Abstract
Prenatal maternal psychological distress increases risk for adverse infant outcomes. However, the biological mechanisms underlying this association remain unclear. Prenatal stress can impact fetal epigenetic regulation that could underlie changes in infant stress responses. It has been suggested that maternal glucocorticoids may mediate this epigenetic effect. We examined this hypothesis by determining the impact of maternal cortisol and depressive symptoms during pregnancy on infant NR3C1 and BDNF DNA methylation. Fifty-seven pregnant women were recruited during the second or third trimester. Participants self-reported depressive symptoms and salivary cortisol samples were collected diurnally and in response to a stressor. Buccal swabs for DNA extraction and DNA methylation analysis were collected from each infant at 2 months of age, and mothers were assessed for postnatal depressive symptoms. Prenatal depressive symptoms significantly predicted increased NR3C1 1F DNA methylation in male infants (β = 2.147, P = 0.044). Prenatal depressive symptoms also significantly predicted decreased BDNF IV DNA methylation in both male and female infants (β = −3.244, P = 0.013). No measure of maternal cortisol during pregnancy predicted infant NR3C1 1F or BDNF promoter IV DNA methylation. Our findings highlight the susceptibility of males to changes in NR3C1 DNA methylation and present novel evidence for altered BDNF IV DNA methylation in response to maternal depression during pregnancy. The lack of association between maternal cortisol and infant DNA methylation suggests that effects of maternal depression may not be mediated directly by glucocorticoids. Future studies should consider other potential mediating mechanisms in the link between maternal mood and infant outcomes.
Keywords: BDNF, depression, DNA methylation, early life adversity, fetal programming, NR3C1
Abbreviations
- BDNF
gene encoding the brain-derived neurotrophic factor protein
- BPA
bisphenol A
- GR
glucocorticoid receptor
- HPA
hypothalamic-pituitary adrenal
- NR3C1
gene encoding the glucocorticoid receptor
Introduction
Psychological wellbeing during pregnancy can have long-term implications for offspring mental and physical health.1-4 In particular, psychological distress, which encompasses feelings of anxiety, depression, and perceived stress, increases risk for a number of adverse offspring outcomes,5-9 including premature birth,10 behavioral difficulties in childhood,5 and psychiatric disorder in adulthood.7,11 However, the mechanism(s) that account for associations between prenatal distress and health outcomes are unclear.
The prevailing mechanistic theory in perinatal psychiatry to account for mood-associated effects in offspring is via alterations of the maternal hypothalamic-pituitary-adrenal (HPA) axis during pregnancy.1,4 Indeed, symptoms of prenatal depression have been associated with increased diurnal salivary cortisol,12 and exaggerated cortisol responses to an acute stressor.13 Moreover, enduring changes in fetal DNA methylation have been proposed to be involved in entraining the fetal HPA axis in response to maternal distress.4,14 Epigenetic variation, including DNA methylation changes in brain regions involved in the regulation of HPA activity, have been observed in animal studies following prenatal exposure to stress,15 maternal separation,16 and in response to variation in mother-infant interactions,17,18 with implications for the HPA response to stress. In particular, increased DNA methylation in the promoter region of the gene encoding the glucocorticoid receptor (Nr3c1) in the hippocampus has been observed, and may account for heightened glucocorticoid levels in offspring.19
The glucocorticoid receptor (GR) plays a critical role in HPA responses to stress through negative feedback on glucocorticoid release. Increased DNA methylation within the Nr3c1 gene results in decreased expression of hippocampal GR, a dampening of negative feedback, and prolonged increase in circulating glucocorticoid levels.19 In rodents, offspring that experience low compared to high levels of postnatal maternal care have increased hippocampal Nr3c1 DNA methylation and reduced Nr3c1 expression. Cross-fostering studies have confirmed that these epigenetic changes emerge in response to maternal care during the postnatal period, and pharmacological studies have indicated that shifts in hippocampal Nr3c1 DNA methylation can modulate the behavioral and physiological response to stress.20 Although the epigenetic effects of maternal care are not restricted to Nr3c1,21,22 this target gene may be particularly relevant to understanding the coordinated biological response observed following exposure to early life adversity.
