Abstract
The receptor tyrosine kinase HER2 is known to play a central role in mitogenic signaling, motivating the development of targeted, HER2-specific therapies. However, despite the longstanding use of antibodies to target HER2, controversies remain concerning antibody/HER2 trafficking behavior in cancer cells. Understanding this behavior has direct relevance to the mechanism of action and effective design of such antibodies. In the current study, we analyzed the intracellular dynamics of trastuzumab, a marketed HER2-targeting antibody, in a panel of breast and prostate cancer cell lines that have a wide range of HER2 expression levels. Our results reveal distinct post-endocytic trafficking behavior of antibody-HER2 complexes in cells with different HER2 expression levels. In particular, HER2-overexpressing cells exhibit efficient HER2 recycling and limited reductions in HER2 levels upon antibody treatment, and consequently display a high level of antibody persistence on their plasma membrane. By contrast, in cells with low HER2 expression, trastuzumab treatment results in rapid antibody clearance from the plasma membrane combined with substantial decreases in HER2 levels and undetectable levels of recycling. A cell line with intermediate levels of HER2 expression exhibits both antibody recycling and clearance from the cell surface. Significantly, these analyses demonstrate that HER2 expression levels, rather than cell origin (breast or prostate), is a determinant of subcellular trafficking properties. Such studies have relevance to optimizing the design of antibodies to target HER2.
Keywords: HER2 degradation, intracellular trafficking
Abbreviations
- ADCs
Antibody drug conjugates
- ADCC
antibody dependent cell-mediated cytotoxicity
- ADCP
antibody dependent cell-mediated phagocytosis
Introduction
In breast cancer, overexpression of the receptor tyrosine kinase (RTK) HER2 is observed in 20–30% of patients and is associated with poor prognosis.1 Monoclonal antibodies such as trastuzumab represent a promising treatment option as they have been shown to be beneficial in a subset of HER2hi breast cancer patients. However, despite considerable interest in the targeting of HER2 with antibodies, there is uncertainty concerning the intracellular trafficking itinerary of trastuzumab and its HER2 target. Understanding these pathways is of direct relevance to elucidating mechanistic aspects of antibody-based HER2-specific therapies.
While a subset of studies report that trastuzumab remains on the cell surface and does not internalize following interaction with HER2,2,3 others claim that trastuzumab internalizes4,5 and subsequently traffics back to the plasma membrane.4 A related unanswered question concerns antibody-induced HER2 degradation; conflicting reports indicate HER2 degradation6-9 or a lack thereof.2,4 To further confound these issues, how anti-HER2 antibodies behave in cells that express intermediate or low levels of HER2 (HER2int or HER2lo, respectively), and whether this differs from the behavior in HER2-overexpressing cells has not been investigated. This not only relates to the druggability of HER2, but might also yield insight into factors that contribute to differences in HER2 expression levels.
The discordant results concerning the intracellular fates of anti-HER2 antibodies have implications for their mechanism of action. For instance, antibody-induced HER2 endocytosis and lysosomal degradation is expected to extinguish HER2 signaling. In addition, for antibody-drug conjugates (ADCs), efficient delivery into the endolysosomal pathway is required.10 By contrast, antibody-HER2 internalization would be expected to negatively affect antibody dependent cell-mediated phagocytosis (ADCP) or antibody dependent cell-mediated cytotoxicity (ADCC), which require antibody persistence on the cell surface.
In addition to HER2-overexpressing cancers, there is interest in targeting HER2 in tumors that express intermediate or low levels of HER2, for which recent data support a role for the HER2 signaling axis in tumorigenesis.11-13 For example, studies have indicated that the heterodimerization of HER2 with HER3, which is one of the most potent activators of the PI3K/Akt pathway known, can play an important role in the pathogenesis of breast and prostate tumors with normal to low HER2 levels.11-13 This, combined with the variability in HER2 expression due to intratumor heterogeneity,14,15 motivates a comparative analysis of anti-HER2 antibody dynamics in cancer cells with a wide range of HER2 expression levels.
In the current study, we performed a quantitative characterization of antibody/HER2 trafficking dynamics in a panel of breast and prostate cancer cell lines. This has been combined with microscopy analyses to define the behavior of the anti-HER2 antibody trastuzumab and HER2 at the level of intracellular trafficking. Our results demonstrate that HER2 can internalize following antibody treatment in all cancer cell lines analyzed. Importantly, both trastuzumab recycling and decreased HER2 levels are observed in HER2hi or HER2int breast cancer cell lines. Unexpectedly, in HER2lo breast and prostate cancer cell lines, the percentage decrease in total HER2 levels is higher than in HER2hi/HER2int cells, with undetectable levels of recycling of internalized trastuzumab combined with efficient entry into degradative, lysosomal compartments. The dynamic behavior of antibody-HER2 complexes in the different cell lines is also consistent with the levels of trastuzumab present on the plasma membrane. In particular, a significant fraction of the antibody persists on the cell surface of HER2hi cells, and this fraction progressively diminishes with decreasing HER2 expression levels. Consequently, the HER2 expression level, rather than cell origin, is a predictor of trafficking behavior. Collectively, these analyses give novel insight into antibody-HER2 dynamics that could have implications for the design of therapeutics.
