-23; PMID:19109173; http://dx.doi.org/ 10.4049/jimmunol.182.1.416 [DOI] [PubMed] [Google Scholar]
- 50.Moreau V, Granier C, Villard S, Laune D, Molina F. Discontinuous epitope prediction based on mimotope analysis. Bioinformatics 2006; 22:1088-95; PMID:16434442; http://dx.doi.org/ 10.1093/bioinformatics/btl012 [DOI] [PubMed] [Google Scholar]
- 51.Saphire EO, Montero M, Menendez A, van Houten NE, Irving MB, Pantophlet R, Zwick MB, Parren PWHI, Burton DR, Scott JK, Wilson IA. Structure of a high affinity “minotope” peptide bound to HIV-1-neutralizing antibody b12 explains its inability to elicit gp120 cross-reactive antibodies. J Mol Biol 2007; 369:696-709; PMID:17445828; http://dx.doi.org/ 10.1016/j.jmb.2007.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bostrom J, Yu SF, Kan D, Appleton BA, Lee CV, Billeci K, Man W, Peale F, Ross S, Wiesmann C, et al. Variants of the antibody Herceptin that interact with HER2 and VEGF at the antigen binding site. Science 2009; 323:1610-4; PMID:19299620; http://dx.doi.org/ 10.1126/science.1165480 [DOI] [PubMed] [Google Scholar]
- 53.Yip YL, Smith G, Koch J, Dubel S, Ward RL. Identification of epitope regions recognized by tumor inhibitory and stimulatory anti-ErbB-2 monoclonal antibodies: implications for vaccine design. J Immnol 2001; 166:5271-8; PMID:11290813; http://dx.doi.org/ 10.4049/jimmunol.166.8.5271 [DOI] [PubMed] [Google Scholar]
- 54.Di Niro R, Ferrara F, Not T, Bradbury ARM, Chirdos F, Marzari R, Sblattero D. Characterizing monoclonal antibody epitopes by filtered gene fragment phage display. Biochem J 2005; 388:889-94; PMID:15720292; http://dx.doi.org/ 10.1042/BJ20041983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mullaney PB, Pallavicini MG, Marks JD. Epitope mapping of neutralizing Botulinum neurotoxin A antibodies by phage display. Infect Immun 2006; 69:6511-14; PMID:11553596; http://dx.doi.org/ 10.1128/IAI.69.10.6511-6514.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J, Bardina L, Shreffler WG, Andreae DA, Ge Y, Wang J, Bruni FM, Fu Z, Han Y, Sampson HA. Development of a novel peptide microarray for large scale epitope mapping of food allergens. J Allergy Clin Immunol 2009; 124:315-22; PMID:19577281; http://dx.doi.org/ 10.1016/j.jaci.2009.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou M, Meyer T, Koch S, Koch J, von Briesen H, Benito JM, Soriano V, Haber A, Bickel M, Dubel S. Identification of a new epitope for HIV-neutralizing antibodies in the gp41 membrane proximal external region by an Env-tailored phage display library. Eur J Immunol 2013; 43:499-509; PMID:23180650; http://dx.doi.org/ 10.1002/eji.201242974 [DOI] [PubMed] [Google Scholar]
- 58.Forsström B, Axnäs BB, Stengele K-P, Bühler J, Albert TJ, Richmond TA, Hu FJ, Nilsson P, Hudson EP, Rockberg J, et al. Proteome-wide epitope mapping of antibodies using ultra-dense peptide arrays. Moll Cell Proteomics 2014; 13:585-97; PMID:24705123; http://dx.doi.org/ 10.1074/mcp.M113.033308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jaeger IS, Kretzschmar I, Korner J, Weiser AA, Mahrenholz CC, Potty A, Kourentzi K, Willson RC, Volkmer R, Preissner R. Mapping discontinuous protein-binding sites via structure-based peptide libraries: combining in silico and in vitro approaches. J Mol Recognit 2013; 26:23-31; PMID:23280614; http://dx.doi.org/ 10.1002/jmr.2237 [DOI] [PubMed] [Google Scholar]
- 60.Yañez J, Arguello M, Osuna J, Soberon X, Gaytan P. Combinatorial codon-based amino acid substitutions. Nucleic Acids Res 2003; 32:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matochko WL, Chu K, Jin B, Lee SW, Whitesides GM, Derda R. Deep sequencing analysis of phage libraries using Illumina platform. Methods 2012; 58:47-55; PMID:22819855; http://dx.doi.org/ 10.1016/j.ymeth.2012.07.006 [DOI] [PubMed] [Google Scholar]


