Abstract
Functional knockdowns mediated by endoplasmatic reticulum-retained antibodies (ER intrabodies) are a promising tool for research because they allow functional interference on the protein level. We demonstrate for the first time that ER intrabodies can induce a knock-down phenotype in mice. Surface VCAM1 was suppressed in bone marrow of heterozygous and homozygous ER intrabody mice (iER-VCAM1 mice). iER-VCAM1 mice did not have a lethal phenotype, in contrast to the constitutive knockout of VCAM1, but adult mice exhibited physiological effects in the form of aberrant distribution of immature B-cells in blood and bone marrow. The capability to regulate knock-down strength may spark a new approach for the functional study of membrane and plasma proteins, which may especially be valuable for generating mouse models that more closely resemble disease states than classic knockouts do.
Keywords: endoplasmatic reticulum, intrabody, in vivo knockdown, KDEL, knockdown, knockout, membrane protein, mouse model, VCAM1
Abbreviations
- ER
endoplasmatic reticulum
- VCAM1
vascular cell adhesion molecule 1
- ES
embryonic stem cell
- FITC
fluorescein isothiocyanate
- PE
phycoerythrin
- CMV
cytomegalovirus
- ROSA26
reverse orientation splice acceptor 26
- SA
streptavidin
Introduction
Although the production of functional antibodies in the cytosol is in many cases not successful due to folding problems,1,2 which makes cytosolic antibody delivery necessary,3 the use of endoplasmatic reticulum-retained antibodies (ER intrabodies) has proven to be a reliable approach to achieve functional knockdowns of membrane proteins in cell culture.4-6 ER intrabodies are recombinant antibodies that carry the peptide motif KDEL, which mediates redirection of proteins in the secretion pathway to the ER.7,8 In nature, KDEL is employed to keep ER-resident proteins such as protein disulfide isomerase (PDI) or ER-resident chaperones in place by preventing them from being secreted.7,8 The ER-retention mechanism is highly efficient7 and ER intrabodies utilize this mechanism to retain their antigen, typically a membrane protein that passes the ER, by providing it with a KDEL ER retention motif through its binding. This retention eventually leads to disappearance of the protein from its natural post-ER location, typically the cell membrane. To provide this knockdown, ER intrabodies do not need Fc functions, so they typically consist of the smallest unit still providing antigen specificity, the single chain fragment variable (scFv). ScFvs have a size of ∼25–30 kDa, and consist of the immunoglobulin VH and VL domains connected with a flexible peptide linker.
Other than genetic knockouts or RNAi mediated knockdowns, ER intrabodies act at the protein level. Therefore, ER intrabodies could specifically be designed to target a particular splice variant or even detect a protein only if one particular post-translational modification is present. Furthermore, specificity of antibodies can be extensively tested in vitro before using them as ER intrabodies, whereas unspecific effects of RNAi are more difficult to predict in advance. RNAi have been shown to lead to aberrant gene expression and an interferon response that leads to non-specific suppression of translation.9-16 Moreover, knockdowns by RNAi may suffer from a low half-life of RNAi,13 whereas ER intrabodies in contrast are more stable in comparison.11 Although the ER intrabody technology has been applied successfully in vitro, its effectiveness has so far not been proven in vivo. The reliability of a report claiming the successful use of an intrabody in vivo17,18 has been questioned.19
In this study, we used ER intrabodies to mediate knockdown of VCAM1 in mice (Fig. 1). VCAM1 is an Ig-superfamily type I transmembrane protein expressed mainly on endothelial cells during inflammation. Two of the 7 Ig domains (Ig-domain 1 and 4) bind to integrin20 to mediate the adhesion of circulating leukocytes to activated endothelial cells during inflammation. Besides the glycosylated full-length protein, splice variants of VCAM1 with only one integrin binding domain exist, and, apart from cell surface expression, there is also a soluble form of VCAM1.21-23 The treatment of several medical conditions may benefit from studying VCAM1 function, including autoimmune diseases,24 asthma, inflammatory bowel disease, multiple sclerosis, arthritis,25 transplant rejection,25,26 atherosclerosis,26 viral infections27 and cancer.28 In a mouse model, the knockout of VCAM1 was found to be lethal in early embryonal development.29 Therefore, conditional knockouts or knockdown strategies allowing regulation of the knockdown strength are required to study the function of VCAM1. Successful ER intrabody induced knockdown of membrane VCAM1 has already been demonstrated in cell culture.5 The aims of this study were to prove the principal feasibility of ER intrabody mediated knockdowns in vivo and to use the method to generate a new mouse model for VCAM1 function.
Figure 1.

The ER intrabody knockdown principle: recombinant scFv-antibody fragments carrying a signal peptide and the ER retention peptide “KDEL” are expressed in transgenic mice. By binding to their antigen (= knock down target) in the ER, they prevent it from being secreted or transported to the cell surface, thereby inducing the knock down phenotypes.
Results
Whereas the genetic knockout of VCAM1 leads to an early embryonally lethal phenotype,29 neither hemizygous nor homozygous mice of the iER-VCAM1 mouse line showed a lethal phenotype (for generation of mouse lines, see Fig. S1). iER-VCAM1 mice were inconspicuous and offspring was distributed according to Mendel. To verify the production of the intrabody, spleen cells from transgenic mice were analyzed by immunostain of lysates from spleen cells (Fig. S2). ER intrabodies were found in the homozygous mouse, but not in both wildtype and a hemizygous iER-STOP-VCAM1 mouse, also proving that the stop-cassette is not leaky.
