Abstract
Inflorescences of a dominant mutant of Arabidopsis Aux/IAA7, axr2, showed negative phototropism with a similar fluence response curve to the positive phototropism of wild-type stems. Application of a synthetic auxin, NAA, and an inhibitor of polar auxin transport, NPA, increased and decreased respectively the magnitude of the phototropic response in the wild type, while in axr2 application of NAA reduced the negative phototropic response and NPA had no effect. Decapitation of the apex induced a small negative phototropism in wild-type stems, and had no effect in axr2 plants. Inflorescences of the double mutants of auxin transporters, pgp1 pgp19, showed no phototropic response, while decapitation resulted in a negative phototropic response. These results suggest that negative phototropism can occur when the level of auxin or of auxin signaling is reduced to a minimal level, and that in plant axial organs the default phototropic response to unilateral blue light may be negative. Expression of axr2 protein by an endodermis-specific promoter resulted in agravitropism of inflorescences in a similar way to that of axr2, but phototropism was normal, confirming that the endodermis does not play a critical role in phototropism.
Keywords: Arabidopsis thaliana, dominant Aux/IAA mutants, gravitropism, hypocotyl, inflorescence, phototropism
Abbreviations
- Aux/IAA
auxin/inodole-3-acetic acid
- TIR1
transport inhibitor 1
- axr2
auxin resistant 2
- NAA
1-naphtha-leneacetic acid
- NPA
1-N-naphthylpthalamic acid
- pgp
p-glycoprotein
- phot
phototropin
- SAUR
small auxin up RNA
- SCR
scarecrow
axr2 is a dominant mutant of the Arabidopsis AUX/IAA7 gene that encodes a transcriptional repressor in TIR1-mediated auxin signaling.1,2 In axr2, the mutated protein is stabilized by a mutation in a degron called domain II, thus resulting in the inhibition of transcription mediated through auxin response factors.2 axr2 is a dwarf, and shows obvious gravitropic defects in roots, hypocotyls and inflorescence stems.1,3 In the present study, we investigated phototropism of axr2 inflorescences, and found that they showed negative phototropism, in contrast to the positive phototropism of wild-type stems.4 The negative phototropic response of axr2 and the positive phototropic response of wild type to unilateral blue-light irradiation showed essentially the same dose-response curves, while phot1 phot2 double mutants showed no phototropic response, as was found for stem phototropism in the double mutant.4 These results suggest that negative phototropism of axr2 stems is mediated through phototropins, as is positive phototropism in the wild type. Application of lanolin paste containing a synthetic auxin, 1-naphthaleneacetic acid (NAA), on shoot apices decreased the magnitude of the negative phototropic response in axr2, but increased the positive phototropic response of wild type (Figs. 1 and 2). Application of an inhibitor of polar auxin transport, 1-N-naphthylpthalamic acid (NPA), had no effect on the positive phototropic response of wild type, and a slight decrease in the negative phototropic response of axr2. Decapitation of the apex, which is likely to be a major source of auxin for stems, resulted in a small negative phototropic response in wild type, but had no effect on the negative phototropic response of axr2. These results suggest that a decreased level of auxin leads to a decrease in the magnitude of positive phototropism, and eventually, when the level of auxin is sufficiently reduced, to negative phototropism. We next examined phototropism in pgp1 pgp19 double mutants (pgpD). PGP encode ABC transporters of auxin, defects of which have been reported to reduce the basipetal transport of auxin in inflorescences.5 pgpD inflorescences showed no phototropic response, while decapitated pgpD inflorescences showed a negative phototropic response, an observation which is consistent with the above suggestion. In fact, the level of expression of auxin-inducible genes, such as IAA14, IAA19 and SAUR50, was reduced in axr2 and pgpD stems compared with that in wild-type stems, and in each genotype, decapitation further decreased the level of expression. Negative phototropism has only been reported in oat coleoptiles, when exposed to unilateral pulse irradiation with a limited range of blue-light fluence.6 Results of the present study suggest that negative phototropism can occur when the level of auxin or of auxin signaling is reduced to a minimal level, and that negative phototropism is the basal response to unilateral blue-light irradiation in plant axial organs.