Results
Variable levels of HER2 following trastuzumab treatment
We first quantitated the levels of HER2 over the course of three days of trastuzumab treatment in a panel of cancer cell lines (Fig. 1). These cell lines have receptor expression levels that range from overexpression of HER2 (HER2hi) to intermediate to low levels of HER2 (HER2int or HER2lo) (Fig. 1A). A flow cytometry-based assay was used to measure the total HER2 levels in cells. Briefly, the cells were treated with trastuzumab for different times and total HER2 levels were quantitated by staining with fluorescently labeled pertuzumab followed by flow cytometry. Pertuzumab and trastuzumab are known to recognize distinct epitopes on domain II and domain IV of the HER2 extracellular domain, respectively.16,17 Confirmatory experiments verified that pertuzumab can indeed be used as a noncompeting, staining antibody to label HER2 in cells treated with trastuzumab (Figure S1).
Figure 1.
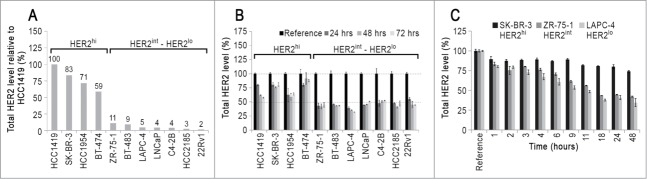
HER2 expression levels and effects of trastuzumab. (A) Relative levels of HER2 in the cell line panel used in this study. Cells were fixed, permeabilized and stained with fluorescently labeled trastuzumab and analyzed by flow cytometry. The fluorescence signal for each cell line is normalized with respect to HCC1419 which had the highest signal and is expressed as a percentage. (B) The effect of trastuzumab treatment on total HER2 levels. Cells were pulsed for the indicated times with 15 μg/ml trastuzumab, harvested and stained with Alexa 647-labeled pertuzumab to determine total HER2 levels by flow cytometry. For each cell line, the HER2 level in untreated cells is taken as reference, and HER2 levels are expressed as a percentage of reference. (C) Kinetics of trastuzumab-mediated reductions in HER2 levels in SK-BR-3, ZR-75–1 and LAPC-4 cells. The cells were treated with 15 μg/ml trastuzumab for the indicated times and at the end of each time point, cells were harvested and total HER2 levels determined as in panel. (B) Error bars indicate SD. The experiments are representative of at least two independent experiments.
Figure 1B shows the HER2 levels, expressed as a percentage of the steady-state (without antibody) levels, following 24–72 h of trastuzumab treatment in the different cell lines. Interestingly, a higher percentage of HER2 remains in the HER2hi cells relative to that in HER2int/lo cells. At 72 h, for example, the level of HER2 is reduced by 60–70% in the HERint/lo cells compared with 10–35% in the HER2hi cells. We verified that this observation is not due to antibody consumption by the HER2hi cells, since the reduction in HER2 levels is similar for a given cell line over a concentration range of 1–100 μg/ml trastuzumab (Figure S2).
We also investigated the reduction in HER2 levels over shorter time scales ranging from 1–48 h of trastuzumab treatment in a subset of cell lines, representing HER2hi (SK-BR-3), HERint (ZR-75–1) and HER2lo (LAPC-4). Figure 1C shows the decrease in HER2 levels within 1 h of trastuzumab treatment. Further, the HER2 levels continue to decrease until 18 h of trastuzumab treatment in ZR-75–1 and LAPC-4 cell lines. Analyses of the kinetics of the reductions in cell surface HER2 and intracellular HER2 levels showed that at all times post-trastuzumab treatment, cell surface HER2 levels decreased at a higher rate than intracellular HER2 levels (Figure S3). This suggests that HER2 internalization precedes degradation.
Trastuzumab recycling correlates with HER2 expression levels
We next investigated whether internalized trastuzumab is recycled back to the plasma membrane in cells with different HER2 expression levels. We found that trastuzumab recycled in all of the HER2hi (SK-BR-3, BT-474, HCC1419 and HCC1954) and HER2int (BT-483 and ZR-75–1) cell lines in our panel (Fig. 2A). However, the HER2lo cell lines LAPC-4, C4–2B and HCC2185, which have 20-, 25-, 33-fold lower levels of HER2 expression, respectively, than HER2hi HCC1419 cells showed distinct behavior. Specifically, we observed that trastuzumab was not recycled at detectable levels in HER2lo cells (Fig. 2B). Interestingly, the HER2int cell line BT-483 exhibits trastuzumab recycling behavior, but with a lower rate than that in HER2hi cells. For comparative purposes, the recycling behavior of transferrin, a prototypical recycling marker, was similar in HER2hi, HER2int and HER2lo cell lines (Figure S4).
Figure 2.
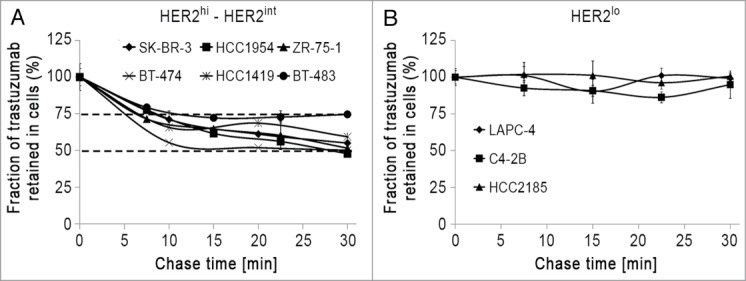
Trastuzumab is differentially recycled in HER2 expressing cell lines. Trastuzumab recycling in HER2hi or HER2int (A) and HER2lo (B) cells are shown. Cells were pulsed with Alexa 488-labeled trastuzumab for 30 min at 37°C. The cells were cooled and incubated with 50 μg/ml anti-Alexa 488 antibody for 30 min to quench the (cell surface) Alexa 488 signal. Cells were chased for the indicated times at 37°C in medium containing 25 μg/ml anti-Alexa 488 antibody. At the end of each time point, the cells were harvested and analyzed by flow cytometry. The fraction retained in cells is calculated by normalizing the fluorescence signal at each time point with the 0 min chase time point signal. Error bars indicate SD. The experiments are representative of at least two independent experiments.