Translational studies examining the association between exposure to early life stress and NR3C1 exon 1F DNA methylation in humans have generated findings consistent with animal studies using both post-mortem brain samples and peripheral tissues.23-25 Increased NR3C1 exon 1F methylation and decreased GR mRNA have been detected in the hippocampus of suicide victims with a history of childhood abuse.23 Increased NR3C1 1F DNA methylation has also been detected in DNA extracted from whole blood samples from young adolescents exposed to physical abuse in childhood,24 and adolescents exposed to stressful life events or trauma.25 This susceptibility to alterations in NR3C1 DNA methylation is also observed in response to prenatal distress. Increased NR3C1 1F DNA methylation has been found in cord blood of infants exposed to maternal depressed mood during pregnancy and this altered epigenetic state was predictive of stress responses in these infants at 3 months of age.26 Increased NR3C1 1F DNA methylation has also been observed in whole blood of young adolescents whose mothers had experienced intimate partner violence during pregnancy.27
Although gene targets associated with the HPA response to stress have predominated epigenetic studies of the impact of early life adversity, the complex behavioral phenotypes that emerge suggest the importance of genes involved in brain development and neuroplasticity, such as brain derived neurotrophic factor (BDNF). BDNF plays an essential role in neurodevelopment and has been linked to psychiatric risk.28 In animal studies, maternal stress during pregnancy induces increased bdnf promoter IV DNA methylation and decreased BDNF expression in the amygdala and hippocampus in offspring that persist into adulthood.29 Persistent epigenetic changes in bdnf, within the cortex of offspring, are also observed in response to abusive caregiving during the postnatal period.29-32 We have recently found that increased BDNF promoter IV DNA methylation in peripheral blood may be a significant biomarker of prenatal adversity that is relevant for both animals and humans.33 However, the impact of prenatal psychological distress on infant BDNF IV DNA methylation has yet to be explored.
The role of epigenetic variation in mediating the effects of early life adversity is an evolving area of study. In the current study, we determine whether exposure to maternal prenatal depression predicts increased infant NR3C1 IF DNA methylation in buccal cells. It has been suggested that biological markers of distress in pregnancy may be more reliable predictors of adverse infant outcomes than self-reported psychological measures.34 Here, we assess whether maternal prenatal cortisol may be a more accurate predictor of infant NR3C1 1F DNA methylation than a self-reported measure of depression. A further aim of this study is to investigate, for the first time in a human cohort, whether depressed mood during pregnancy may have implications for infant BDNF IV DNA methylation.
Results
Sample characteristics
The demographic characteristics of this sample are presented in Table 1. All mothers in this study were primiparous; average age was 32.04 (SD 4.35). This primarily Caucasian (89.5%) group of women was highly educated (49.1% had a postgraduate degree). No participants reported smoking cigarettes during pregnancy; however, 21.1% reported consuming 1-5 units of alcohol per week. No participants were using antidepressant or steroid-based medications while pregnant. A total of 89.5% of participants reported that their pregnancy had been planned, and 29.8% reported a previous history of mental health disorders. There were no significant differences between the demographic characteristics of the control group (n = 37) and depression-symptom group (n = 20). The groups only differed on prenatal and postnatal EPDS score; with a significantly higher EPDS score in the depression-symptom group compared to the control group [Table 1; (t(55) = 2.297, P < 0.001) and (t(55) = −2.469, P < 0.05), respectively]. There were no group differences in prenatal salivary cortisol responses to the infant distress stimulus (Table 1; t(55) = 1.097, P = 0.277) or log AUC of diurnal cortisol (Table 1; t(55) = −1.471, P = 0.147). Days-of-gestation was not correlated with either log AUC of diurnal cortisol (r = 0.224, P = 0.107), or mean cortisol change in response to the stressor (r = 0.026, P = 0.849).
Table 1.