As intrabodies were produced, and thus the prerequisite for a phenotypic knockdown was given, cell samples from mice were analyzed for cell surface expression of VCAM1. Apart from induced expression of VCAM1 due to inflammation,30 VCAM1 is mainly expressed in bone marrow.31 Cells were therefore prepared from bone marrow, stained for cell-surface VCAM1 and analyzed by flow cytometry. Anti-VCAM1 detection antibodies were shown to bind to another epitope than the ER intrabody clone to VCAM15 (Fig. S4) to exclude any potential false negative results due to masking of cell surface VCAM1 by intrabodies that were released by cell lysis or by overwhelming the KDEL sorting apparatus.VCAM1 was found to be reduced on the cell surface in bone marrow of ER intrabody-expressing mice compared to control mice as revealed by staining with the anti-VCAM1 antibody clone MAB6434 (Fig. S3) and the biotinylated antibody clone 429, where detection via biotin provided a clearer difference due to the absence of background signals (Fig. 2A). The ablation of VCAM1 on the cell surface of ER intrabody-expressing mice was confirmed by the analysis of 6 control mice (either wildtype or iER-STOP-VCAM1 mice) and 9 ER intrabody-expressing mice (either heterozygous or homozygous iER-VCAM1 mice). It is noteworthy that depletion of VCAM1 from the cell surface of bone marrow cells occurred in mice that were homozygous as well as heterozygous for ER intrabody expression.
Figure 2.

(A) Cell surface expression of VCAM1 in bone marrow. Cell surface-VCAM1 is ablated in bone marrow of mice that are homozygous as well as heterozygous for the ER-intrabody gene (detected by anti-VCAM mab429). 6 control mice (either wt or iER-STOP-VCAM1 mice) and 9 intrabody-expressing mice (heterozygous or homozygous iER-VCAM1 mice) were analyzed (one representative experiment is shown). A comparison of wildtype, heterozygous and homozygous iER-intrabody mice was performed in 3 independent experiments with one mouse per genotype each. (B) Blood counts. White blood cells (WBC) and lymphocytes (LYM) are significantly increased in ER-intrabody-expressing mice (heterozygous or homozygous) compared to control mice (wildtype or iER-STOP-VCAM1 mice). P-values are 0.0013 for white blood cells and 0.00093 for lymphocytes. Standard deviations are shown as error bars. In total 4 control mice and 8 iER-VCAM1 mice were analyzed. Of the 8 iER-VCAM1 mice that have been analyzed, 6 were heterozygous and 2 were homozygous. Bloodcounts were performed based on impedance technology (VetScan Hematology System, Abaxis).
To further analyze the effect of ER intrabody production on the phenotype, we investigated whether the depletion of VCAM1 observed in bone marrow also results in a physiological effect. VCAM1 on non-lymphoid cells in the bone marrow serves as an anchor that retains immature B cells in the bone marrow.32 The analysis of conditional genetic VCAM1 knockout mice revealed an elevated number of immature B cells in the peripheral blood, while immature B cells in the bone marrow were reduced.32
To determine whether ER intrabody-expressing mice exhibit the same phenotype, blood and bone marrow were analyzed for their content of B-cell progenitors. Blood counts were analyzed from 4 control mice (wildtype or iER-STOP-VCAM1) and 8 iER-VCAM1 mice (6 heterozygous and 2 homozygous) to determine whether the expected differences between control mice and ER intrabody-expressing mice could be found. The analysis of the number of white blood cells and lymphocytes per volume was found to be significantly different in control mice and ER intrabody mice, with a p-value of 0.0013 for white blood cells and 0.00093 for lymphocytes (Fig. 2B).
To further characterize the subsets of B cells in the peripheral blood, cells from blood were stained for the surface markers B220, IgM and IgD. For analysis, B220+ cells in the lymphocyte gate33 were plotted according to their IgM and IgD expression. Analysis of B220+ cells revealed a slight increase in IgM+ IgD− cells of ER intrabody-expressing cells compared to controls (Fig. 3). In contrast, ER intrabody-expressing cells exhibited a slight decrease in IgM+ IgD+ cells compared to controls in accordance with the expected shift toward more immature B cells. In 3 independent experiments the clearest difference was always observed when comparing controls with mice that were homozygous for the ER intrabody, hinting at a possible quantitative effect of the intrabody expression level.
Figure 3.

Analysis of B-cells in the peripheral blood. The fraction of immature B-cells (IgM+ IgD−) is increased in animals that are heterozygous or homozygous for the ER-intrabody gene. Mature B-cells (IgM+ IgD+) in turn are decreased in ER-intrabody mice compared to controls. In 3 independent experiments the difference between ER-intrabody mice and controls was observed to be most pronounced in homozygous iER-VCAM1 mice.
To determine whether the additional immature B cells in the blood are missing in the bone marrow, cells were isolated from bone marrow and classified according to their expression of the pan-B cell marker B220 and IgM by flow cytometric analysis. Cells from the lymphocyte gate33 were then plotted according to their IgM and B220 expression in order to allow distinction of different B-cell subsets, which are distinguished by letters where A denotes the most immature and F the most mature fraction of B-cells.34 There was a clear decrease in the B-cell fraction F in ER intrabody-expressing mice, the largest difference again being noticeable between control mice and mice that were homozygous for the ER intrabody (Fig. 4). The percentage of all B220+ cells in the lymphocyte gate was slightly decreased in ER intrabody-expressing mice compared to controls, as would be expected if progenitor cells are prematurely released from the bone marrow.
Figure 4.
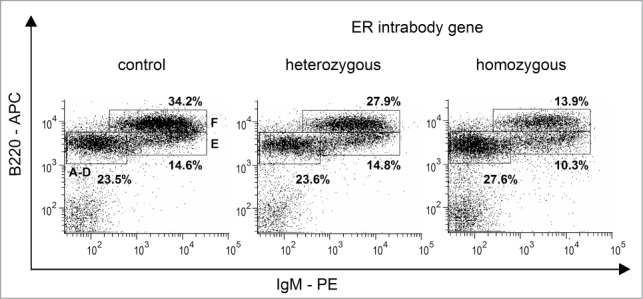
Analysis of B cells in Bone marrow. Bone marrow of iER-VCAM1 mice exhibited a reduction in the population of most mature B cells (fraction F). In 3 independent experiments the difference between ER-intrabody mice and controls was observed to be most pronounced in homozygous iER-VCAM1 mice.