Trastuzumab recycling leads to increased persistence on the cell surface
The differences in trastuzumab recycling behavior in HER2hi and HER2int/lo cells raised the question as to how this affects surface levels of trastuzumab-HER2 complexes, prompting an analysis of trastuzumab persistence on the plasma membrane of cells with different HER2 expression levels (Fig. 3). In all of the cells, trastuzumab levels at the cell surface decrease by ∼15% within the first 60 min. However, the behavior at later time points is very different: in the HER2hi SK-BR-3 cell line, a significant fraction of trastuzumab (∼75%) remains at the cell surface after 18 h, whereas this fraction is only around 10% in the HER2lo LAPC-4 cell line. Further, in the HER2int ZR-75–1 cell line, which has 2-fold higher HER2 levels than LAPC-4 cells and both recycles and retains trastuzumab within cells, ∼40% of trastuzumab remains on the cell surface after 18 h.
Figure 3.
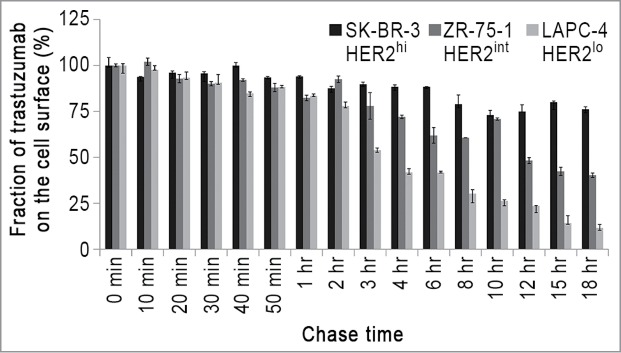
Trastuzumab shows differences in persistence on the cell surface of HER2-expressing cells. Cells were pulsed with 15 μg/ml trastuzumab for 10 min and chased for the indicated times, then harvested, fixed, stained with Alexa 647-labeled anti-human IgG conjugate and analyzed using flow cytometry. The trastuzumab levels are normalized with respect to the 0 min chase time point. Error bars indicate SD. The experiments are representative of at least two independent experiments.
Importantly, the observed reduction in trastuzumab levels across the different cell lines cannot be accounted for by dissociation of the antibody from HER2 on the cell surface. Specifically, the amount of trastuzumab present in the chase medium collected at the end of each time point is ≤5% of the initial amount bound to the cells (data not shown).
Trastuzumab trafficks differently in SK-BR-3 and LAPC-4 cells
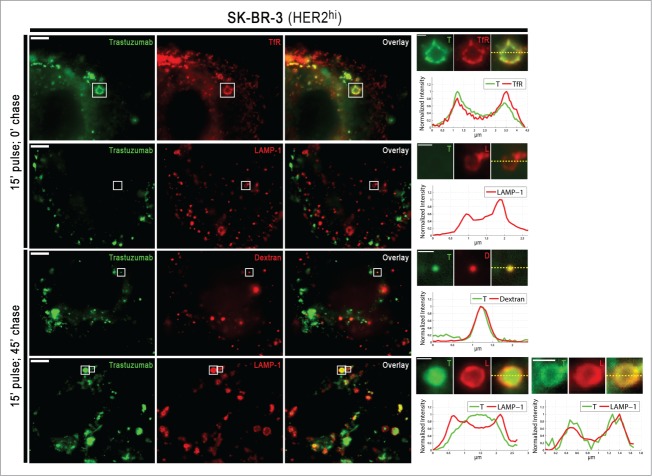
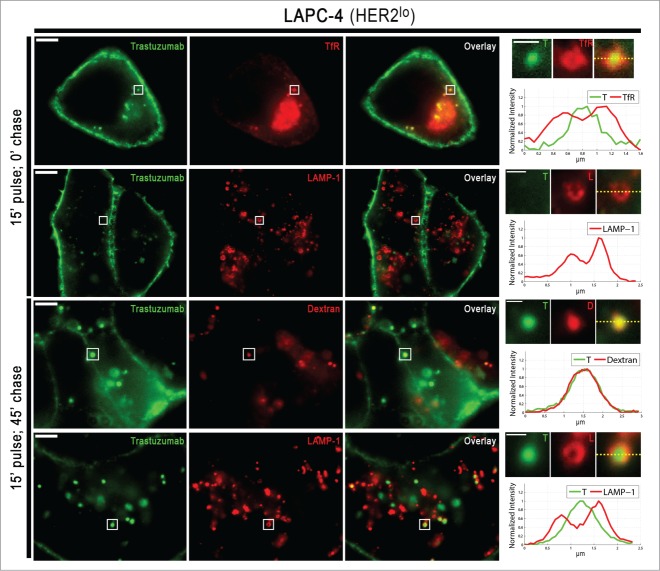
The marked differences in the trastuzumab recycling behavior between HER2hi and HER2lo cells prompted us to investigate the intracellular distribution of trastuzumab in these cells. Cells were pulsed with trastuzumab for 15 min and stained with markers for early (transferrin receptor) and late endosomes/lysosomes (LAMP-1) following no chase or a 45 min chase period. In addition, cells were pulse-chased with the fluid phase marker, dextran, to label the luminal compartments of lysosomes. The relatively high HER2 expression in the HER2hi cells resulted in robust plasma membrane staining for trastuzumab, which obscures visualization of the internalized antibody in intracellular compartments (Figure S5). To overcome this, Alexa 488-labeled trastuzumab was used, enabling selective quenching of surface levels using an anti-Alexa 488 antibody.4,18
Figures 4 and 5 show the intracellular distribution of internalized trastuzumab in SK-BR-3 and LAPC-4 cells. Control experiments with Alexa 488-labeled human anti-HEL IgG1 (isotype control) confirmed that trastuzumab internalization is HER2-mediated and not due to non-specific fluid-phase uptake (Figure S6). Following 15 min of treatment with trastuzumab, the antibody can be detected in the lumena of transferrin receptor-positive endosomes in LAPC-4 cells, whereas in SK-BR-3 cells it is localized on the limiting membranes of these compartments (Figs. 4,5). The difference in endosomal localization of trastuzumab in these cell lines is congruent with the recycling behavior of this antibody in the two cell types. Specifically, luminal delivery of trastuzumab following internalization into early endosomes in LAPC-4 cells is expected to result in trafficking into the late endosomal/lysosomal pathway rather than entry into recycling transport carriers that segregate from endosomes.19,20 Importantly, control experiments indicated that the difference in behavior across cell lines is not due to the presence of the quenching antibody (Figure S7).
