Abstract
Fast-growing hairy root cultures of Picrorhiza kurroa induced by Agrobacterium rhizogenes offers a potential production system for iridoid glycosides. In present study we have investigated the effects of various nutrient medium formulations viz B5, MS, WP and NN, and sucrose concentrations (1–8%) on the biomass and glycoside production of selected clone (14-P) of P. kurroa hairy root. Full strength B5 medium was found to be most suitable for maximum biomass yield on the 40th day of culture (GI = 32.72 ± 0.44) followed by the NN medium of the same strength (GI = 22.9 ± 0.43). Secondary metabolite production was 1.1 and 1.3 times higher in half strength B5 medium respectively in comparison to MS medium. Maximum biomass accumulation along with the maximum picroliv content was achieved with 4% sucrose concentration in basal medium. RT vitamin and Thiamine-HCl effected the growth and secondary metabolite production of hairy roots growing on MS medium but did not show any effect on other media. The pH of the medium played significant role in growth and secondary metabolite production and was found to be highest at pH 6.0 while lowest at pH 3.0 and pH 8.0. To enhance the production of biomass and Picroliv 5 liter working capacity bioreactor was used, 27-fold (324 g FW) higher growth was observed in bioreactor than shake flask and secondary metabolite production was similarly enhanced.
Keywords: agrobacterium rhizogenes, glycosides, hairy roots, kutkoside, picroside I, picrorhiza kurroa
Introduction
Picrorhiza kurroa Royle ex Benth, Scrophularaceous- a medicinal plant commonly known as Kutki or Kadu is endemic to the alpine Himalayan range. This plant has already been declared as an endangered species due to its indiscriminate over exploitation.1 It is the representative source of herbal compound, called Kutkin that has significant anti inflammatory, hepato-protective, anticholestatic, antiulcerogenic, antiasthematic, antidiabitic and immuno-regulatory properties.1-3 Kutkin, is a mixture of 2 C9- iridoid glycosides, i.e. picroside I (6-O-trans Cinnamoylcatalpol) and kutkoside (10-O-Vaniloylcatalpol),4 it is usually extracted from the dried underground parts of 3–4 y old P. kurroa plants.3 The medicinally important components of P. kurroa are picroside I, picroside II, picroside III, picroside IV, apocynin, androsin, catechol, and kutkoside.3,5
Agrobacterium rhizogenes induced hairy root cultures have attracted much attention in recent years as advantageous resource of useful plant based compounds.6 Hairy root culture has revolutionized the role of in vitro plant organ culture for the secondary metabolite production.7 A major characteristic of hairy root is their general ability to produce the desired phytochemicals concomitantly with growth. This is relevant because, unlike the production being repressed during the growth phase of dedifferentiated cell culture, it is possible to get constant and standardized production of the desired phytochemicals from growing hairy roots.8
The biosynthetic potential of the transformed roots is genetically controlled but it has frequently been observed that such transformed roots are sensitive to medium composition with respect to both biomass yield and secondary metabolite productivity.5,9,10 Several groups have shown that besides selection of superior hairy root clones with higher than average productivity, media optimization could enhance the biomass as well as secondary metabolite yield.5,10
We previously reported A. rhizogenes mediated transformation of this medicinally imperative plant species for the production of the desired phytochemicals (i.e., Picropside I and Kutkoside).5
In present study we optimized the different parameters to enhance productivity of picroliv from hairy roots of P. kurroa. We investigated the effect of different media composition at full and half strengths of salts with or without supplementation of the additional vitamin source with different concentrations and different carbon sources for the production enhanced potential both in terms of biomass and secondary metabolites during different growth phases of selected hairy root line (14-P) of P. kurroa. The current research finding also highlights the significance of optimum conditions for hairy root cultures to investigate its feasibility for the large scale culture in bioreactor and culture conditions on growth and active ingredient (i.e., kutkoside and picroside I) production.
Materials and Methods
Plant material and culture conditions
Root clone 14-P initiated from Agrobacterium rhizogenes strain LBA 9402 mediated leaf-disc transformation were used which was obtained after screening of several clones for their biomass as well as secondary metabolite productivity.5 The hairy root cultures were maintained in 50 ml modified B5 liquid medium (3% sucrose) pH 5.8 in 250 ml Erlenmeyer flasks. The cultures were incubated on a shaker (90 rpm) under standard cool white fluorescent tubes with a flux rate of 35 µmols 16-h photoperiod at 25 ± 20C. The roots were regularly sub-cultured after a period of 4 weeks.
Effect of different basal media
Four different media formulations, namely MS (Murashige and skoog), Gamborg's B5, NN (Nistch & Nistch) at both full and half strengths were used for optimizing the yield potential of the selected hairy root clone 14-P. Best calculated inoculums density of 1.8 g fresh weight of freshly grown root were inoculated in triplicate to 50 ml of hormone-free full or half concentrations of the basal media (data un-published). The growth analyses of the roots were done at 20 d interval up to 60 d.
Growth kinetics
Fresh weight (FW) and dry weight (DW) of the cultures for growth kinetic analysis were determined by following the methods as described earlier.11 All the experiments were performed in triplicates. Hairy roots were harvested and washed, dried on blotting paper, and weighed to determine the fresh weights. Formula for calculating the growth index is given as:
Growth Index = Final fresh Wt - Initial fresh Wt / Initial fresh Wt × 100
Extraction of glycosides
The time course production of the desired secondary metabolites, i.e., kutkoside and picroside I, was determined by subjecting dried root samples to chemical extraction process.
The extraction of glycosides and HPLC analysis for kutkoside and picroside I was carried out according to the procedure reported earlier with minor modifications.12
Quantification of glycosides by HPLC
HPLC analysis for kutkoside and picroside I was carried out on reverse phase HPLC (Agilent HP 1100) on C18column (Waters Co. USA) as described earlier.5 For the examination of kutkoside and picroside I, known amount of stock solution of pure compounds (K and P) was added in P. kurroa root extract and the quantification repeated thrice. Selected wavelength was 265 nm, close to the absorption maxima of the compound (K and P). Concentrations of analyates were estimated at different intervals and no change was observed for the studied time
Effect of varying initial pH of the medium
The effects of pH on the yield potentials of the selected hairy root clone were studied through adjusting the pH of the half strength B5 medium supplemented with 4.0% sucrose before autoclaving to 4.0±0.1, 5.0±0.1, 6.0±0.1, 7.0±0.1 and 8.0±0.1. The medium with initial pH of 5.86 served as control. All the cultures were grown under the conditions specified earlier. After 40 d of incubation, roots were harvested.
Effect of optimal sucrose concentration
The effects of 8 different concentrations of sucrose, starting from 1 to 8 % were tested through its supplementation to the best suited media formulation, i.e. half- strength B5, on the yield potentials of the 14-P hairy root clone after 40 d of culture. The same root clone grown in the medium supplemented with 3% sucrose served as control. The culture conditions were same as in earlier experiments. After 40 d of incubation, roots were harvested.