Discussion
In contrast to the VCAM1-knockout mouse models, the iER-VCAM1 mice did not have a lethal phenotype. Although iER-VCAM1 mice exhibited no lethal phenotype, intrabody production was shown and led to a marked and expected phenotype, the suppression of VCAM1 on the cell surface of bone marrow cells from adult mice. This proves the principal feasibility of the ER intrabody knockdown approach in vivo. There are several possible explanations for the non-appearance of the lethal phenotype in iER-VCAM1 mice. Intrabody expression levels in vivo can be expected to be different from the so far described quite efficient in vitro systems5 since there is a maximum of 2 copies of the transgene per cell in the transgenic mice, whereas the usual in vitro approaches used transiently transfected cells that may contain much more copies and additionally may benefit from higher expression levels provided by the CMV promoter compared to the endogenous ROSA26-promoter used in vivo. Further, although the endogenous promoter of the ROSA26 locus should be active at the embryonal stage that is critical for the VCAM1-knockout induced lethality,29,35 it remains to be analyzed whether expression levels of the ER intrabody are sufficient for maximum knockdown at this embryonal stage. Even in vitro, the ER intrabody-mediated knockdown was not complete in spite of its high efficiency (94% knockdown in vitro5). Only 1.5 ± 0.7 bonds of VCAM1 to its receptor was found to be sufficient to mediate cell-binding,36 so few remaining VCAM1 molecules may have been enough to allow for normal placental development and to circumvent the lethal phenotype.
Low residual expression levels have also helped to prevent the lethal phenotype in a mouse model in which the 4th Ig domain of VCAM1 was deleted. Expression levels of VCAM1 in this mouse model were observed to be around 2–8%, which is similar to the remaining expression observed for the ER intrabody mediated knockdown of VCAM1 in vitro. The lethal phenotype of mice with a deletion of the 4th Ig domain of VCAM1 corresponds to the splice variant of VCAM1, which has even reduced activity compared to full-length VCAM1. Nevertheless, lethality is reduced in these mice compared to the complete knockout of VCAM1, although survival rates are strain dependent, with 6% for C57BL/6 and 129, 24–25% for Balb/c and 29% for 129-Balb/c and 129-BL/6 hybrids.26 This mouse model with low VCAM1 expression levels exhibited higher survival rates, which may explain the non-appearance of a lethal phenotype in iER-VCAM1 mice.
Functional consequences of the ER intrabody mediated knockdown are clearly seen in adult animals. The number of white blood cells and lymphocytes was significantly increased in the peripheral blood of iER-VCAM1 mice compared to controls, as expected from what is known about inducible VCAM1 knockout mice.32 The observed increase of immature B-cells in the peripheral blood of iER-VCAM1 mice compared to controls had also been found in induced VCAM1 knockout mice, although more pronounced than in iER-VCAM1 mice.32 The less pronounced effect observed in the blood of iER-VCAM1 mice compared to induced VCAM1 knockout mice points to an intermediate phenotype induced by the ER intrabody.
The clearest difference in the fraction of immature B cells in the peripheral blood was observed between control mice and homozygous mice. Although the cell surface expression of VCAM1 was similar in both heterozygous and homozygous iER-VCAM1 mice, the slightly more prominent fraction of immature B cells in the blood of homozygous iER-VCAM1 mice hints at 2 different levels of knockdown efficiency at the physiological level. This may be caused by tiny differences in the cell surface expression of VCAM1 between heterozygous and homozygous mice, which are too small to be revealed by cell surface stainings. The analysis of bone marrow for its content of immature B cells pointed to a similar phenomenon. While most mature B cells (fraction F) in the bone marrow were decreased in accordance with their release into the blood, the reduction of cells from fraction F was most prominent in homozygous iER-VCAM1 mice.
Avoiding the lethal phenotype that made analysis of adult VCAM1 knockout mice impossible, the ablation of cell surface VCAM1 by the ER intrabody led to a phenotype on the functional level. This demonstrates the effectiveness of ER intrabody-mediated knockdowns in vivo and its potential for studying otherwise lethal phenotypes. Further, since a stronger phenotype was seen in homozygous mice, which are expected to produce higher intrabody amounts than heterozygous mice, both observations strongly suggest that ER intrabodies allow not only a novel approach for the study of VCAMI by knock downs on the protein level, but also the future possibility to regulate the strength of the knock down - a feature so far not achievable. Initial analysis of ER intrabody expression levels in bone marrow and spleen of a limited set of mice supported the hypothesis of a gene dosis effect (Fig. S5), although this needs further confirmation. If aradual knockdown of VCAM1 could indeed be achieved, this may serve as a model for different degrees of immune impairment as VCAM1 is required for the migration of immune cells into inflamed tissues30 and for the recirculation of mature B cells into the bone marrow.32 Furthermore, a gradual knockdown would be a valuable tool for studying signaling receptors that cause different cellular responses depending on the amount of receptor on the cell surface. An example for this type of cell surface receptors are p75NTR and TrkA, the ratio of which is believed to determine cellular response.37 The iER-VCAM1 mice may additionally be used as a model system for VCAM1-impairment to improve the natalizumab-based therapy of multiple sclerosis and Crohn's disease, which suffers from severe side effects in the form of an increased risk for lethal brain infections.38,39 The iER-VCAM1 mice may also aid in the study of atherosclerosis, the initiation of which critically depends on the amount of VCAM1.40
As hypomorphic ablation of proteins is often closer to pathologic conditions, ER intrabody-mediated knockdowns may allow generation of new mouse models that are closer to disease states than those based on the knockout of genes. Depletion of VCAM1 has also been achieved by using RNAi.8,40 However, the RNAi-mediated knockdown of VCAM1 suffered from off-target effects of RNAi that appeared in the form of impaired cell growth and the activation of stress pathways, including the upregulation of programmed death ligand 1 and BiP.9 In contrast, the overexpression of an ER intrabody did not result in ER-stress as was found previously by analyzing the unfolded protein response.6 Gradual knockdowns by ER intrabodies will launch a new generation of functional studies in vivo by allowing the analysis of qualitative consequences from quantitative changes on the protein level. Apart from quantitative differences due to heterozygous or homozygous expression of an ER intrabody, the degree of knockdown may also be tuned by changing affinity and expression levels of antibodies at will by antibody engineering, which today can be achieved with little effort.41 Since several large scale antibody generation initiatives ongoing today provide the antibody genes right away,41,42 their straightforward use to generate graded knock downs in mice provide a new level for the study of membrane and plasma protein function in vivo.