Figure 4.
Subcellular trafficking behavior of trastuzumab in SK-BR-3 cells. Cells were pulsed with Alexa 488-labeled trastuzumab for 15 min, washed and chased for either 0 or 45 min. At the end of each time point, the cells were cooled and incubated with 50 μg/ml anti-Alexa 488 antibody to quench the (cell surface) Alexa 488 signal. The cells were fixed, permeabilized and stained for transferrin receptor (TfR) and LAMP-1. Boxed regions for each set of images are presented on the right hand side of each row as expanded images of individual endosomes/lysosomes. These individual endosomes/lysosomes are shown for trastuzumab (T), TfR, LAMP-1 (L) or dextran (D). Normalized fluorescence intensities along the yellow dotted lines in the overlays are presented in the fluorescence intensity plots. In all panels, trastuzumab is pseudocolored green and endosomal staining (TfR, LAMP-1 or Dextran) is pseudocolored red. Scale bars represent 5 μm (large panels) or 1 μm (expanded regions of interest).
Figure 5.
Subcellular trafficking behavior of trastuzumab in LAPC-4 cells. LAPC-4 cells were treated in the same way as Figure 4, except that quenching antibody was not used. Boxed regions for each set of images are presented on the right hand side of each row as expanded images of individual endosomes/lysosomes. These individual endosomes/lysosomes are shown for trastuzumab (T), TfR, LAMP-1 (L) or dextran (D). Normalized fluorescence intensities along the yellow dotted lines in the overlays are presented in the fluorescence intensity plots. In all panels, trastuzumab is pseudocolored green and endosomal staining (TfR, LAMP-1 or Dextran) is pseudocolored red. Scale bars represent 5 μm (large panels) or 1 μm (expanded regions of interest).
Consistent with the undetectable levels of recycling of internalized trastuzumab in LAPC-4 cells, following 15 min of antibody treatment and a 45 min chase period, trastuzumab is present in the lumena of LAMP-1+ late endosomes/lysosomes (Fig. 5). In SK-BR-3 cells, trastuzumab that is retained in cells following an analogous pulse-chase can be detected on the limiting membranes and lumena of LAMP-1+ endosomes (Fig. 4). In earlier studies,21 we have demonstrated that recycling receptors can segregate from LAMP-1+ late endosomes in cells, suggesting that the membrane associated trastuzumab in SK-BR-3 cells may constitute part of the recycling pool. In both cell types, luminal trastuzumab colocalized with fluorescently labeled dextran, which under the conditions of the experiments, was used as a marker for the lysosomal lumen (Figs. 4,5).
Discussion
The overexpression of HER2 in malignancies frequently results in an aggressive disease course. This has motivated the development of both single agent and combination therapies directed toward targeting HER2. Further, recent studies have indicated that the HER2 signaling axis can play a role in tumorigenesis in HER2int or HER2lo cells.11-13 However, despite HER2 being a commonly targeted marker by antibody therapeutics in cancer, the intracellular dynamics of antibody-HER2 complexes is not well defined. In particular, controversy concerning antibody/HER2 behavior in HER2hi cells persists,2-5 and there is a paucity of data for HER2int/lo cells. Here, we have systematically analyzed trastuzumab-HER2 trafficking and dynamics in a panel of cell lines with widely varying HER2 expression levels.
In the current study, we demonstrate that trastuzumab-HER2 complexes internalize in all cell lines analyzed, which vary over 50-fold in their expression levels of this receptor. However, following internalization the behavior of trastuzumab-HER2 complexes differs and is dependent on HER2 expression levels. Specifically, in HER2hi cells trastuzumab is efficiently recycled. Nevertheless, a small fraction of antibody-HER2 complexes enters the lysosomal pathway. By contrast, in HER2lo cells, trastuzumab recycling cannot be detected under the conditions of the assay and internalized antibody-HER2 complexes enter the lysosomal pathway. Trastuzumab-HER2 complexes in HER2int cells demonstrate properties that are between those of HER2hi and HER2lo cells, with both endocytic recycling and an intermediate loss of trastuzumab-HER2 complexes from the cell surface occurring.