Figure 1.
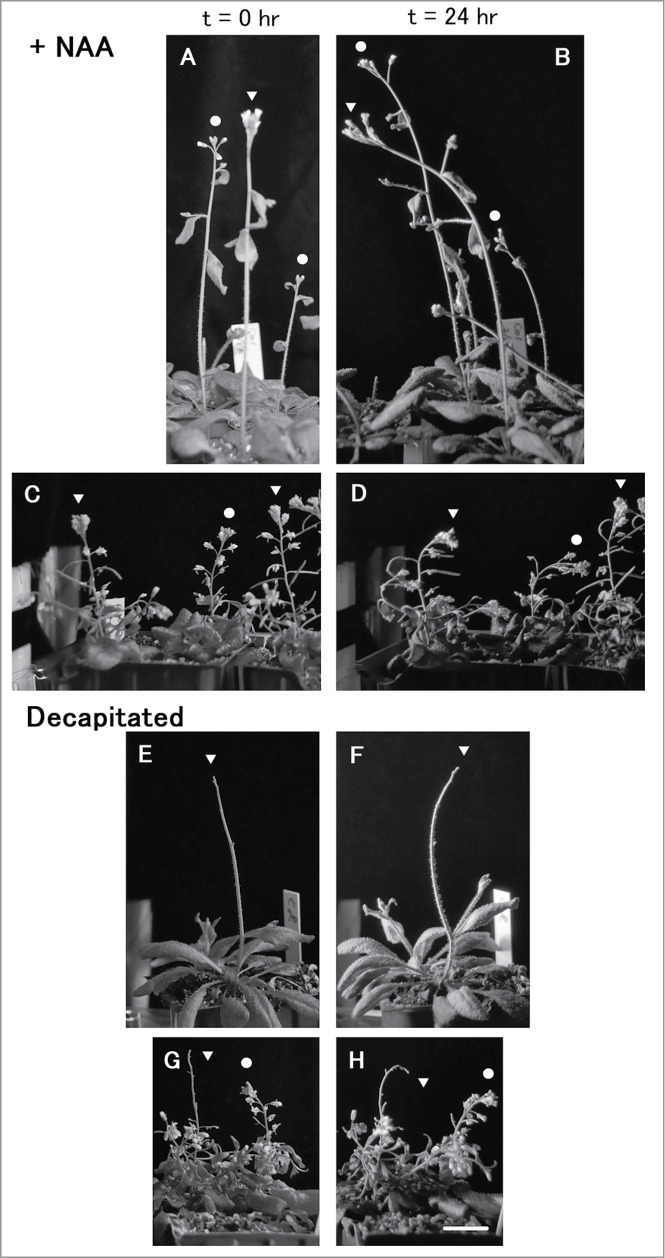
Phototropism of the wild-type (A, B, E and F) or axr2-1 (C, D, G and H) inflorescences affected by NAA application on the shoot apical region (A to D) or decapitation (E to H). Images were taken before (A, C, E and G) and after unilateral blue-light irradiation at 53 μmol s−1 m−2 for 24 hr from the left side (B, D, F and H). Stems without any treatments are indicated by circles, and NAA-treated or decapitated stems are indicated by triangles. Bar, 2 cm. Pictures are taken from the study of Sato et al.8
Figure 2.