Materials and Methods
Cloning and preparation of the gene targeting construct
The vector TVrosa26‑LMP1 was generously provided by Martin Hafner from the HZI Braunschweig. The gene targeting construct TVrosa26-aVCAM-KDEL was obtained by PCR amplification of the VCAM-1 specific single chain variable fragment scFv6C7.1 fused to the C-terminal ER retention sequence KDEL5 gene using primers NS30‑for and NS31‑rev and pCMV-aVCAMscFv-KDEL (NS24-20) as a template. The vector TVrosa26-LMP1 and PCR-products were digested with AscI and digested DNA fragments were ligated using T4-DNA-Ligase (Promega). The correct sequence of the plasmid TVrosa26- aVCAM-KDEL was confirmed by sequencing with primers NS29, NS12 and NS33. Endotoxin-free DNA preparations were isolated using the Endofree Plasmid preparation kit from Qiagen. DNA concentrations were quantified by absorption measurements (NanoDrop, Peqlab) and gel electrophoresis. The plasmid was linearized by digesting 200 μg of the endotoxin-free DNA preparation with the restriction enzyme KpnI in React4-buffer (Invitrogen). DNA from this reaction was purified by phenol/chloroform extraction, precipitated by isopropanol and dissolved in phosphate buffered saline (PBS). Integrity, yield and purity of linearized DNA were assessed by gel electrophoresis and measurement of absorption (Table 1).
Table 1.
Primers.ama168x
| Name of oligonucleotide | Sequence |
|---|---|
| NS29-for | 5′-GAGGTCTGATGACACGGCCAC-3′ |
| NS30‑for | 5′‑ATATATGGCGCGCCCGTGGCCACCATGGGATG-3′ |
| NS31‑rev | 5′-ATATATGGCGCGCCTCCCCAGCATGCCTGCTA-3′ |
| NS33-rev | 5′-GCCTGCCCAGAAGACTCC-3′ |
| P48-OH | 5′-GGCTCCTCAGAGAGCCTCGGCTAG-3′ |
| P49-OH | 5′-ACCGCGAAGAGTTTGTCCTCAACC-3′ |
| P287 | 5′-ACCAGGTTCGTTCACTCATGG-3′ |
| P288 | 5′-AGGCTAAGTGCCTTCTCTACAC-3′ |
| P400 | (5′‑CAGTAGTCCAGGGTTTCCTTGATG-3′) |
| P38-OH | (5′‑CATCAAGGAAACCCTGGACTACTG-3′) |
| P50-OH | (5′‑AAAGTCGCTCTGAGTTGTTAT-3′) |
| P51-OH | (5′‑GGAGCGGGAGAAATGGATATG-3′) |
Transfection, selection and isolation of ES-cells
Feeder-free cultivation of HM-1 ES-cells was performed according to protocols of Joyner42 and Magin et al.43 ES cells were passaged at least once after thawing and before electroporation. Subconfluent cells were harvested by trypsinization and ∼1 × 107 ES-cells were mixed with 20–30 μg of the linearized gene targeting construct in PBS and incubated on ice before electroporation. Electroporation was performed at 200 V and 500 μF using a Gene Pulser II (BioRad). Cells were kept on ice for 10 min after the pulse, transferred to medium and distributed to 4 10 cm cell culture dishes. Medium was changed daily and, starting from the second day after electroporation, a selection pressure of 200 μg/ml G418 was applied. Surviving ES cell colonies were isolated after 8–10 d of selection by trypsinization, transferred to a 96-well cell culture plate and screened for the transgene.
ES-cell screening by PCR
Positive ES-cell clones were identified by PCR analysis using primers P48-OH and P49-OH, which should result in a PCR product of ∼1.3 kb in size if homologous recombination of the transgene with the genome has occurred.
ES-cell screening by Southern Blot
In order to confirm the presence of the transgene on DNA level, positive clones were analyzed by Southern Blot. Genomic DNA of clones 7, 14 and 67 was digested after expansion with the restriction enzyme EcoRI in EcoRI-buffer (Fermentas) and transferred by Southern Blot to a Hybond-N Nylon membrane (Amersham, GE Healthcare) after separation of the fragments by gel electrophoresis. DNA fragments were immobilized by UV-crosslinking (UV-Stratalinker 2400, Stratagene) onto the membrane. The 5′ probe (∼600 bp PacI/EcoRI-fragment) was radioactively labeled using the Random Primed Labeling Kit (Roche) and hybridized with the Southern Blot membrane. After washing and exposition on Imager Screens, the radioactive signals were recorded using Phospho Imager (Cyclone Storage Phosphor System, Packard). For all PCR-positive clones, a signal was detected at the size of 7.15 kb that is expected if homologous recombination was successful (15 kb expected for wildtype). As wildtype and mutant signals from clone 67 were of different intensity, this appeared to be a mixed clone. Mouse generation was continued with clone 7.