Our observations concerning trastuzumab-HER2 dynamics, combined with the microscopy analyses, have led us to propose the following intracellular trafficking model for trastuzumab-HER2 complexes (Fig. 6). In HER2hi cells, internalized trastuzumab trafficks to early endosomes (transferrin receptor-positive) and remains on the limiting membrane, whereas in HER2lo cells the antibody is delivered into the lumen of these compartments within 15 min following uptake. By analogy with earlier studies of the trafficking of the recycling receptor, FcRn,19,22 trastuzumab in HER2hi cells is therefore expected to segregate from these endosomes via tubulovesicular transport carriers into the endocytic recycling pathway. The lumenal localization of trastuzumab-HER2 complexes in HER2lo cells, which could occur by either multivesicular body formation23 or HER2 cleavage, results in lysosomal delivery. The molecular mechanisms that lead to these differences in behavior across the different cell lines are not understood and require further analyses. In addition, we also observe that following one hour, trastuzumab can be detected on the limiting membrane of LAMP-1+ endosomes in SK-BR-3 cells. In earlier studies, we have shown that FcRn can localize to the limiting membrane and recycle from LAMP-1 positive late endosomes,21 suggesting that trastuzumab-HER2 complexes may traffic in an analogous manner in these HER2hi cells.
Figure 6.
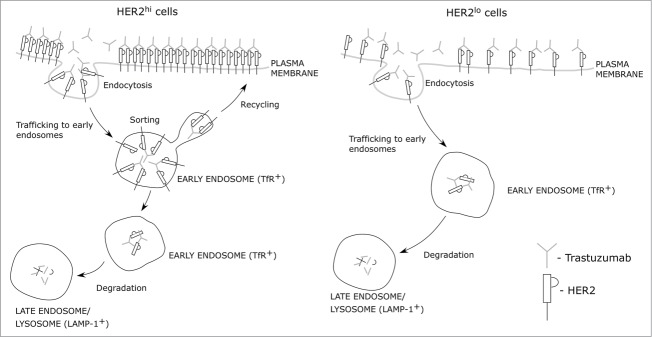
Model for antibody-HER2 trafficking. The schematic shows the intracellular trafficking behavior of antibody-HER2 complexes in cells with different HER2 expression levels.
Importantly, differences in trastuzumab-HER2 trafficking between HER2hi and HER2lo cells are not due to variations in cancer cell type, since HER2lo cells of both prostate and breast origin show similar characteristics. Nevertheless, efficient HER2 recycling and consequently alterations in the ratio of recycling/degradation could contribute to the maintenance of high surface HER2 levels in HER2hi cells. This could have fundamental relevance to understanding how cells convert to the HER2-addicted, tumorigenic state. An alternative possibility, that is not mutually exclusive, is that HER2 overexpression is initiated by other factors, and the increased numbers of internalized HER2 molecules in HER2-overexpressing cells relative to HER2int or HER2lo cells could directly affect the intracellular trafficking behavior of this potent signaling receptor. Further, although our observations demonstrate differential trafficking of trastuzumab within cells with different levels of HER2 expression, they do not exclude an additional contribution of variations in HER2 biosynthetic rates to the total HER2 levels following trastuzumab exposure.
The internalization resistance of trastuzumab that has been described previously in HER2hi cells using fluorescence microscopy2,3 can be explained by our observations of fast endocytic recycling of antibody-HER2 complexes, which when combined with very high levels of HER2 expression on the plasma membrane, obscure the visualization of internalized antibody-receptor complexes in cells. Specifically, to visualize internalized HER2 in these cells, we have found it necessary to quench the signal from fluorescently labeled HER2 on the plasma membrane. More generally, our observations concerning the extent of reduction in HER2 expression levels across different cell lines reflect the divergent reports5,8 concerning antibody-mediated effects on HER2 in which cell lines with widely varying HER2 expression levels were used.
Prior results concerning the lack of HER2 internalization of trastuzumab in HER2hi SK-BR-3 cells2,4,8 appear discrepant given the reported efficacy of trastuzumab as an ADC (trastuzumab emtansine, Kadcyla®), which requires the antibody to traffick into the endolysosomal pathway. Although a lower percentage of trastuzumab is internalized in HER2hi cells (SK-BR-3) relative to HER2lo cells (LAPC-4), the absolute numbers of internalized receptors would be much higher in SK-BR-3 cells due to a 16-fold difference in HER2 levels. These observations indicate that ADCs can be used to target receptors that inefficiently enter the endolysosomal pathway if the receptor level is sufficiently high. As a corollary, lower level receptors could be druggable with ADCs if their internalization/lysosomal delivery is high. Collectively, these studies indicate the need for subcellular trafficking studies of receptors to inform the design of ADCs.
Although directing antibody into the endolysosomal pathway is desirable for the effective delivery of ADCs, the relatively fast clearance of trastuzumab from the plasma membrane of HER2lo cells when compared with HER2hi cells would be expected to have a negative effect on antibody effector function. For instance, in prostate cancer HER2 overexpression is rarely observed, and these tumors typically exhibit low surface HER2 levels.24 Thus, antibody-mediated clearance mechanisms such as phagocytosis and ADCC may be significantly abrogated in such tumors. This may also contribute to the disappointing results observed in clinical trials with anti-HER2 antibody treatments in prostate cancer.25,26
In conclusion, our observations provide a quantitative characterization of anti-HER2 antibody dynamics and trafficking in HER2-expressing cancer cells. Significantly, the analyses reveal fundamental differences in subcellular trafficking behavior that are dependent on the expression levels of HER2. In turn, the findings in the current study have relevance to the design and optimization of HER2-directed therapeutics.