Maternal demographic and salivary cortisol variables
| Demographic Variables | Control Group (N = 37) | Depressive symptom group (N = 20) |
|---|---|---|
| Age (mean, SD) | 32.14 (4.33) | 31.85 (4.50) |
| Education (n, %) | ||
| A-level | 1 (2.7) | 0 |
| Undergraduate degree | 16 (43.2) | 9 (45) |
| NVQ | 2 (5.4) | 1 (5) |
| Postgraduate degree | 18 (48.6) | 10 (50) |
| Ethnicity (n, %) | ||
| Caucasian | 35 (84.6) | 16 (80) |
| Black | 0 | 1 (5) |
| Asian | 1 (2.7) | 3 (15) |
| Mixed Race | 1 (2.7) | 0 |
| Alcohol units/week (n, %) | ||
| None | 28 (75.7) | 17 (85) |
| 1–5 | 9 (24.3) | 3 (15) |
| Cigarettes/week (n, %) | ||
| None | 33 (89.2) | 18 (90) |
| Did not respond | 4 (10.8) | 2 (10) |
| Weeks of gestation (mean, SD) | 28.4 (12.4) | 29.9 (6.67) |
| Planned Pregnancy (n, %) | 33 (89.2) | 18 (90) |
| Previous history of mental health problems (n, %) | 10 (27) | 7 (35) |
| Prenatal EPDS (depression) | 4.68 (2.82) | 12.2 (2.12) |
| Postnatal EPDS (depression) | 6.22 (3.30) | 8.95 (4.96) |
| Salivary Cortisol Variables (nmol/l) | ||
| Mean change in response to stressor (mean, range) | −0.54 (−8.63 − 5.66) | −1.13 (−4.13 − 3.07) |
| Log AUC diurnal cortisol (mean, range) | 15.81 (6.70 − 25.66) | 17.79 (6.61 − 31.32) |
| EPDS: Edinburgh Postnatal Depression Scale, NVQ: National Vocational Qualification |
Of the 57 infants included in this study, 25 (43.9%) were male and 32 (56.1%) were female. Mean birth weight was 3.33 kg (SD = 0.64), and 21 mothers (36.8%) reported delivery complications. Mothers who experienced prenatal depressive symptoms reported significantly more delivery complications than controls (x2(1) = 4.0657, P < 0.05). Examples of reported delivery complications include the development of preeclampsia resulting in an induced birth, signs of fetal distress leading to an assisted delivery, and a retained placenta. There were no significant differences between women who did and did not report delivery complications on measures of prenatal cortisol or postnatal depression.
Maternal prenatal depressive symptoms and infant NR3C1 1F and BDNF IV DNA methylation
None of the maternal demographic variables, including prenatal depression, significantly correlated with DNA methylation at any CpG site. In the regression analyses, mean DNA methylation of all 10 CpG sites within NR3C1 1F was first used as the outcome variable. There was a significant effect of the prenatal depression*gender interaction to predict mean NR3C1 1F DNA methylation (β = −0.350, P = 0.017); see Table 2. To investigate this interaction further, the data was split by gender, and the regression model repeated. For male infants only, prenatal depression significantly predicted mean NR3C1 DNA methylation (β = 2,147, P = 0.044), such that those infants exposed to prenatal depressive symptoms had increased NR3C1 1F DNA methylation across the 10 CpG sites. These results are shown graphically in Figure 1. The regression model was reconstructed using percentage methylation at each individual CpG site as the outcome variable. At CpG sites 2 and 9, a prenatal group*gender interaction also significantly predicted DNA methylation [(β = −0.285, P < 0.05) and (β = −0.330, P < 0.05) respectively]. The data was split by gender and the model was repeated for the 2 sites. At CpG2, the effect of prenatal depression approached significance for males (β = 0.395, P = 0.067), but not females (β = −0.239, P = 0.231). At CpG9 the effect of prenatal depression in predicting DNA methylation in the females approached significance (β = −0.360, P = 0.066), but was not significant for the males (β = 0.288, P = 0.191).
Table 2.
Regression model using prenatal depression and infant gender to predict infant NR3C1 IF and BDNF IV methylation
| Unstandardized coefficients |
|||||||
|---|---|---|---|---|---|---|---|
| Model | B | Std. error | Standardized coefficients Beta | t | Sig. | ||
| Outcome: Mean NR3C1 1F methylation | |||||||
| 1 | Batch number | 0.238 | 0.234 | 0.167 | 1.208 | 0.233 | |
| Postnatal depression | 0.338 | 0.40 | 0.117 | 0.845 | 0.402 | ||
| 2 | Batch number | 0.263 | 0.241 | 0.155 | 1.089 | 0.282 | |
| Postnatal depression | 0.352 | 0.432 | 0.122 | 0.815 | 0.419 | ||
| Prenatal depression | 0.066 | 0.184 | 0.053 | 0.36 | 0.720 | ||
| Infant gender | 0.069 | 0.185 | 0.054 | 0.372 | 0.711 | ||
| 3 | Batch number | 0.157 | 0.233 | 0.093 | 0.676 | 0.502 | |
| Postnatal depression | 0.427 | 0.412 | 0.148 | 1.037 | 0.305 | ||
| Prenatal depression | −0.024 | 0.179 | −0.019 | −0.132 | 0.896 | ||
| Infant gender | 0.114 | 0.177 | 0.089 | 0.642 | 0.524 | ||
| Prenatal depression*gender | −0.435 | 0.176 | −0.350 | −2.475 | 0.017 | ||
| Outcome: Mean BDNF IV methylation | |||||||
| 1 | Batch number | 0.114 | 0.217 | 0.072 | 0.524 | 0.602 | |
| Postnatal depression | −0.405 | 0.382 | −0.145 | −1.061 | 0.294 | ||
| 2 | Batch number | 0.253 | 0.205 | 0.16 | 1.232 | 0.224 | |
| Postnatal depression | −0.402 | 0.376 | −0.144 | −1.069 | 0.290 | ||
| Prenatal depression | −0.419 | 0.164 | −0.336 | −2.565 | 0.013 | ||
| Infant gender | −0.322 | 0.158 | −0.271 | −2.034 | 0.047 | ||
| 3 | Batch number | 0.274 | 0.209 | 0.173 | 1.31 | 0.196 | |
| Postnatal depression | −0.421 | 0.38 | −0.151 | −1.107 | 0.274 | ||
| Prenatal depression | −0.427 | 0.165 | −0.343 | −2.59 | 0.013 | ||
| Infant gender | −0.296 | 0.165 | −0.249 | −1.796 | 0.079 | ||
| Prenatal depression*gender | 0.100 | 0.162 | 0.084 | 0.620 | 0.538 | ||
Figure 1.