Effect of different carbon sources on yield potential of the 14-P hairy root clone
The effect of 9 different carbon sources glucose, fructose, sorbitol, mannitol, ribose, lactose, rhamnose, galactose and market grade sugar, each supplemented to the half strength B5 medium at the 4% concentration were tested on the yield potential of the 14-P hairy root clone after 40 d of culture. Medium supplemented with sucrose as a carbon source was treated as control. The culture conditions were same as in earlier performed experiments. After 40 d of culture, roots were harvested for estimation of FW, DW and the yield of the desired secondary metabolites, i.e., picroside I and kutkoside.
Effect of vitamin supplementation
Effect of vitamins Thiamine HCl (10 mg/l) and modified RT vitamin (2 ml/l) stock solution were studied by supplementation to the half or full concentration of MS, B5 or NN media on the growth as well as secondary metabolite productivity of the 14-P hairy roots clone. Cultures maintained in the respective media without the vitamin supplementation served as control.
Optimization of critical parameters for up-scaling hairy root clone 14-P of P. kurroa in bioreactor
In the present study, attempts were made for up-scaling the selected 14-P hairy root clone of P. kurroa in a 5 liter bench top fermenter (model Bioflo-3000 from M/s New Brunswick Scientific Co. Inc.., USA) through applying the design modifications, already established for the scaling-up of hairy root cultures of other plant systems and optimization of critical parameters. The marine impeller was placed in the upper chamber of the glass vessel (about 10 cm above the mesh but dipped in the media) and turbine blade impeller was placed in the lower section of the vessel. The two spargers were placed below the blades; the air-flow rate was fixed (0.5 liter/min) and the agitation speed was kept constant at 50 rpm.
Culture conditions
In the bioreactor vesse,l 3 Ls of half -strength B5 medium with 4% market-grade sugar was used. Initial inoculum of 4.0 g (FW) per liter was kept constant. The initial inoculum was obtained from 15 d old hairy root cultures and was inoculated aseptically through the optimally-flamed inoculation-port of the bioreactor under a sterile laminar-hood. Culture temperature was maintained constantly at 25 ± 2° C by circulating cold water from a chiller attachment through the cooling jacket. Roots were grown in dark. The culture period was maintained till the 30th day of inoculation. The same hairy root clone was also inoculated in 50ml of the same medium in 250ml Erlenmeyer flasks with the initial inoculum of 0.18 g (FW) and was cultured for 30 d at 25 ± 2° C under dark condition on a shaker at 80 rpm.
Processing of hairy roots
After 30 d of sterile run, the roots were harvested through dismantling the vessel-assembly, washed under running tap water, blotted dry between filter papers and weighed to determine the FW. The harvested root samples were air-dried at room temperature (30 – 35°C) for 7 d followed by vacuum-desiccation for uniform drying and then the DW of the samples were determined. The dried hairy roots samples of uniform weight (i.e., 1g DW) were powdered and extracted and analyzed through HPLC as per the already stated method in-order to quantify the contents of the 2 specific glycosides, i.e., kutkoside and picroside I. The shake flask grown roots were also processed accordingly in triplicate which served as control.
Statistical analysis
The variation in biomass yield (growth index) and secondary metabolite content (% DW content of kutkoside and picroside I), were compared using critical difference test, following the procedure of Randomized Complete Block Design (RCBD). The statistical analysis was performed using in-house developed ANOVA statistical packages.
Results
Effect of different basal media on growth
An almost linear increment in the root biomass was noted in all the media combination tested up to the optimum growth phase, which differed among the different basal media used (Fig. 1). Full strength B5 medium was found to be most suitable for maximum biomass yield on the 40th day of culture of the 14-P hairy root clone (GI 32.72 ± 0.44) followed by the NN medium of the same strength (GI 22.9 ± 0.43). On the contrary, the MS and WP media were found to be unsuitable for the growth of this particular root clone, both of which extended the exponential phase up to the 60th day of culture. The half strength compositions, however, performed better than the respective full strengths in terms of the final biomass yield. In comparison to the highest recorded GI with the B5 medium at the optimum growth phase, a 2.68 and 4.17 times reduction was recorded in the half strengths of MS and WP media respectively at their respective optimum growth phase (Fig. 1).
Figure 1.
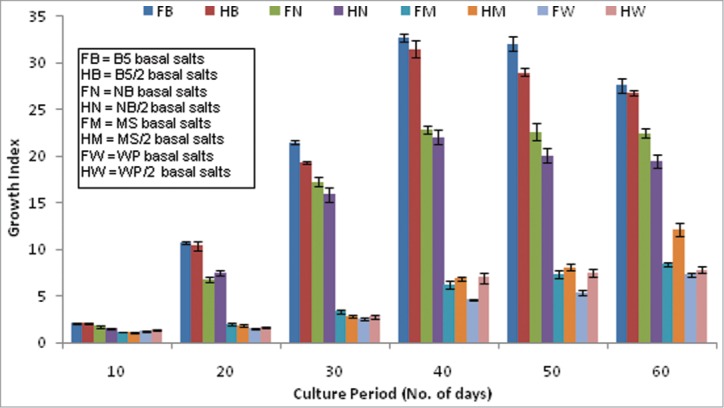
Influence of 4 different basal media formulations at respective full and half concentrations on the growth performance of hairy root clone (14-P) of P. kurroa during extended growth phases.
Effect of different basal media on secondary metabolite production
The secondary metabolite productivity of this selected root clone, especially with respect to the 2 major compounds of interest (i.e., kutkoside and picroside I), seemed to be quite sensitive to the changes in the basal media formulations. In all the media combinations tested during the present course of study, the optimum metabolite production phase synchronized with the respective optimum growth phases. The same basal media that favored the maximum biomass yield of the 14-P root clone at full strengths (i.e., B5 medium) were found to be most effective for the optimum level of the desired secondary metabolite production but at its half strength. The half strength B5 medium produced 1.1 and 1.3 times higher levels of the kutkoside and picroside I, respectively, than the same in the full strength B5 medium at the optimum production phase (Fig. 2).
Figure 2.
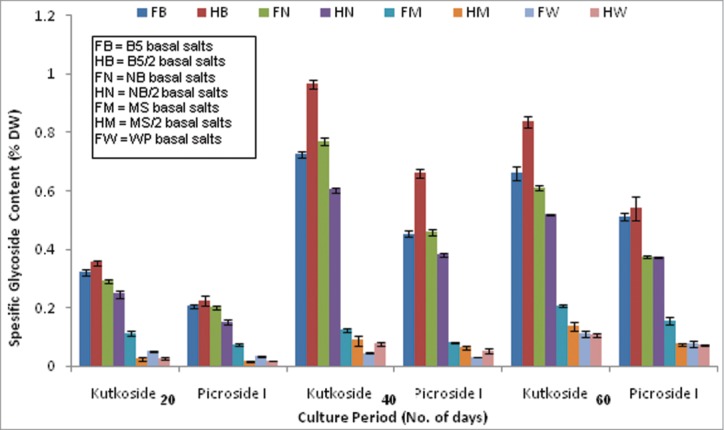
Influence of 4 different basal media formulations at respective full and half concentrations on the specific glycoside (Kutkoside & Picroside I) productivity.