Materials and Methods
Antibodies, reagents and cell lines
Trastuzumab (Roche) and pertuzumab (Roche) were obtained from the UT Southwestern Pharmacy. Antibodies were labeled with Alexa Fluor dyes (Life Technologies) using standard protocols. Human IgG1 (isotype control) was purified from the supernatant of a NS0 stable cell line expressing human anti-hen-egg-lysosome (HEL) antibody.27 The following antibodies were used for immunofluorescence staining: rabbit anti-LAMP-1 antibody (Abcam; ab24170), anti-mouse-EEA-1 antibody (BD Biosciences #610456), anti-transferrin receptor antibody (Invitrogen; #13–6800) and mouse anti-LAMP-1 antibody (DSHB – Univ of Iowa; H4A3). All secondary antibody conjugates were purchased from Life Technologies. The cell lines SK-BR-3, BT-474, 22Rv1, LNCaP and ZR-75–1 were obtained from the ATCC and maintained as recommended in ATCC protocols. The cell lines HCC1419, HCC1954, HCC2185 and BT-483 were generously provided by Drs. Adi Gazdar, John Minna and Kenneth Huffman (University of Texas Southwestern Medical Center at Dallas, Dallas, TX) and maintained in RPMI supplemented with 5% fetal bovine serum. The cell lines C4–2B and LAPC-4 were provided by Dr. N. Sharifi (Lerner Research Institute, Cleveland, OH) and maintained in RPMI and modified IMEM, respectively, supplemented with 10% fetal bovine serum and antibiotics.
Measurement of cell surface, intracellular and total HER2 levels
Cells treated with 15 μg/ml trastuzumab were harvested by trypsinization and fixed with 2% PFA for 20 min at 4°C. To detect cell surface HER2 levels, the cells were stained with 10 μg/ml fluorescently labeled pertuzumab for 30 min at 4°C. To detect total HER2 levels, harvested cells (from separate wells) were fixed as described above, permeabilized with 0.1% saponin for 20 min at 4°C and stained with fluorescently labeled pertuzumab for 30 min at 4°C. The cells were analyzed using flow cytometry (FACSCaliber, Becton Dickinson) to measure the fluorescence signal corresponding to either cell surface or total HER2 levels. Intracellular HER2 levels were determined by calculating the difference between the signal corresponding to total HER2 levels and that of cell surface HER2 levels. For each cell line, all data points are normalized with respect to the signal in untreated cells (labeled as reference) and are expressed as a percentage.
Measurement of trastuzumab levels on the cell surface
Cells were pulsed with 15 μg/ml trastuzumab for 10 min at 37°C, washed three times and chased in medium for different times at 37°C. The cells were harvested, fixed as described above and then stained with 5 μg/ml Alexa 647-labeled anti-human IgG for 30 min at 4°C. The cells were analyzed using flow cytometry (FACSCalibur, Becton Dickinson) to measure the fluorescence signal corresponding to cell surface trastuzumab levels. The fluorescence signal for all of the time points was normalized with respect to the 0 min chase time point and expressed as a percentage.
Trastuzumab and transferrin recycling assay
The recycling experiments were based on fluorescence quenching of Alexa 488 using an anti-Alexa 488 polyclonal antibody (Life Technologies; A11094) and were performed as described previously.4,18 Briefly, cells were pulsed with 2 or 10 μg/ml Alexa 488-labeled trastuzumab (degree of labeling = 1) for 30 min at 37°C to allow receptor-mediated endocytosis of the antibodies. The cells were placed on ice, washed three times with ice cold HBSS buffer and incubated on ice for 10 min in HBSS buffer. The cells were then pulsed with 50 μg/ml anti-Alexa 488 antibody for 25 min with agitation to quench the Alexa 488 fluorescence signal on the cell surface. The cells were washed twice with room temperature PBS and chased for different times at 37°C in pre-warmed medium containing 25 μg/ml anti-Alexa 488 antibody to quench the Alexa 488 signal when the antibody recycles from the cell interior back to the plasma membrane. To assess transferrin recycling, cells (in separate wells) were pulsed with 5 μg/ml of Alexa 647-labeled transferrin and subjected to the same treatment as for trastuzumab-treated cells except that anti-Alexa 488 antibody was not used and also cells were chased in medium with or without 0.25 mg/ml unlabeled transferrin. At the end of each chase phase, the cells were harvested by trypsinization, pelleted by centrifugation, and immediately analyzed using flow cytometry (FACSCalibur, Becton Dickinson) to determine the amount of trastuzumab or transferrin present inside the cells. The fraction of recycled trastuzumab or transferrin was calculated by normalizing the fluorescence signal at each time point with the signal pertaining to the 0 min chase time point and is expressed as a percentage.