Generation of chimeras by blastocyst injection
ES-cells of the positive clone 7 in M2 medium were injected in 3.5 day old blastocysts and morulae (C57BL/6 background), which had been obtained after superovulation by hormones followed by rinsing the uterus with M2 medium. On average, 12 ES cells per embryo were injected. For incubation at 37°C, 5% CO2 and saturated humidity, embryos were transferred to M16 medium. After a phase of recovery, surviving embryos were transferred in M2-medium into the uteri of pseudopregnant CD1-foster mothers. In total, 81 of the injected embryos were transferred into 6 foster mothers. Of the 17 pups from 5 litters, of which 12 remained, 5 chimeric animals were identified. Four of the 5 chimeric animals could be bred and included a 25% chimeric female and 3 × 100% chimeric males. The degree of chimerism was estimated according to the color of the fur, black originating from the recipient blastocyst and brownish or whitish fur originating from the ES-cells.
Establishing the mouse line iER-STOP-VCAM1
To test whether the transgene had been transmitted to the germline, the 4 chimeric animals were mated with C57BL/6 mice. Germline transmission after mating chimeras with C57BL/6 is indicated by a brownish color of the fur of the offspring. One of the chimeric males did not lead to offspring, but germline transmission was found in one of the remaining 2 chimeric males. The identity of the animal was confirmed by PCR analysis and Southern Blot. Genotyping via PCR was performed using the primers P50-OH, P51-OH and P38-OH, which results in a PCR product of 600 bp in length for the wildtype allele and a fragment of 250 bp in length for the recombinant allele. Southern Blot analysis with the 5′-probe (∼600 bp PacI/EcoRI fragment) confirmed germline transmission by a signal at the correct size of 7.15 kb for homologous recombination (wildtype 15 kb). The mouse line iER-STOP‑VCAM1 was established from this founder animal by continuous backcrossing to C57BL/6. All mice used in the experiments described here were in backcrossing generation N5 or N6.
Cre-deletion of the STOP-cassette for generation of the iER-VCAM1 mouse line
Deletion of the STOP-cassette was achieved by mating with females of the K14-Cre mouse line, which expresses Cre in its oocytes. In this way the STOP-cassette can be eliminated ubiquitously, although the allele for Cre does not necessarily have to be inherited. Offspring was genotyped by PCR for the Cre-allele, for the ROSA26 wildtype and mutant allele and for the presence or absence of the STOP-cassette. Genotyping for the Cre allele was performed using primers P287 and P288, which results in a PCR product sized 238 bp. The wildtype and mutant allele of the ROSA26 locus was identified using primers P50-OH, P51-OH and P38‑OH. The presence or absence of the STOP-cassette was distinguished using primers P400 and P51-OH, which result in a PCR-product of ∼1.7 kb in size if Cre-deletion was successful. Mice with successfully deleted STOP-cassette but without the allele for Cre (the female 8452 and the male 8455) were chosen to establish the iER-VCAM1 mouse line, which constitutively expresses the ER intrabody. The mouse line was established by continuous backcrossing with C57BL/6. Homozygous animals for experiments were obtained by intercrossings.
Preparation of cells from organs
Mice were handled according to the guidelines by FELASA. For analyses of cells from different tissues, mice were sacrificed and organs were prepared. Blood was taken from the heart and transferred to potassium-EDTA containing tubes (1.3 mL, Sarstedt). For bloodcounts 75 μL of blood were taken retrobulbar from living animals and transferred to tubes containing 5 μL of 0.1 M sodium-EDTA. Tissues were meshed with a syringe plug and meshed organs were filtered through a 70 μm pore size cell strainer (Falcon) with PBS. Cells were singled out by pipetting up and down, the cell suspension was filled up to 10 mL with PBS and cells were sedimented for 5 min at 500 × g. Sedimented cells were then resuspended in 20 mL erythrocyte lysis buffer (10× stock solution: 0.1 M KHCO3, 1.5 M NH4Cl and 1 mM EDTA), incubated for 5 min on ice and sedimented again for 5 min at 500 × g. Cells were then washed by resuspending pellets in 10 mL PBS and sedimented for 5 min at 500 × g. Pellets were then resuspended in PBS containing 1 μg/100 μL streptavidin (SA, SERVA) and incubated for at least 30 min on ice during counting of cells in order to deplete free biotin. Cells were counted using a counting chamber (Neubauer) and 1 × 106 cells per sample were transferred to flow cytometry tubes (Sarstedt). For blood samples, aliquots corresponding to 80–100 μL whole blood were used per sample. Erythrocyte lysis of blood was either performed before distribution of aliquots to flow cytometry tubes or was performed by adding 3.5 mL of erythrocyte lysis buffer per flow cytometry tube.
Flow cytometric analysis
Cells were kept on ice and centrifuged at 4°C during the whole staining procedure until measurement. Cells were washed with 3.5 mL FC-buffer (5 mM EDTA, 0.5% BSA in PBS) to remove SA from the solution. After sedimentation for 5 min at 500 × g cells were incubated with the first antibody (biotinylated anti-VCAM1 clone 429 from BD PharMingen, anti-VCAM MAB6434 from RnD Systems, anti-CD19-PE clone 1D3 from eBioscience, Isotype Rat IgG UNLB from Beckman Coulter, biotinylated Rat IgG2A Isotype control from BD PharMingen) for 1 h in a total volume of 100 μL. Cells were then washed twice with FC-buffer and subsequently incubated for 30 min in a total volume of 100 μL with either a secondary antibody (goat anti-Rat-APC from Dianova), SA‑FITC (Anaspec) or, if the first antibody was fluorescently labeled, then with FC-buffer only. Cells were washed with FC-buffer and resuspended in 500 μL FC-buffer before measurement.