Effect of vitamin supplementation to different basal media formulations
The half or full strengths of the MS medium supplemented either with Thiamin HCl or RT vitamins, supported active proliferation of hairy root clone. After 40 d almost 2 to 2.5 times improvement in GI was noticed as compared to the respective strengths of standard MS medium. However the improved GI, recorded after vitamin supplementation still remained lower than the standard B5 composition without vitamin supplementation (Figs. 3a, b).
Figure 3.
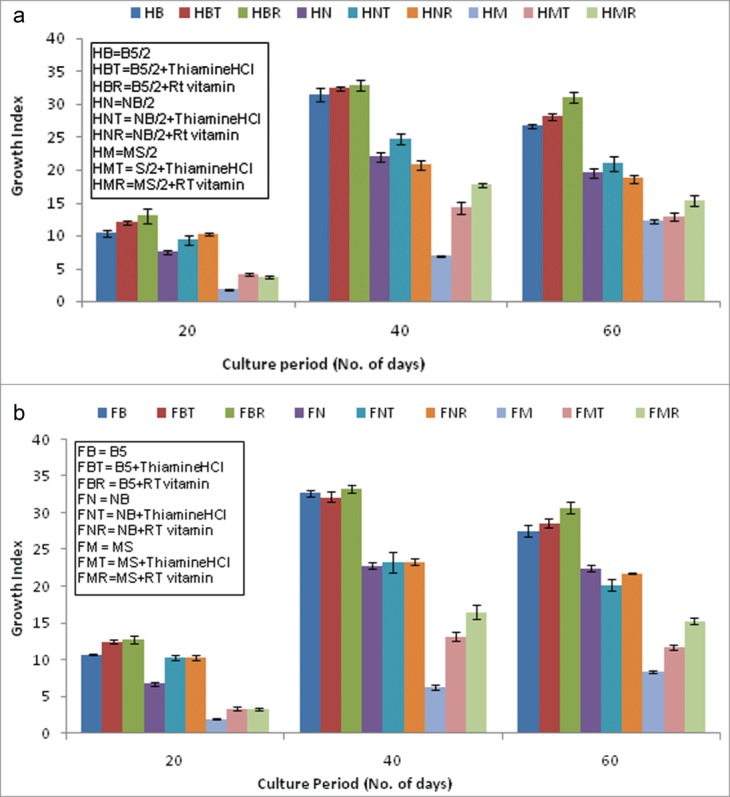
(A) Effect of T-HCl (10 mg/l) and RT vitamin (2ml/l of stock) supplementation to 3 different basal media at their respective half concentrations on growth kinetics. (B) Effect of T-HCl (10 mg/l) and RT vitamin (2ml/l of stock) supplementation to 3 different basal media at their respective full concentrations on growth kinetics.
The addition of RT vitamins or Thiamine HCl greatly influenced the overall secondary metabolite productivity in half or full strengths of MS medium (Figs. 4a, b). Nonetheless, these improved levels lag far behind (2.0–4.0 times) than the respective half or full strengths of standard B5 medium without any additional vitamin supplementation. In case of biomass yield, supplementation either with RT or Thiamine HCl to the full or half strengths of B5 or NN media failed to exert any statistically significant effect on the individual as well as total secondary metabolite productivity (Figs. 4a, b).
Figure 4.
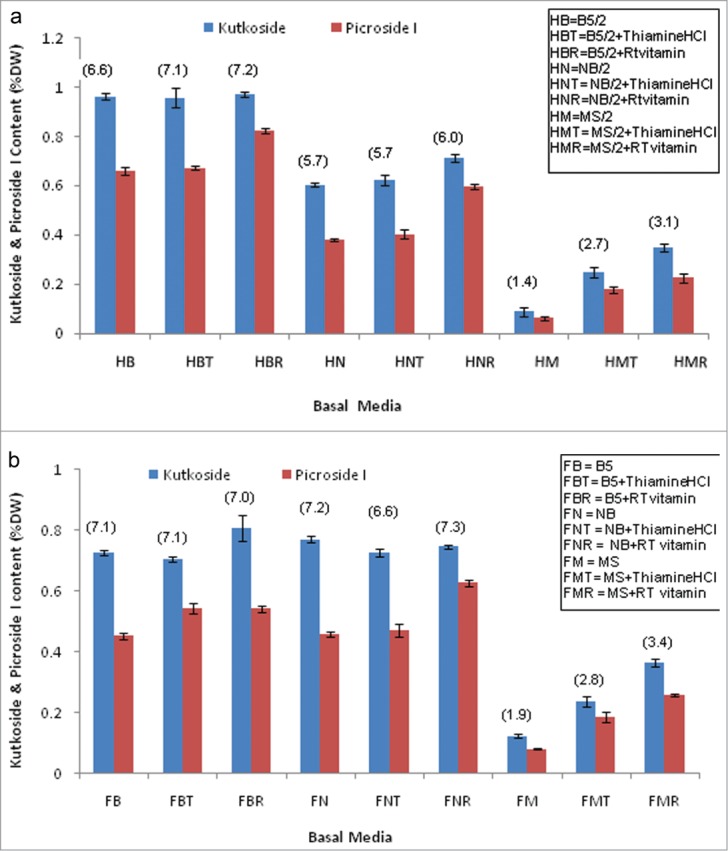
(A) Effect of T-HCl (10 mg/l) and RT vitamin (2ml/l of stock) supplementation to 3 different basal media at their respective half concentration on the Kutkoside and picroside I contents. (B) Effect of T-HCl (10 mg/l) and RT vitamin (2ml/l of stock) supplementation to 3 different basal media at their respective full concentrations on the Kutkoside and picroside I contents.
Effect of the medium pH
The effect of initial pH of medium was investigated on growth indices and secondary metabolite production of hairy root clone. Medium of different pH (3.0, 4.0, 5.0, 6.0, 7.0, and 8.0) were used for optimum growth and production phase (i.e. 40th day of culture) (Fig. 5).
Figure 5.
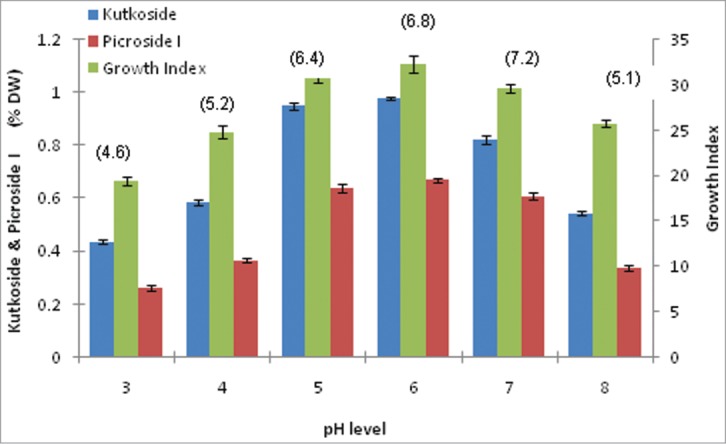
Comparison of growth kinetics and Picroliv contents (paranthesis) in hairy root clone in B5/2 medium with variable initial pH.