Percent methylation at each CpG site (1–10) within the examined NR3C1 1F region for the depression-exposed and control infants. (A) shows the values for the male infants and (B) the female infants.
A similar model was constructed using mean BDNF IV DNA methylation across the 5 assessed CpG sites as the outcome variable. Here, prenatal depression significantly predicted mean BDNF promoter IV DNA methylation (β = −2.590, P = 0.013); however, there was no significant effect of gender (β = −1.796, P = 0.079) or a prenatal depression*gender interaction (β = 0.620, P = 0.538); see Table 2. This model was reconstructed using % DNA methylation at each CpG site as the outcome variable. Prenatal depression significantly predicted % DNA methylation at CpG3 only (β = −3.244, P = 0.002), such that those infants exposed to antenatal depression had decreased methylation at CpG3. These results are presented graphically in Figure 2.
Figure 2.
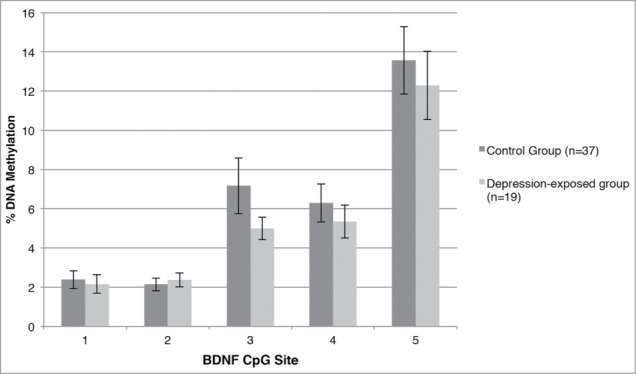
Percent methylation at each CpG site within the examined BDNF IV region for the depression-exposed and control infants.
Maternal prenatal cortisol and infant NR3C1 1F and BDNF IV DNA methylation
Two measures of maternal cortisol were available: mean change in response to a stressor and the log AUC for diurnal cortisol. Regression models were constructed as previously described, but each measure of maternal cortisol was used in the place of prenatal depression, and days-of-gestation was entered as a covariate. Neither measure of cortisol significantly predicted mean infant NR3C1 IF DNA methylation (mean change: β = 0.219, P = 0.828; log AUC cortisol: β = 0.296, P = 0.769), and neither did a cortisol*gender interaction (mean change: β = 1,227, P = 0.226; log AUC cortisol: β = 0.436, P = 0.665).
Similarly, neither cortisol measure significantly predicted mean BDNF promoter IV DNA methylation (mean change: β = −0.639, P = 0.532; log AUC cortisol: β = −0.069, P = 0.945), and neither did a cortisol*gender interaction (mean change: β = 1.345, P = 0.185; log AUC cortisol β = −1.633, P = 0.109).