Competition test anti-VCAM1 detection antibodies
HEK293T cells were maintained in DMEM (PAA) with 8% FCS (PAA) and 1% Penicillin/Streptomycin (PAA) and transfected with the VCAM1 expression plasmid pcDNA-VCAM1-739 (NS20-1). For transfection, 10 μg of DNA were mixed with 25 μL of a 1 mg/mL polyethylenimine solution (25 kDa linear polyethyleneimine from Polysciences, Warrington, PA, USA) in a total of 1 mL of serum-free DMEM medium (PAA) and incubated for 30 min at room temperature. The transfection mix was then added to the cells in a 10 cm cell culture dish. After 24 h, cells were detached with Trypsin/EDTA (PAA), counted and 4 × 105 cells were used per sample. Cells were washed with 3.5 mL FC-buffer (5 mM EDTA, 0.5% BSA in PBS). Sedimentation took place at 500 × g for 5 min at 4°C. Cells were then stained with a dilution series of 6C7.1 in the first staining step. Cells from samples that were destined for determining the amount of bound 6C7.1 cells were washed twice with 3.5 mL FC-buffer, and the mouse anti-Myc antibody clone Myc1-9E10 was added in a total volume of 100 μl for the second staining step. Cells from samples destined for testing whether binding of anti-VCAM1-biotin clone 429 is blocked by 6C7.1 were not washed, but 10 μl of a 1:10 dilution of the anti-VCAM1-biotin clone 429 (BD PharMingen) were directly added to the mixture of cells and 6C7.1 solution for the second staining step. Incubation took place for 1 h on ice for the second staining step. All samples were then washed twice with 3.5 mL FC-buffer and detection of 6C7.1 was then performed using an anti-Mouse APC conjugated antibody (Allophycocyanin-conjugated AffiniPure F(ab’)2 Fragment goat anti-Mouse IgG, Fc Fragment specific from Jackson ImmunoResearch), while detection of anti-VCAM1-biotin clone 429 was performed using SA-FITC (Anaspec). After incubation for 30 min on ice for the third staining step cells were washed twice with 3.5 mL FC-buffer again and resuspended in 500 μL FC-buffer for measurement.
Immunoblot
Cell pellets were resuspended in PBS and SDS was subsequently added to a final concentration of 5% (v/v) in order to lyse cells. The lysate was boiled at 95°C for 5 min and 1:1 diluted in 5x Laemmli buffer (50% v/v glycerol, 10% w/v SDS, 25% v/v β-mercaptoethanol, 20% v/v Tris-HCl pH 6.8, 0.05% w/v bromphenol blue). Lysate with Laemmli buffer was boiled again for 5 min at 95°C and samples were then analyzed using a 12% SDS-polyacrylamide gel and blotted to a methanol‑rinsed PVDF membrane (Carl Roth, Karlsruhe). The membrane was then incubated in 2–5% skimmed milk in PBS for 1 h or overnight for blocking protein‑free areas on the membrane. Incubation with the first antibody (9E10 and anti-Tubulin) took place for 1 h at RT and antibodies were diluted in 2% skimmed milk in PBS. ER intrabodies were detected via their myc-tag by the mouse anti-Myc antibody clone Myc1-9E10 and tubulin was detected as a loading control by the mouse anti-tubulin antibody T 6074 from Sigma. Blots were washed 3x for 5 min each with PBS 0.05% Tween 20 before incubation with the secondary antibody (anti-Mouse HRP conjugated, A0168 from Sigma). The membrane was then washed twice shortly with PBS and developed using a 1:1 mixture of a luminal enhancer solution and stable peroxide solution (SuperSignal West Pico, Pierce). The development of the signals was captured using the ChemiDoc imaging system (BioRad).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Werner Müller, Congcong Zhang, Marina Pils, Hermann Riedesel, Thomas Schirrmann, Michael Hust, Konrad Büssow, Sandra Düber, Dagmar Wirth and Thomas Böldicke for their support and helpful discussions. We thank Doris Meier for the introduction to bone marrow preparation and isolation of cells from mouse tissue. We are grateful for cell material from mice provided by Franz Vauti for initial tests to set up the staining procedure. We thank Petra Beyer for introduction to mouse sectioning and Lothar Gröbe for introduction to the flow cytometry device at HZI. The plasmid TVrosa26‑LMP1 was generously provided by Martin Hafner. We thank Susanne Etzrodt for breeding and tailing of mice. We thank Kirsten Kleemann and Janine Schreiber for assistance with taking blood samples.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Marschall AL, Frenzel A, Schirrmann T, Schüngel M, Dübel S. Targeting antibodies to the cytoplasm. MAbs 2011; 3:3-16; PMID:21099369; http://dx.doi.org/ 10.4161/mabs.3.1.14110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stocks M. Intrabodies as drug discovery tools and therapeutics. Curr Opin Chem Biol 2005; 9:359-65; PMID:15979379; http://dx.doi.org/ 10.1016/j.cbpa.2005.06.003 [DOI] [PubMed] [Google Scholar]