The pH levels ranging from 5.0 to 7.0 did not apparently affect the growth indices as well as the kutkoside and picroside I productivities. The yields of both biomass and 2 specific glycosides were highest at pH 6.0 and lowest at pH 3.0. While at pH 4.0 and 8.0, intermediate levels of production was noted in root biomass and secondary metabolite production (Fig. 5).
Effect of sucrose concentration
Attempts were made to further enhance the productivity of root clone, by the use of different concentrations of sucrose. Among the tested sucrose concentrations, the 4% level supported the highest GI, which was statistically insignificantly with that recorded either with the 3% or 5% levels during the same growth period (Table 1 and Figs. 6a, b).The lowest growth was recorded with the 0.5% level and the growth increment was found to be directly proportional with the increase in sucrose concentrations from 0.5 to 4% level and inversely proportional with the sucrose levels above 5% (Table 1 and Figs. 6a, b).
Table 1.
Effect of sucrose concentration on growth and glycoside content in hairy root clone (14-P) of P. kurroa after 40th days of culture
| Sucrose Concentration (%) | Fresh weight (gm) | Dry Weight (gm) | Growth Index | Glycoside (% DW) | Kutkoside (% DW) | Picroside I (% DW) |
|---|---|---|---|---|---|---|
| 0.5 | 0.84 ± 0.01 | 0.082 ± 0.001 | 4.68 ± 0.07 | 1.44 ± 0.09 | 0.077 ± 0.004 | 0.056 ± 0.005 |
| 1.0 | 1.45 ± 0.12 | 0.141 ± 0.012 | 8.05 ± 0.67 | 3.27 ± 0.16 | 0.207 ± 0.010 | 0.173 ± 0.017 |
| 2.0 | 2.46 ± 0.09 | 0.244 ± 0.010 | 13.66 ± 0.48 | 4.22 ± 0.13 | 0.349 ± 0.013 | 0.236 ± 0.014 |
| 3.0 | 5.33 ± 0.09 | 0.528 ± 0.009 | 29.59 ± 0.49 | 6.28 ± 0.12 | 0.858 ± 0.009 | 0.570 ± 0.068 |
| 4.0 | 5.67 ± 0.17 | 0.542 ± 0.012 | 31.35 ± 1.11 | 6.57 ± 0.14 | 0.964 ± 0.013 | 0.661 ± 0.015 |
| 5.0 | 5.26 ± 0.10 | 0.518 ± 0.010 | 29.20 ± 0.54 | 5.52 ± 0.18 | 0.670 ± 0.017 | 0.375 ± 0.016 |
| 6.0 | 3.73 ± 0.13 | 0.365 ± 0.016 | 20.72 ± 0.75 | 5.27 ± 0.13 | 0.632 ± 0.012 | 0.403 ± 0.010 |
| 7.0 | 2.55 ± 0.16 | 0.252 ± 0.013 | 14.14 ± 0.89 | 4.40 ± 0.18 | 0.603 ± 0.015 | 0.393 ± 0.011 |
| 8.0 | 2.62 ± 0.14 | 0.252 ± 0.010 | 14.53 ± 0.77 | 4.69 ± 0.10 | 0.601 ± 0.016 | 0.342 ± 0.006 |
| CD 5% | 0.26 | 0.025 | 1.52 | 0.31 | 0.027 | 0.056 |
| CD 1% | 0.36 | 0.034 | 2.09 | 0.43 | 0.037 | 0.077 |
Figure 6.
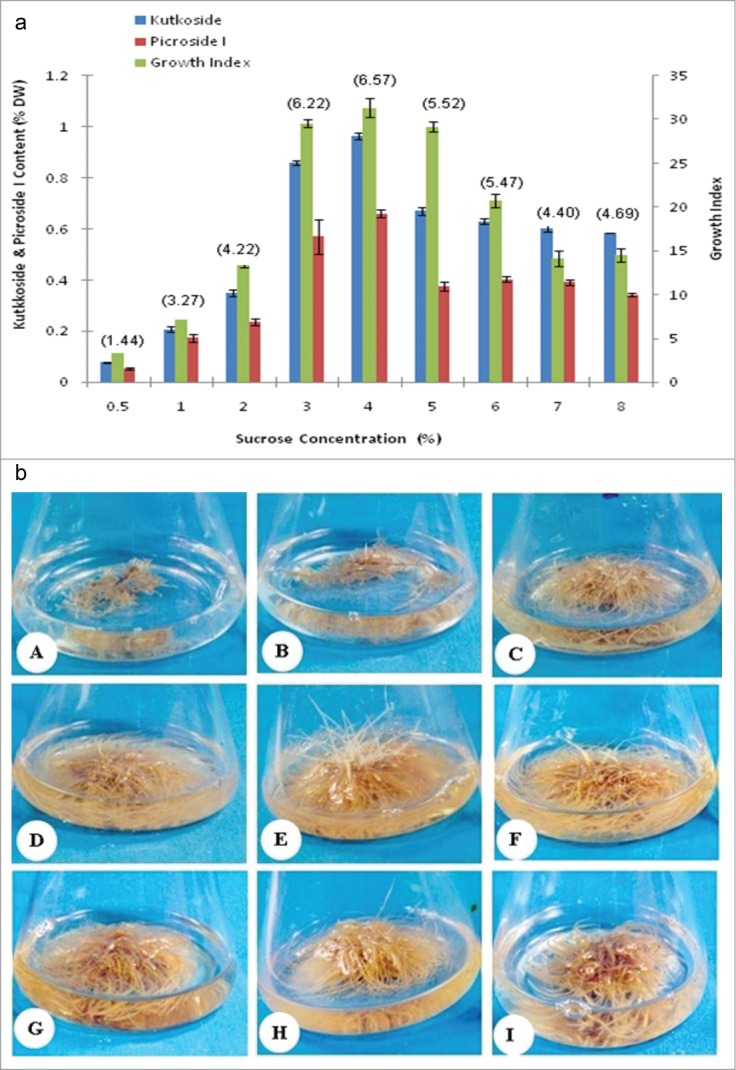
(A) Comparison of growth kinetics and Picroliv contents (paranthesis) in hairy root clone in B5/2 medium supplemented with different concentrations of sucrose. (B) Growth in hairy root clone in B5/2 medium supplemented with different concentrations of sucrose. A: 0.5%, B: 1%, C: 2%, D: 3%, E: 4%, F: 5%, G: 6%, H: 7%, and I: 8%.
There were almost 1.44 and 1.76 times enhancement in kutkoside and picroside I production respectively with optimal 4% of sucrose as compared to 5% sucrose level (Table 1 and Fig. 6A). However, the production levels of these 2 compounds did not differ significantly at higher sucrose concentrations (Fig. 6A).