Immunofluorescence microscopy
The cells were seeded in glass-bottomed dishes (Mattek Corporation) pre-coated with poly-l-lysine and grown in phenol-red free medium for two days. The cells were pulsed for 15 min with either 5 μg/ml Alexa 488-labeled trastuzumab (SK-BR-3 or LAPC-4 cells) or 10 μg/ml Alexa 555-labeled trastuzumab (LAPC-4 cells) at 37°C and chased for the indicated times. To assess fluid phase uptake, the cells (in separate dishes) were pulsed for 15 min with 5 μg/ml Alexa 488-labeled human anti-HEL IgG (SK-BR-3) or 10 μg/ml Alexa 555-labeled human anti-HEL-IgG (LAPC-4) at 37°C. To quench the signal from the cell surface bound Alexa 488-labeled trastuzumab, cells were washed twice with ice-cold HBSS, incubated on ice for 10 min in ice-cold HBSS and incubated for 30 min on ice in medium containing 50 μg/ml anti-Alexa 488 antibody. Cells were then washed with PBS, fixed with 1.7% paraformaldehyde for 10 min at 37°C, permeabilized with 0.025% saponin for 10 min at room temperature, then stained and counter-stained with primary and secondary antibodies, respectively, each for 25 min at 37°C in 1% BSA in PBS. Cells were stored in 1% BSA in PBS at 37°C and imaged within two days.
Cells were imaged using a Zeiss Axiovert 200M microscope fitted with a Zeiss 100× 1.4NA planapochromat objective and an ORCA CCD camera (Hamamatsu). All image processing was performed in the MIATool software package.28 Image intensities were linearly adjusted for each channel and the individual channels were overlaid to create the color images. The region showing the cell of interest was cropped and exported in uncompressed tiff format. To analyze the location of trastuzumab in endosomes or lysosomes, fluorescence intensity plots were generated by subtracting the background fluorescence intensity from the signal in regions of interest along the displayed dotted yellow lines. The color images were then assembled in Canvas software (ACD Systems) and saved in tiff file format.
Acknowledgments
We thank Drs. Adi Gazdar, John Minna and Kenneth Huffman (Hamon Center for Therapeutic Oncology Research, University of Texas Southwestern Medical Center at Dallas, Dallas, TX) for generously providing BT-483 and the HCC cell lines. We also thank Dr. Nima Sharifi (Lerner Research Institute, Cleveland, OH) for generously providing C4–2B and LAPC-4 cell lines, and Sungyong You for help with microscopy image acquisition. This research was supported in part by a grant from the Cancer Prevention and Research Institute of Texas (grant RP110069).
Author Contributions
SR, RJO and ESW designed the project, SR and DK performed the experiments and analyzed the data, and all authors wrote the manuscript.
Supplementary Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235:177-82; PMID:3798106; http://dx.doi.org/ 10.1126/science.3798106 [DOI] [PubMed] [Google Scholar]
- 2. Longva KE, Pedersen NM, Haslekås C, Stang E, Madshus IH. Herceptin-induced inhibition of ErbB2 signaling involves reduced phosphorylation of Akt but not endocytic down-regulation of ErbB2. Int J Cancer 2005; 116:359-67; PMID:15800944; http://dx.doi.org/ 10.1002/ijc.21015 [DOI] [PubMed] [Google Scholar]
- 3. Hommelgaard AM, Lerdrup M, van Deurs B. Association with membrane protrusions makes ErbB2 an internalization-resistant receptor. Mol Biol Cell 2004; 15:1557-67; PMID:14742716; http://dx.doi.org/ 10.1091/mbc.E03-08-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Austin CD, De Mazière AM, Pisacane PI, van Dijk SM, Eigenbrot C, Sliwkowski MX, Klumperman J, Scheller RH. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell 2004; 15:5268-82; PMID:15385631; http://dx.doi.org/ 10.1091/mbc.E04-07-0591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rudnick SI, Lou J, Shaller CC, Tang Y, Klein-Szanto AJ, Weiner LM, Marks JD, Adams GP. Influence of affinity and antigen internalization on the uptake and penetration of Anti-HER2 antibodies in solid tumors. Cancer Res 2011; 71:2250-9; PMID:21406401; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cuello M, Ettenberg SA, Clark AS, Keane MM, Posner RH, Nau MM, Dennis PA, Lipkowitz S. Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Cancer Res 2001; 61:4892-900; PMID:11406568 [PubMed] [Google Scholar]
- 7. Henson ES, Hu X, Gibson SB. Herceptin sensitizes ErbB2-overexpressing cells to apoptosis by reducing antiapoptotic Mcl-1 expression. Clin Cancer Res 2006; 12:845-53; PMID:16467098; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-0754 [DOI] [PubMed] [Google Scholar]
- 8. Scaltriti M, Verma C, Guzman M, Jimenez J, Parra JL,Pedersen K, Smith DJ, Landolfi S, Ramon y, Cajal S, Arribas J, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene 2009; 28:803-14; PMID:19060928; http://dx.doi.org/ 10.1038/onc.2008.432 [DOI] [PubMed] [Google Scholar]
- 9. Tseng PH, Wang YC, Weng SC, Weng JR, Chen CS, Brueggemeier RW, Shapiro CL, Chen CY, Dunn SE, Pollak M, et al. Overcoming trastuzumab resistance in HER2-overexpressing breast cancer cells by using a novel celecoxib-derived phosphoinositide-dependent kinase-1 inhibitor. Mol Pharmacol 2006; 70:1534-41; PMID:16887935; http://dx.doi.org/ 10.1124/mol.106.023911 [DOI] [PubMed] [Google Scholar]