- 3.Marschall A, Zhang C, Frenzel A, Schirrmann T, Hust M, Perez F, Dubel S. Delivery of antibodies to the cytosol: debunking the myths. MAbs 2014; 6:943-56; PMID:24848507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Böldicke T. Blocking translocation of cell surface molecules from the ER to the cell surface by intracellular antibodies targeted to the ER. J Cell Mol Med 2007; 11:54-70; PMID:17367501; http://dx.doi.org/ 10.1111/j.1582-4934.2007.00002.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strebe N, Guse A, Schüngel M, Schirrmann T, Hafner M, Jostock T, Hust M, Müller W, Dübel S. Functional knockdown of VCAM-1 at the posttranslational level with ER retained antibodies. J Immunol Methods 2009; 341:30-40; PMID:19038261; http://dx.doi.org/ 10.1016/j.jim.2008.10.012 [DOI] [PubMed] [Google Scholar]
- 6.Zhang C, Helmsing S, Zagrebelsky M, Schirrmann T, Marschall AL, Schüngel M, Korte M, Hust M, Dübel S. Suppression of p75 neurotrophin receptor surface expression with intrabodies influences Bcl-xL mRNA expression and neurite outgrowth in PC12 cells. PLoS One 2012; 7:e30684; PMID:22292018; http://dx.doi.org/ 10.1371/journal.pone.0030684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelham HR. The dynamic organisation of the secretory pathway. Cell Struct Funct 1996; 21:413-9; PMID:9118249; http://dx.doi.org/ 10.1247/csf.21.413 [DOI] [PubMed] [Google Scholar]
- 8.Wilson D, Lewis M, Pelham H. pH dependent binding of KDEL to its receptor in vitro. J Biol Chem 1993; 268:7465-8; PMID:8385108 [PubMed] [Google Scholar]
- 9.Alam AK, Florey O, Weber M, Pillai RG, Chan C, Tan PH, Lechler RI, McClure MO, Haskard DO, George AJ. Knockdown of mouse VCAM-1 by vector-based siRNA. Transpl Immunol 2006; 16:185-93; PMID:17138052; http://dx.doi.org/ 10.1016/j.trim.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 10.Bridge AJ, Pebernard S, Ducraux A, Nicoulaz AL, Iggo R. Induction of an inteferon 638 response by RNAi vectors in mammalian cells. Nat Genet 2003; 34:263-4; PMID:12796781; http://dx.doi.org/ 10.1038/ng1173 [DOI] [PubMed] [Google Scholar]
- 11.Cao T, Heng BC. Commentary: intracellular antibodies (Intrabodies) versus RNA interference for therapeutic applications. Ann Clin Lab Sci 2005; 35:227-9; PMID:16081577 [PubMed] [Google Scholar]
- 12.Couzin J. Molecular biology. RNAi shows cracks in its armor. Science 2004; 306:1124-5; PMID:15539580; http://dx.doi.org/ 10.1126/science.306.5699.1124 [DOI] [PubMed] [Google Scholar]
- 13.Fish RJ, Kruithof EK. Short-term cytotoxic effects and long-term instability of RNAi 557 delivered using lentiviral vectors. BMC Mol Biol 2004; 5:9; PMID:15291968; http://dx.doi.org/ 10.1186/1471-2199-5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pebernard S, Iggo RD. Determinants of interferon-stimulated gene induction by RNAi vectors. Differentiation 2004; 72:103-11; PMID:15066190; http://dx.doi.org/ 10.1111/j.1432-0436.2004.07202001.x [DOI] [PubMed] [Google Scholar]
- 15.Sledz CA, Williams BR. RNA interference and double-stranded RNA activated pathways. Biochem Soc Trans 2004; 32:952-6; PMID:15506933; http://dx.doi.org/ 10.1042/BST0320952 [DOI] [PubMed] [Google Scholar]
- 16.Sledz CA, Holko M, de Veer MJ, Silverman RH, Williams BR. Activation of the 636 interferon system by short-interfering RNAs. Nat Cell Biol 2003; 5:834-9; PMID:12942087; http://dx.doi.org/ 10.1038/ncb1038 [DOI] [PubMed] [Google Scholar]
- 17.Sato M, Iwaya R, Ogihara K, Sawahata R, Kitani H, Chiba J, Kurosawa Y, Sekikawa K. Intrabodies against the EVH1 domain of Wiskott-Aldrich syndrome protein inhibit T cell receptor signaling in transgenic mice T cells. FEBS J 2005; 272:6131-44; PMID:16302976; http://dx.doi.org/ 10.1111/j.1742-4658.2005.05011.x [DOI] [PubMed] [Google Scholar]
- 18.Sawahata R, Sato M, Kitani H. Cytoplasmic expression and specific binding of the VHVL single domain intrabodies in transfected NIH3T3 cells. Exp Mol Pathol 2009; 86:51-6; PMID:19094983; http://dx.doi.org/ 10.1016/j.yexmp.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 19.Cardinale A, Biocca S. Can intrabodies be targeted to the secretory compartment interact with a cytosolic protein? A comment on the article by Sawahata et al “Cytoplasmic expression and specific binding of the VHVL single domain intrabodies in transfected NIH2T3 cells”, Exp. Mol. Pathol. 2008 Nov 27 ahead of print. Exp Mol Pathol 2009; 86:138; PMID:19348064; http://dx.doi.org/ 10.1016/j.yexmp.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 20.Kilger G, Needham LA, Nielsen PJ, Clements J, Vestweber D, Holzmann B. Differential regulation of alpha 4 integrin-dependent binding to domains 1 and 4 of vascular cell adhesion molecule-1. J Biol Chem 1995; 270:5979-84; PMID:7534304; http://dx.doi.org/ 10.1074/jbc.270.11.5979 [DOI] [PubMed] [Google Scholar]
- 21.Cybulsky MI, Fries JWU, Williams AJ, Sultan P, Eddy R, Byers M, Shows T, Gimbrone MA, Collins T. Gene structure, chromosomal location, and basis for alternative mRNA splicing of the human VCAM1 gene. Proc Natl Acad Sci U S A 1991; 88:7859-63; PMID:1715583; http://dx.doi.org/ 10.1073/pnas.88.17.7859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 1989; 59:1203-11; PMID:2688898; http://dx.doi.org/ 10.1016/0092-8674(89)90775-7 [DOI] [PubMed] [Google Scholar]