Discussion
Maternal depression is predictive of adverse psychiatric and physical outcomes in offspring7-10 and it has been speculated that elevated maternal glucocorticoids may induce these effects.1,4,14 Moreover, there is increasing evidence for the role of epigenetic variation in the lasting consequences of early life adversity19,22,23,26 In the current study, we find a sex-specific effect of maternal prenatal depressive symptoms on DNA methylation within the NR3C1 gene, with elevated NR3C1 1F DNA methylation in male, but not female, infants exposed to maternal prenatal depression. Analysis of the BDNF promoter IV region revealed that both male and female infants exposed to prenatal depression had decreased DNA BDNF IV methylation. However, for both NR3C1 and BDNF, maternal cortisol did not predict infant DNA methylation changes.
Variation in DNA methylation within the NR3C1 1F region has been reported in both animal15,18,19 and human23-27 studies, suggesting that this epigenetic marker is developmentally sensitive to the quality of the environment. These studies have consistently reported increased DNA methylation of NR3C1 1F and our findings compliment previous reports examining the epigenetic impact of maternal depressed mood during pregnancy.26 While previous studies have indicated increased NR3C1 1F DNA methylation in cord blood samples at birth, here we illustrate that increased NR3C1 1F DNA methylation associated with prenatal depression persists into the postnatal period in infant buccal cells, independent of postnatal depressed maternal mood, and is particularly evident in male infants. Previous studies in humans have not examined the question of sex differences in environmentally-induced NR3C1 1F DNA methylation,23-27 and although animal studies have primarily examined these effects exclusively in males, there is emerging evidence suggestive of a particular epigenetic vulnerability in males in response to prenatal33 and postnatal35 experiences. These findings compliment a body of literature, which suggests that males may more be at risk of adverse outcomes compared to females as a result of exposure to maternal distress during pregnancy.36-40 Therefore, sex differences in NR3C1 DNA methylation could, in part, explain stronger associations between prenatal mood disturbance and more adverse outcomes in males. Importantly, the differentially methylated region of NR3C1 contains the binding site for the transcription factor NGFI-A (Fig. 1; CpG sites 8 and 9) and is directly involved in the DNA methylation-mediated regulation of NR3C1 expression.23 Although it is unlikely that DNA methylation differences occurring at a single gene could account for the increased risk for complex outcomes in later life, our findings suggest that epigenetic disruption at this locus may be functionally relevant to the impact of maternal depression on offspring development.
Previous studies in animals have suggested that increased bdnf IV DNA methylation emerges as a consequence of elevated prenatal stress29 and Bisphenol A (BPA) exposure.16 In contrast, we report that in human infants, exposure to prenatal depression results in decreased infant BDNF IV methylation at CpG3. This CpG site is adjacent to the binding site of the transcription factor CREB (Fig. 1), which controls BDNF transcription via DNA methylation-dependent mechanism.41 Although species differences in the regulation of this gene may account for our findings, recent analyses indicate increased DNA methylation in human cord blood samples within the CREB binding site associated with elevated in utero BPA exposure, which is consistent with the direction of reported effects in rodents.33 An alternative explanation for these findings may involve the biphasic epigenetic changes that have been observed to occur across development.42
The decreased BDNF methylation we observe in infants exposed to prenatal depression could reflect a molecular basis for the rapid maturation that may be induced by adverse prenatal events. BDNF is a critical growth factor involved in neurogenesis, and so the timing of regulation and expression of BDNF serves as a marker of developmental time. Stress during pregnancy induces an increased risk of preterm delivery43 and may lead to an advanced pace of neurodevelopment.44-46 Consistent with this, children who have experienced maternal deprivation in early life have been shown to have mature connectivity between the amygdala and medial prefrontal cortex that is comparable to an adolescent phenotype,47 suggestive of more rapid brain development. Further exploration of this hypothesis from both a molecular and neurodevelopmental level may provide insights into the mechanisms that underlie adversity-induced changes in brain maturation.
A prevailing theory in the field of perinatal psychiatry is that prenatal depression impacts on fetal development via increased circulating maternal glucocorticoids.1,4,34,48 However, it is important to note that investigations of maternal depression and cortisol in pregnancy have reported mixed results.12,13,49-54 Nonetheless, it has been suggested that biological markers of prenatal stress may be better indicators of offspring outcomes than self-report measures.34 Thus, we expected that maternal cortisol would significantly predict infant NR3C1 IF and BDNF IV methylation. However, we found no evidence to support this hypothesis. Moreover, in our cohort, maternal depressive symptoms were not correlated with elevated diurnal or stress-induced glucocorticoid levels. The lack of group differences in glucocorticoid levels and the relatively low variability of cortisol levels between individuals may account for this finding. However, this initial investigation suggests that perhaps maternal cortisol does not account for the association between prenatal depression and changes in infant DNA methylation, and future studies should consider other potential mediating pathways, such as changes in maternal immune activation.