Effect of different carbon sources on yield potential of the selected hairy root clone
The effect of 10 different carbohydrate sources namely rhamnose, mannitol, ribose, sorbitol, lactose, galactose, glucose, fructose, sucrose and market grade sugar, on the biomass as well as kutkoside and picroside I production was determined. Since sucrose is most frequently used carbon source for the hairy root culture, it has been used as control. The highest growth rate was obtained with market grade sugar which was 1.01 times higher than that noted with sucrose. In terms of the total glycoside yield, market grade sugar appeared to be the most suitable carbohydrate source which exhibited 1.2, 1.3 and 1.4 times higher yield of total glycoside, kutkoside and picroside I respectively (Figs. 7A and B).
Figure 7.
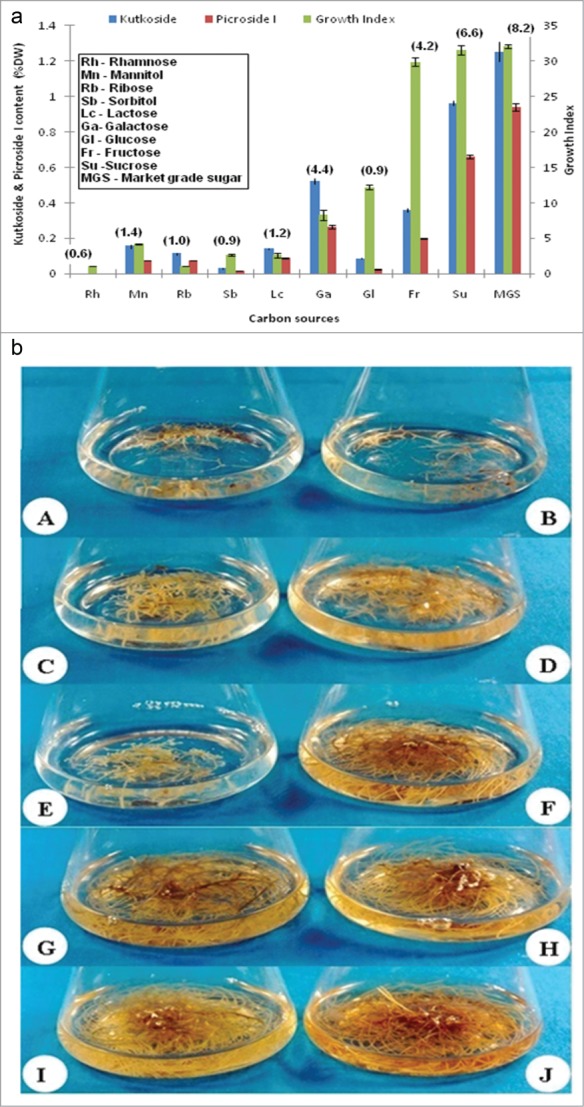
(A) Comparison of growth kinetics and Picroliv contents (paranthesis) in hairy root clone in B5/2 medium with different carbon sources. (B) Growth patterns in hairy root clone in B5/2 medium with different carbon sources. A: Rhamnose, B: Mannitol, C: Ribose, D: Sorbitol, E: Lactose, F: Galactose, G: Glucose, I: Fructose, J: Sucrose and K: Market grade sugar.
Among the 2 hexose monosacharide's tested, fructose performed significantly better than glucose both in terms of growth as well as secondary metabolite yield. A 2.4 and 4.6-fold higher biomass and total glycosides were observed respectively. A 4.1 and 8.3-fold higher yields of the kutkoside and picroside I respectively was obtained with fructose as compared to glucose supplementation (Figs. 7A and B). However, a 1.6, 2.7, and 3.3 times reduction in the total glycoside, kutkoside and picroside I productivities was noted through fructose supplementation as the carbohydrate source in comparison to that in standard 4% sucrose containing medium while the GI with both these carbohydrate sources remained within the comparable limits (Figs. 7A and B).
The lowest growth rate and secondary metabolite yield were obtained with ribose and rhamnose. In case of lactose and sorbitol biomass yield was 11.94 and 11.63 times lower than sucrose, respectively. The kutkoside and picroside I content were however 4.5 and 5.2 times higher in lactose containing media than sorbitol containing media (Figs. 7A and B). The growth, kutkoside and picroside I content of the hairy root clone in the mannitol containing media were 7.5, 6.2, and 8.1 times lesser than control, respectively.
The growth index of the hairy root clone in the galactose containing media was 3.6 and 3.8 times lesser than the fructose and sucrose containing media, respectively. However, the kutkoside and picroside I content were 1.45 and 1.33 times higher in the galactose containing media than fructose containing media.
Quantification of glycosides by HPLC
Peaks corresponding to compounds kutkoside (K) and picroside I (P) were base line separated and symmetrical. Retention times were 14.56 and 21.62 min and recoveries were 98 and 97% for compounds K and P, respectively (Fig. 8).
Figure 8.
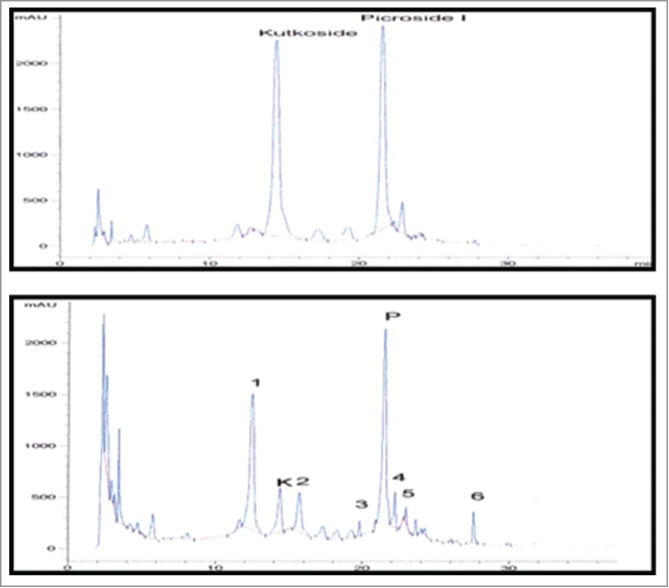
Chromatograms showing HPLC separation of compounds Kutkoside (K) and Picroside I (P) in: (top panel) an artificial mixture of compounds; (bottom panel) extract from transformed dried hairy root.
Optimization of critical parameters for up-scaling of hairy root clone in bioreactor
Attempts were made to optimize conditions in terms of air flow rate and agitation speed for the up-scaling of hairy root clone. It was cultivated in a structurally modified mechanically agitated bioreactor. The run was carried out with the marine impeller in the upper chamber and with 2 spargers, 27-fold growth enhancements (324 g FW) could be obtained after the same period of 30 d of culture through reduction in the air-flow rate (0.5 l/min) and also in the agitation speed (50rpm). Although in terms of fold increase, this growth rate was slightly lower than that of the shake flask grown roots which exhibited 28.4-fold growth increases within the same growth period (Fig. 9), the biomass yield per liter of the former was higher than the later (Table 2). In terms of secondary metabolite contents, comparable results were obtained in bioreactor run with shake-flask grown roots (Table 2).