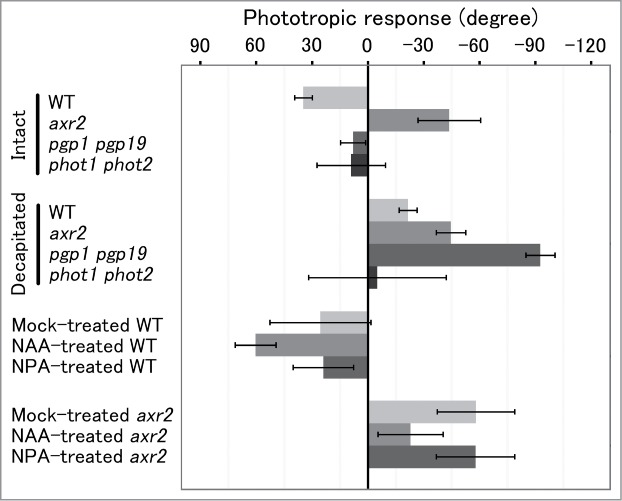
Phototropic responses of intact or decapitated inflorescences or inflorescences treated with NAA or NPA. Phototropic curvature was induced by unilateral blue-light irradiation at 53 μmol s−1 m−2 for 24 hr. Ninety degrees indicates the direction of the blue-light source, and 0 degrees indicates vertically upward. When inflorescences exhibited circumnutation, the value indicated is a mean of angles observed in one period. phot1 phot2 is a double mutant of phototropins. Data are reconstituted from those in Sato et al.8
We also examined gravitropism in axr2 inflorescences, and found that this was partially restored under white-light. In fact, wild-type stems also showed a faster gravitropic response in the light than in the dark. In spite of the agravitropic nature of axr2 stems, amyloplasts in the axr2 endodermis sedimented normally. Either application of NAA or NPA, or decapitation slowed down gravitropism in wild type and axr2 inflorescence stems; in some cases, it had no significant effect. These results suggest that levels of auxin or auxin signaling which are limiting for phototropic responses in stems are almost optimal for stem gravitropism.
To determine the significance (if any) of the endodermis in stem phototropism, we made transgenic Arabidopsis (pSCR:GFP-axr2) that expressed a GFP-axr2 fusion protein driven by the endodermis-specific SCARECROW promoter. Gravitropism in the inflorescences of the transgenic lines was compromised, as in axr2, but the phototropic response was normal. Furthermore, neither gravitropism nor phototropism in etiolated hypocotyls of the transgenic lines was affected. These results confirm a previous finding that the endodermis plays a critical role in stem gravitropism, but not in phototropism.7
Funding
This work was supported in part by a Grant-in-Aid from Ministry of Education, Culture, Sports, Science and Technology, Japan to K.T.Y. (19060008). S.S. was supported by Research Fellowship for Young Scientists of Japan Society for the Promotion of Science.
References
- 1. Wilson AK, Pickett FB, Turner JC, Estelle M. A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol Gen Genet 1990; 222:377-83; PMID:2148800; http://dx.doi.org/ 10.1007/BF00633843 [DOI] [PubMed] [Google Scholar]
- 2. Nagpal P, Walker LM, Young JC, Sonawala A, Timpte C, Estelle M, Reed JW. AXR2encodes a member of the Aux/IAA protein family. Plant Physiol 2000; 123:563-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li N. The dual- and opposing effect of ethylene on the negative gravitropism of Arabidopsis inflorescence stem and light-grown hypocotyls. Plant Sci 2008; 175:71-86. [Google Scholar]
- 4. Kagawa T, Kimura M, Wada M. Blue light-induced phototropism of inflorescence stems and petioles is mediated by phototropin family members phot1 and phot2. Plant Cell Physiol 2009; 50:1774-85; PMID:19689999; http://dx.doi.org/ 10.1093/pcp/pcp119 [DOI] [PubMed] [Google Scholar]
- 5. Noh B, Murphy AS, Spalding EP. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 2001; 13:2441-54; PMID:11701880; http://dx.doi.org/ 10.1105/tpc.13.11.2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iino M. Pulse-induced phototropism in oat and maize coleoptiles. Plant Physiol 1988; 88:823-28; PMID:16666391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fukaki H, Fujisawa H, Tasaka M. SGR1, SGR2, and SGR3: novel genetic loci involved in shoot gravitropism in Arabidopsis thaliana. Plant Physiol 1996; 110:945-55; PMID:8819871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sato A, Sasaki S, Matsuzaki J, Yamamoto KT. Light-dependent gravitropism and negative phototropism of inflorescence stems in a dominant Aux/IAA mutant of Arabidopsis thaliana, axr2. J Plant Res 2014; 127:627-39; PMID:24938853; http://dx.doi.org/0.1007/s10265-014-0643-1 [DOI] [PubMed] [Google Scholar]