- 10. Sievers EL, Senter PD. Antibody-drug conjugates in cancer therapy. Annu Rev Med 2013; 64:15-29; PMID:23043493; http://dx.doi.org/ 10.1146/annurev-med-050311-201823 [DOI] [PubMed] [Google Scholar]
- 11. Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2002; 2:127-37; PMID:12204533; http://dx.doi.org/ 10.1016/S1535-6108(02)00097-1 [DOI] [PubMed] [Google Scholar]
- 12. Agus DB, Scher HI, Higgins B, Fox WD, Heller G, Fazzari M, Cordon-Cardo C, Golde DW. Response of prostate cancer to anti-Her-2/neu antibody in androgen-dependent and -independent human xenograft models. Cancer Res 1999; 59:4761-4; PMID:10519379 [PubMed] [Google Scholar]
- 13. Arteaga CL. Can trastuzumab be effective against tumors with low HER2/Neu (ErbB2) receptors? J Clin Oncol 2006; 24:3722-5; PMID:16847283; http://dx.doi.org/ 10.1200/JCO.2006.06.5268 [DOI] [PubMed] [Google Scholar]
- 14. Potts SJ, Krueger JS, Landis ND, Eberhard DA, Young GD, Schmechel SC, Lange H. Evaluating tumor heterogeneity in immunohistochemistry-stained breast cancer tissue. Lab Invest 2012; 92:1342-57; PMID:22801299; http://dx.doi.org/ 10.1038/labinvest.2012.91 [DOI] [PubMed] [Google Scholar]
- 15. Seol H, Lee HJ, Choi Y, Lee HE, Kim YJ, Kim JH, Kang E, Kim SW, Park SY. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: its clinicopathological significance. Mod Pathol 2012; 25:938-48; PMID:22388760; http://dx.doi.org/ 10.1038/modpathol.2012.36 [DOI] [PubMed] [Google Scholar]
- 16. Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr., Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 2003; 421:756-60; PMID:12610629; http://dx.doi.org/ 10.1038/nature01392 [DOI] [PubMed] [Google Scholar]
- 17. Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 2004; 5:317-28; PMID:15093539; http://dx.doi.org/ 10.1016/S1535-6108(04)00083-2 [DOI] [PubMed] [Google Scholar]
- 18. Spangler JB, Neil JR, Abramovitch S, Yarden Y, White FM, Lauffenburger DA, Wittrup KD. Combination antibody treatment down-regulates epidermal growth factor receptor by inhibiting endosomal recycling. Proc Natl Acad Sci U S A 2010; 107:13252-7; PMID:20616078; http://dx.doi.org/ 10.1073/pnas.0913476107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ober RJ, Martinez C, Vaccaro C, Zhou J, Ward ES. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J Immunol 2004; 172:2021-9; PMID:14764666; http://dx.doi.org/ 10.4049/jimmunol.172.4.2021 [DOI] [PubMed] [Google Scholar]
- 20. Ward ES, Martinez C, Vaccaro C, Zhou J, Tang Q, Ober RJ. From sorting endosomes to exocytosis: association of Rab4 and Rab11 GTPases with the Fc receptor, FcRn, during recycling. Mol Biol Cell 2005; 16:2028-38; PMID:15689494; http://dx.doi.org/ 10.1091/mbc.E04-08-0735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gan Z, Ram S, Vaccaro C, Ober RJ, Ward ES. Analyses of the recycling receptor, FcRn, in live cells reveal novel pathways for lysosomal delivery. Traffic 2009; 10:600-14; PMID:19192244; http://dx.doi.org/ 10.1111/j.1600-0854.2009.00887.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prabhat P, Gan Z, Chao J, Ram S, Vaccaro C, Gibbons S, Ober RJ, Ward ES. Elucidation of intracellular recycling pathways leading to exocytosis of the Fc receptor, FcRn, by using multifocal plane microscopy. Proc Natl Acad Sci U S A 2007; 104:5889-94; PMID:17384151; http://dx.doi.org/ 10.1073/pnas.0700337104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol 2007; 23:519-47; PMID:17506697; http://dx.doi.org/ 10.1146/annurev.cellbio.23.090506.123319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Minner S, Jessen B, Stiedenroth L, Burandt E, Köllermann J, Mirlacher M, Erbersdobler A, Eichelberg C, Fisch M, Brümmendorf TH, et al. Low level HER2 overexpression is associated with rapid tumor cell proliferation and poor prognosis in prostate cancer. Clin Cancer Res 2010; 16:1553-60; PMID:20179235; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-2546 [DOI] [PubMed] [Google Scholar]
- 25. de Bono JS, Bellmunt J, Attard G, Droz JP, Miller K, Flechon A, Sternberg C, Parker C, Zugmaier G, Hersberger-Gimenez V, et al. Open-label phase II study evaluating the efficacy and safety of two doses of pertuzumab in castrate chemotherapy-naive patients with hormone-refractory prostate cancer. J Clin Oncol 2007; 25:257-62; PMID:17235043; http://dx.doi.org/ 10.1200/JCO.2006.07.0888 [DOI] [PubMed] [Google Scholar]
- 26. Ziada A, Barqawi A, Glode LM, Varella-Garcia M, Crighton F, Majeski S, Rosenblum M, Kane M, Chen L, Crawford ED. The use of trastuzumab in the treatment of hormone refractory prostate cancer; phase II trial. Prostate 2004; 60:332-7; PMID:15264245; http://dx.doi.org/ 10.1002/pros.20065 [DOI] [PubMed] [Google Scholar]
- 27. Foote J, Winter G. Antibody framework residues affecting the conformation of the hypervariable loops. J Mol Biol 1992; 224:487-99; PMID:1560463; http://dx.doi.org/ 10.1016/0022-2836(92)91010-M [DOI] [PubMed] [Google Scholar]
- 28. Chao J, Ward ES, Ober RJ. A software framework for the analysis of complex microscopy image data. IEEE Trans Inf Technol Biomed 2010; 14:1075-87; PMID:20423810; http://dx.doi.org/ 10.1109/TITB.2010.2049024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.