Figure 9.
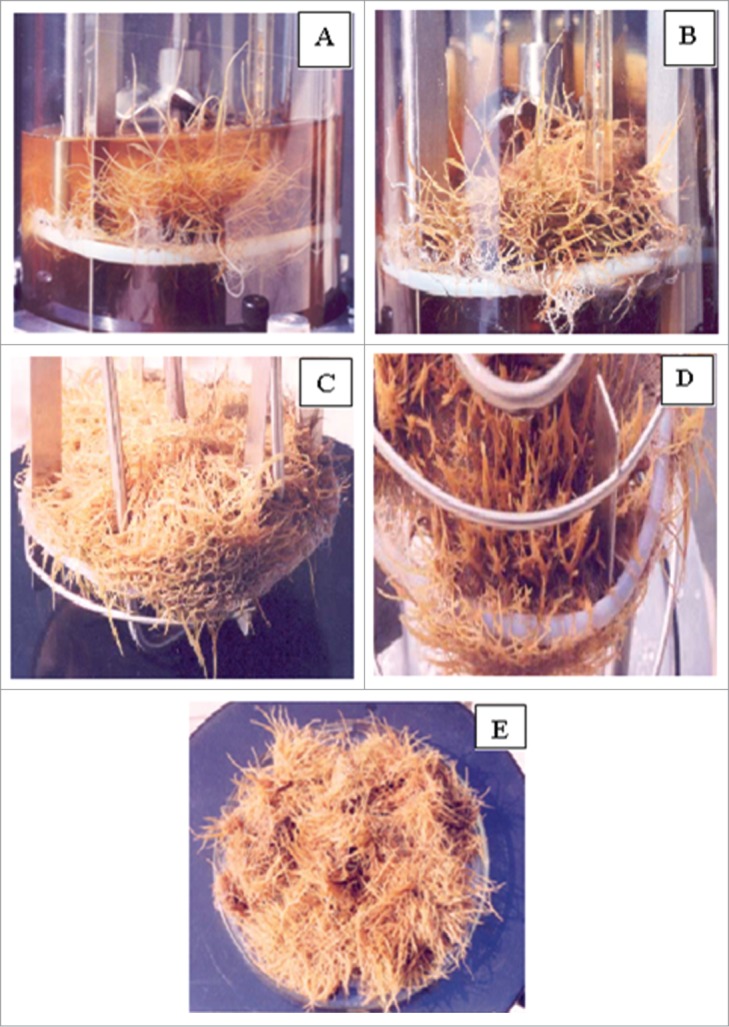
(A and B) Up- scaling of hairy root clone in a modified mechanically agitated, air-sparged bioreactor with inclusion of support matrix; (C) root growth in the upper chamber; (D) root growth in the lower chamber by penetrating through the nylon mesh septum; and (E) final root biomass after 30 d of batch culture.
Table 2.
Comparative analysis of growth performance and secondary metabolite yields of the hairy root clone after 30 d
| Parameters | 250 ml flask | 5 Liter bioreactor |
|---|---|---|
| Medium Volume | 50 ml | 3.0 l |
| Inoculum Weight (g) | 0.18 | 12.0 |
| Final fresh weight (g) | 5.12 | 186 |
| Biomass yield (g/l) | 102.4 | 62 |
| Picroliv Content (% DW) | 7.3 | 6.56 |
| Kutkoside Content (% DW) | 1.14 | 0.92 |
| Picroside I Content (% DW) | 0.85 | 0.72 |
Discussion
The growth promoting effect of the B5 medium has already been reported with respect to the hairy root cultures of several medicinal plant species,13 while limited success has been reported with respect to the NN medium so far which includes Cassia occidentalis and Swertia chirata.14 The MS medium has widely been reported to perform poorly in terms of its effects on the growth behavior of the hairy root cultures of several medicinal plant species.13,15 It however rendered positive effects on the hairy root cultures of Plumbago zeylanica,11 Anethum graveolens,16 and Linum mucronatum ssp. Mucronatum.17 Contrasting results were observed in case of the hairy root cultures of Hyoscyamus albus,18 Atropa belladonna19 and Coleus forskohlii20 where the WP medium exhibited positive influence on the final biomass yields. The influence of different basal media formulations on the secondary metabolite productivities of hairy root cultures had also been noted earlier in case of Trachelium caeruleum21 and Polygonum tinctorium.22
In accordance to earlier reported observations involving hairy root cultures of several plants for the optimum phase for production of secondary metabolite were synchronized with the respective optimum growth phase in all the media combinations tested during the present study.13, 23 B5 medium was found to be most effective for the optimum level of the desired secondary metabolite production, but at its half concentrations as noted in case of Centranthus rubber24 and Trigonella foenum-graecum25 hairy root cultures. The addition of vitamins to different media reiterate the fact that supplementation with any media additives beyond the optimal requirement level of any specific hairy root clone might prove to be ineffective in influencing the overall performance of the same. It is also observed in case of Catharunthus roseus hairy root cultures where no net change in the alkaloid content was observed with the changes in the concentration of vitamins.26 Nevertheless, enhancement in secondary metabolite productivity had also been reported earlier through supplementation of the half strength B5 medium formulation with the vitamin composition of the full strength media.27 In case of Polygonum tinctorium22 hairy root cultures, lower pH levels influenced the biomass productivities in a similar fashion. In accordance to present observations, the secondary metabolite production was negatively influenced by lower pH levels in case of hairy root cultures of Polygonum tinctorium.22
The present study also facilitated further improvements in the biomass as well as secondary metabolite productivities through optimization of the medium pH prior to autoclaving, the knowledge of which will be of immense help for the up-scaling studies.