- 23.Pigott R, Dillon LP, Hemingway I, Gearing AJH. Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatant of cytokine activated culture endothelial cells. Biochem Biophys Res Comm 1992; 178:584-9; http://dx.doi.org/ 10.1016/0006-291X(92)91234-H [DOI] [PubMed] [Google Scholar]
- 24.McMurray RW. Adhesion molecules in autoimmune disease. Semin Arthritis Rheum 1996; 25:215-33; PMID:8834012; http://dx.doi.org/ 10.1016/S0049-0172(96)80034-5 [DOI] [PubMed] [Google Scholar]
- 25.Schreiner EP, Oberhauser B, Foster CA. Inhibitors of vascular cell adhesion molecule-1 expression. Expert Opin Ther Patents 2003; 13:149-66; http://dx.doi.org/ 10.1517/13543776.13.2.149 [DOI] [Google Scholar]
- 26.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos J-C, Connelly PW, Milstone DS. A major role for VCAM-1 but not ICAM-1 in early atherosclerosis. J Clin Invest 2001; 107:1255-62; PMID:11375415; http://dx.doi.org/ 10.1172/JCI11871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ou R, Zhang M, Huang L, Flavell RA, Koni PA, Moskophidis D. Regulation of immune response and inflammatory reactions against viral infection by VCAM-1. J Virol 2008; 82:2952-65; PMID:18216105; http://dx.doi.org/ 10.1128/JVI.02191-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu T-C. The role of vascular cell adhesion molecule-1 in tumor immune evasion. Cancer Res 2007; 67:6003-6; PMID:17616653; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwee L, Baldwin HS, Shen HM, Stewart CL, Buck C, Buck CA, Labow MA. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development 1995; 121:489-503; PMID:7539357 [DOI] [PubMed] [Google Scholar]
- 30.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007; 7:678-89; PMID:17717539; http://dx.doi.org/ 10.1038/nri2156 [DOI] [PubMed] [Google Scholar]
- 31.Simmons PJ, Masinovsky B, Longenecker BM, Berenson R, Torok-Storb B, Gallatin WM. Vascular cell adhesion molecule-1 expressed by bone marrow stromal cells mediates the binding of hematopoietic progenitor cells. Blood 1992; 80:388-95; PMID:1378318 [PubMed] [Google Scholar]
- 32.Leuker CE, Labow M, Muller W, Wagner N. Neonatally induced inactivation of the vascular cell adhesion molecule 1 gene impairs B cell localization and T cell-dependent humoral immune response. J Exp Med 2001; 193:755-68; PMID:11257141; http://dx.doi.org/ 10.1084/jem.193.6.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forster I, Vieira P, Rajewsky K. Flow cytometric analysis of cell proliferation dynamics in the B cell compartment of the mouse. Int Immunol 1989; 1:321-31; PMID:2518724; http://dx.doi.org/ 10.1093/intimm/1.4.321 [DOI] [PubMed] [Google Scholar]
- 34.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med 1991; 173:1213-25; PMID:1827140; http://dx.doi.org/ 10.1084/jem.173.5.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 1999; 21:70-1; PMID:9916792; http://dx.doi.org/ 10.1038/5007 [DOI] [PubMed] [Google Scholar]
- 36.Zwartz G, Chigaev A, Foutz T, Larson RS, Posner R, Sklar LA. Relationship between molecular and cellular dissociation rates for VLA-4VCAM-1 interaction in the absence of shear stress. Biophys J 2004; 86:1243-52; PMID:14747358; http://dx.doi.org/ 10.1016/S0006-3495(04)74198-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Twiss JL, Wada HG, Fok KS, Chan SD, Verity AN, Baxter GT, Shooter EM, Sussman HH. Duration and magnitude of nerve growth factor signaling depend on the ratio of p75LNTR to TrkA. J Neurosci Res 1998; 51:442-53; PMID:9514198; http://dx.doi.org/ 10.1002/(SICI)1097-4547(19980215)51:4%3c442::AID-JNR4%3e3.0.CO;2-C [DOI] [PubMed] [Google Scholar]
- 38.Berger JR. Natalizumab and progressive multifocal leucoencephalopathy. Ann Rheum Dis 2006; 65 Suppl 3:iii48-53; PMID:17038473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med 2005; 353:369-74; PMID:15947079; http://dx.doi.org/ 10.1056/NEJMoa051782 [DOI] [PubMed] [Google Scholar]
- 40.Dansky HM, Courtenay BB, Lominska C, Sikes JL, Kao C, Weinsaft J, Cybulsky MI, Smith JD. Adhesion of monocytes to arterial endothelium and initiation of atherosclerosis are critically dependent on vascular cell adhesion molecule-1 gene dosage. Arterioscler Thromb Vasc Biol 2001; 21:1662-7; PMID:11597942; http://dx.doi.org/ 10.1161/hq1001.096625 [DOI] [PubMed] [Google Scholar]
- 41.Bradbury AR, Sidhu S, Dübel S, McCafferty J. Beyond natural antibodies: the power of in vitro display technologies. Nat Biotechnol 2011; 29:245-54; PMID:21390033; http://dx.doi.org/ 10.1038/nbt.1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joyner AL. Gene Targeting: A Practical Approach. Oxford University Press, 2000; 2nd edition; ISBN-13: 978-0199637928; ISBN-10: 019963792X. [Google Scholar]
- 43.Magin TM, McWhir J, Melton DW. A new mouse embryonic stem cell line with good germ line contribution and gene targeting frequency. Nucleic Acids Res 1992; 20:3795-6; PMID:1641353; http://dx.doi.org/ 10.1093/nar/20.14.3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