The growth promoting effect of 4% sucrose level was also noted earlier in case of hairy root cultures of Artemisia annua28 and Plumbago zeylanica.11 Generation of osmotic stress by higher sucrose concentration might be responsible for the attenuation in root biomass yield as observed earlier.29 Our findings support the results in Psoralea,29 Swertia chirata14 hairy roots, but are in contrast to those of Christen et al,18 who reported that the growth of the Hyoscyamus albus hairy roots were not affected by different sucrose concentrations. The FW/DW ratio of Datura stramonium hairy root culture is greatly affected by the level of sucrose supplied relative to the total ion content of the medium. The present findings extend earlier observations involving the hairy root cultures of Centranthus rubber24 and Polygonum tinctorium22 where the higher sucrose concentrations beyond the optimum level resulted in diminution of the desired secondary metabolite contents. While, in case of Catharanthus roseus hairy root cultures, contradictory results were seen where continuous stimulation of secondary metabolite productivity could be noted with increasing sucrose concentrations although biomass productivity decreased. In another study involving Swertia chirata hairy root cultures, addition of sucrose at 6% and 9% levels respectively resulted in better growth rates and increased total but unaltered relative amarogentin content compared to 3% sucrose level.14 However in another instance, diosgenin production by the Trigonella foenum–graecum was increased by reducing the sucrose concentration from 3% to 1%.25
The inability to grow with glucose as the sole carbon source has earlier been reported for hairy root cultures of Daucus carota and Armoracia rustica30 which was in accordance with our observations. Comparatively better influence of fructose than glucose on the growth performance of hairy root cultures had been reported earlier in case of Tagetes laxa.31 In another report of Rubia tinctorum hairy root culture, fructose supported the best biomass yield which was even better than that in sucrose containing medium, but instead of fructose, galactose supported the maximum pigment content.32 Contradictory result is also available where the Catharanthus roseus hairy roots grew faster in medium with sucrose than one with fructose, at the same time 2-fold enhancements in the secondary metabolite productivity in fructose containing medium was observed as compared to sucrose containing media.33 Similar reduced growth behavior with galactose was also noted in case of Catharanthus roseus hairy root cultures.34 Successful utilization of market grade sugar had also been reported earlier for the biomass as well as secondary metabolite yields from the hairy root cultures of Atropa accuminata and Valeriana wallichii.35
In contrast to cell suspension cultures, hairy root cultures are characterized by high biosynthetic capacity and genetic as well as biochemical stability. Hairy roots can also synthesize more than one secondary metabolite and therefore prove economical for commercial exploitation.9 High level of lateral branching results in faster growth rate, exceeding that of nearly all the non-transformed roots and even that of cell suspension cultures. The up-scaling of hairy root cultures in bioreactors is being attempted worldwide involving several medicinal plant species.9,35 The mechanically agitated reactors with immobilization structures favored growth characteristics of hairy root cultures of several medicinal plant species. In most of the studies the immobilization structure had been used to isolate the roots from the stirrer mechanism in-order to avoid shear damage of the roots by direct contact with the impeller.23
In the present study, a special type of marine impeller was used which normally causes minimum shear damage to the root tissues and hence the impeller had been effectively placed within the root growing areas of the reactor vessel. The inclusion of the nylon mesh septum served the purpose of immobilization and ensured uniform distribution of the root tissue. The present optimization study led to higher biomass yield (g/1) in the 5 liter bioreactor than that in 250 ml shake flask.
This study once again emphasized the fact that for optimization of bioreactor performance, the morphological and physiological properties of the specific hairy root clone of any particular plant system need to be taken into consideration as these characters strongly influence the final productivities of the concerned root clone, both in terms of biomass as well as the secondary metabolite of interest.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Bhat WW, Lattoo SK, Rana S, Razdan S, Dhar N, Dhar RS, Vishwakarma RA.. Efficient plant regeneration via direct organogenesis and Agrobacterium tumefaciens-mediated genetic transformation of Picrorhiza kurroa: an endangered medicinal herb of the alpine Himalayas. InVitro Cell. Develop. Biol. - Plant, 2012; 48:295-303 [Google Scholar]
- 2.Sood H, Chauhan RS. High frequency callus induction and plantlet regeneration from different explants of Picrorhiza kurroa - a medicinal herb of Himalayas. Afric. J. Biotechnol., 2009, 8:1965-72 [Google Scholar]
- 3.Verma PC, Basu V, Gupta V, Saxena G, Rahman LU.. Pharmacology and Chemistry of a Potent Hepatoprotective Compound Picroliv Isolated from the Roots and Rhizomes of Picrorhiza kurroa Royle ex Benth. (Kutki). Current Pharma. Biotechnol., 2009; 10:641-9 [DOI] [PubMed] [Google Scholar]
- 4.Kumar V, Mehrotra N, Lal J, Gupta R.. Pattern profiling of the herbal preparation picroliv using liquid chromatography-tandem mass spectrometry. J. Chromato. A, 2004; 1045:145-52; http://dx.doi.org/ 10.1016/j.chroma.2004.06.021 [DOI] [PubMed] [Google Scholar]
- 5.Verma PC, Rahman L, Negi AS, Jain DC, Khanuja SPS, Banerjee S.. Agrobacterium rhizogenes mediated transformation of Picrorhiza kurroa Royle ex Benth: Establishment and selection of superior hairy root clone. Plant Biotechnol. Rep., 2007; 1:169-74 [Google Scholar]
- 6.Guillon S, Tremouillaux GJ, Pati PK, Rideau M, Gantet P.. Harnessing the potential of hairy roots: dawn of a new era. Trends Biotechnol., 2006; 24:403-9; PMID:16870285; http://dx.doi.org/ 10.1016/j.tibtech.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 7.Georgiev MI, Pavlov AI, Bley T.. Hairy root type plant in vitro systems as sources of bioactive substances. App. Microbiol. Biotechnol., 2007; 74:1175-85; http://dx.doi.org/ 10.1007/s00253-007-0856-5 [DOI] [PubMed] [Google Scholar]
- 8.Bourgaud F, Gravot A, Milesi S, Gontier E.. Production of plant secondary metabolites: a historical perspective. Plant Sci., 2001; 161:839-51; http://dx.doi.org/ 10.1016/S0168-9452(01)00490-3 [DOI] [Google Scholar]
- 9.Giri A, Narasu ML.. Transgenic hairy roots: recent trends and applications. Biotechnol. Advan., 2000, 18:1-22; http://dx.doi.org/ 10.1016/S0734-9750(99)00016-6 [DOI] [PubMed] [Google Scholar]
- 10.Mishra J, Bhandari H, Singh M, Rawat S, Agnihotri RK, Mishra S, Purohit S.. Hairy root culture of Picrorhiza kurroa Royle ex Benth.: a promising approach for the production of picrotin and picrotoxinin. Acta Physiol. Planta., 2011, 33:1841-6; http://dx.doi.org/ 10.1007/s11738-011-0724-x [DOI] [Google Scholar]
- 11.Verma PC, Singh D, Rahman LU, Gupta MM, Banerjee S.. In vitro-studies in Plumbago zeylanica: rapid micropropagation and establishment of higher plumbagin yielding hairy root cultures. J. Plant Physiol., 2002; 159:547-52; http://dx.doi.org/ 10.1078/0176-1617-00518 [DOI] [Google Scholar]
- 12.Gupta PP. Picroliv. Drugs of the Future, 2001; 26:25-31 [Google Scholar]
- 13.Azlan GJ, Marziah M, Radzali M, Johari R.. Establishment of Physalis minima hairy roots culture for the production of Physalins. Plant Cell, Tiss. Org. Cult., 2002; 69:271-8; http://dx.doi.org/ 10.1023/A:1015662118877 [DOI] [Google Scholar]
- 14.Keil M, Hartle B, Guillaume A, Psiorz M.. Production of amarogentinin root cultures of Swertia chirata. Planta Med., 2000, 66:452-7; PMID:10909267; http://dx.doi.org/ 10.1055/s-2000-8579 [DOI] [PubMed] [Google Scholar]
- 15.Saleh NM, Thuc LV.. Assessment of hairy roots induction in Solenostemon scutellarioides leaves by different strains of Agrobacterium rhizogenes. Afric. J. Biotechnol., 2009; 8:3519-23 [Google Scholar]
- 16.Pedro AG, Santos A, Cristina F, Pedro ML, Lourenço JG, Barroso L, Pedro G, Margarida OM, Schripsema J, Stanley GD, et al.. Hairy root cultures of Anethum graveolens (dill): establishment, growth, time-course study of their essential oil and its comparison with parent plant oils. Biotechnol. Lett., 2002; 24:1031-6; http://dx.doi.org/ 10.1023/A:1015653701265 [DOI] [Google Scholar]
- 17.Samadi A, Carapetian J, Heidari R, Jafari M, Hassanzadeh AG.. Hairy Root Induction in Linum mucronatum ssp. mucronatum, an Anti-Tumor Lignans Producing Plant. Not. Botan. Horti Agrobot. Cluj, 2012; 40:125-31 [Google Scholar]
- 18.Christen P, Aoki T, Shimomura K.. Characteristics of growth and tropane alkaloid production in Hyoscyamus albus hairy roots transformed with Agrobacterium rhizogenes A4. Plant Cell Rep., 1992; 11:597-600; PMID:24213359; http://dx.doi.org/ 10.1007/BF00236380 [DOI] [PubMed] [Google Scholar]
- 19.Bonhomme V, Laurain-Mattar D, Lacoux J, Fliniaux M, Jacquin-Dubreuil A.. Tropane alkaloid production by hairy roots of Atropa belladonna obtained after transformation with Agrobacterium rhizogenes and Agrobacterium tumefaciens containing rol A, B, C genes only. J. Biotechnol., 2000; 81:151-8; PMID:10989174; http://dx.doi.org/ 10.1016/S0168-1656(00)00287-X [DOI] [PubMed] [Google Scholar]
- 20.Li W, Koike K, Asada Y, Yoshikawa T, Nikaido T.. Rosmarinic acid production by Coleus forskohlii hairy root cultures. Plant Cell, Tiss. Org. Cult., 2005; 80:151-5; http://dx.doi.org/ 10.1007/s11240-004-9541-x [DOI] [Google Scholar]
- 21.Murakami Y, Shimomura K, Yoshihira K, Ishimaru K.. Polyacetylenes in hairy root cultures of Trachelium caeruleum L. J. Plant Physiol., 1998; 152:574-6; http://dx.doi.org/ 10.1016/S0176-1617(98)80279-4 [DOI] [Google Scholar]
- 22.Young AC, Yu HS, Song JS, Chun HK, Park SU.. Indigo production in hairy root cultures of Polygonum tinctorium Lour. Biotechnol. Lett., 2000; 22:1527-30; http://dx.doi.org/ 10.1023/A:1005668625822 [DOI] [Google Scholar]
- 23.Sudo H, Yamakawa T, Yamazaki M, Aimi N, Saito K.. Bioreactor production of camptothecin by hairy root cultures of Ophiorrhiza pumila. Biotechnol. Lett., 2002; 24:359-63; http://dx.doi.org/ 10.1023/A:1014568904957 [DOI] [Google Scholar]
- 24.Granicher F, Christen P, Kapetanidis I.. Production of valepotriates by hairy root cultures of Centranthus rubber DC. Plant Cell Rep., 1995; 14:294-8; PMID:24186763; http://dx.doi.org/ 10.1007/BF00232031 [DOI] [PubMed] [Google Scholar]
- 25.Peraza LF, Rodríguez MM, Arias CC, Bessiere JM, Calva CG.. Sotolone production by hairy root cultures of Trigonella foenum-graecum in airlift with mesh bioreactors. J. Agricul. Food Chem., 2001; 49:6012-9; http://dx.doi.org/ 10.1021/jf010818j [DOI] [PubMed] [Google Scholar]
- 26.Vazquez FF, Moreno VO, Miranda HML, Coello CJ, Loyola VVM. Catharanthine and ajmalicine synthesis in Catharanthus roseus hairy root cultures. Plant Cell, Tiss. Org. Cult., 1994, 38:273-9; http://dx.doi.org/ 10.1007/BF00033887 [DOI] [Google Scholar]
- 27.Morgan AJ, Barney CS, Penn AH, Shanks JV.. Effect of buffered media upon growth and alkaloid production of Catharanthus roseus hairy roots. Appl. Microbio. Biotech., 2000; 53:262-5; http://dx.doi.org/ 10.1007/s002530050018 [DOI] [PubMed] [Google Scholar]
- 28.Weathers PJ, Hemmavanh DD, Walcerz DB, Cheetham RD, Smith TC.. Interactive effects of nitrate and phosphate salts, sucrose, and inoculum culture age on growth and sesquiterpene production in Artemisia annua hairy root cultures. In Vitro Cell Develop. Biol. Plant, 1997; 33:306-12; http://dx.doi.org/ 10.1007/s11627-997-0056-0 [DOI] [Google Scholar]
- 29.Nguyen C, Bourgaud F, Forlot P, Guckert A.. Establishment of hairy root cultures of Psoralea species. Plant Cell Rep., 1992; 11:424-7; PMID:24201547; http://dx.doi.org/ 10.1007/BF00234375 [DOI] [PubMed] [Google Scholar]
- 30.Taya M, Kino-Oka M, Tone S, Kobayashi T.. Kinetic model of branching growth of plant hairy root. J. Chem. Eng. Japan, 1989; 22:668-70 [Google Scholar]
- 31.Rodriguez TJ, Giullelti AM.. In vitro thiophene production by transformed root cultures of Tagetus laxa (Cabrera). Biotechnol. Lett., 1995; 17:1337-42 [Google Scholar]
- 32.Kino-Oka M, Mine K, Taya M, Tone S, Ichi T.. Production and release of anthraquinone pigments by hairy roots of Maddar (Rubia tinctorium L.) under improved culture conditions. J. ferment. Bioengi., 1994; 77:103-6; http://dx.doi.org/ 10.1016/0922-338X(94)90219-4 [DOI] [Google Scholar]
- 33.Jung KH, Kwak SS, Kim SW, Lee H, Choi CY, Liu JR.. Improvement of the catharanthine productivity in hairy root cultures of Catharanthus roseus by using monosaccharide as carbon source. Biotechnol. Lett., 1992; 14:695-700; http://dx.doi.org/ 10.1007/BF01021645 [DOI] [Google Scholar]
- 34.Rao SR, Ravishankar GA.. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Advan., 2002; 20:101-53 [DOI] [PubMed] [Google Scholar]
- 35.Cui XH, Murthy HN, Jin YX, Yim YH, Kim JY, Paek KY.. Production of adventitious root biomass and secondary metabolites of Hypericum perforatum L. in a balloon type airlift reactor. Biores. Tech., 2011; 102:10072-79; http://dx.doi.org/ 10.1016/j.biortech.2011.08.044 [DOI] [PubMed] [Google Scholar]