The prospective longitudinal design and the inclusion of both biological and psychological measures of maternal prenatal distress as predictors of infant methylation are significant strengths of this study. This is also the first study to replicate the previous finding of increased NR3C1 DNA methylation in response to maternal depression26 using buccal tissue, which has significant implications for assessing epigenetic effects in larger cohorts. However, a number of limitations should be considered. It is unclear how epigenetic variation in the periphery (i.e., blood, buccal cells) relates to epigenetic variation within the brain. Studies of consistency of methylation profiles across peripheral tissues have produced conflicting results, but are generally limited by small sample sizes and a candidate gene approach.55,56 Recently, Davis and colleagues (2012) have analyzed DNA methylation patterns in human blood and post-mortem brain tissue and found that between-tissue variation in DNA methylation greatly exceeded between-individual differences.57 The current study is also limited by the modest sample size and reliance on self-reported mood rather than on a clinical diagnosis. Previous studies of prenatal depression have suggested that clinical diagnoses correlate more reliably with biological markers of depression, such as awakening cortisol, than do self-report measures.12 Therefore, using clinical diagnoses to define the depressive symptom group may have revealed a potential relationship between depressed mood, maternal glucocorticoids, and epigenetic outcomes.
Furthermore, it is likely that a number of postnatal environmental influences, such as parental care, abuse or sleep disturbance, may have implications for infant DNA methylation. Unfortunately, these measures were not available in the current dataset. However, future studies should comprehensively document postnatal environmental variables, to both control for postnatal effects but also in order to fully understand potential mechanisms by which early environmental factors impact on infant DNA methylation. Further, assessments of infant and childhood behavior are required fully disseminate the role of epigenetic mechanisms in mediating associations between prenatal depressed mood and offspring outcomes.
In conclusion, this study found that antenatal symptoms of depression have implications for changes in infant NR3C1 1F and BDNF IV DNA methylation. Conversely, measures of maternal cortisol were not related to infant DNA methylation. Thus, these initial findings suggest that maternal glucocorticoids may not mediate the association between antenatal depression and infant epigenetic regulation. Future studies should consider other potential mediating pathways that likewise contribute to coordinated physiological and behavioral responses to the environment, such as the maternal immune system and sympathetic nervous system activity.
Methods and Materials
Participants
The participants (N = 57) were derived from a larger cohort of pregnant women participating in a longitudinal study based in Oxford, UK. This study was designed to investigate the effects of antenatal mood disturbance on maternal and infant stress responses. This study was reviewed and approved by the South Central Oxford B Research Ethics Committee (REF: 12/SC/0473), and all participants provided informed consent.
Procedures
Participants were assessed during either the second or third trimester of pregnancy. Self-reported symptoms of depression were obtained using a paper-based questionnaire (Edinburgh Postnatal Depression Scale) and maternal salivary cortisol samples were collected in 2 contexts: in response to a stressor (infant distress video) and diurnally. Participants were visited at home approximately 2 months after birth. Self-report measures of postnatal depression from the mother and 2 buccal swabs from each infant were collected.
Measures
Maternal mood
Edinburgh Postnatal Depression Scale (EPDS). The EPDS is the most widely used self-report questionnaire to identify symptoms of depression during the peripartum period. The scale consists of 10 items that describe common symptom of depression; however, it does not include somatic symptoms of depression, such as change in appetite, which are commonly experienced in pregnancy. Each item is scored from 0 to 3, with a maximum score of 30. A cut-off score of 10 is frequently used to identify a group ‘at risk’ of depression.58-62 A recent study has shown that using a cut-off of 10 in the second and third trimester of pregnancy provides a good balance between sensitivity (70-79%) and specificity (96-97%).62
Maternal prenatal cortisol
Response to infant distress stimulus. During either the second or third trimester, participants were invited to an afternoon test session between the hours of 2 P.M. and 7 P.M., and asked to watch a short film depicting distressed young infants, all under the age of 6 months. The film was 6 minutes in length and included 8 short clips of crying infants. The clips were taken from online sources with permission from the owners. This video has been used in a previous study13 and found to induce significant salivary cortisol responses in a group of late first/early second trimester pregnant women with symptoms of depression. Saliva samples were collected at 5 time points during the test session using saliva collection aids and plastic cryovials (Salimetrics, UK). Two samples were taken before the film, approximately 20 minutes apart. The third sample was taken immediately post-film, and the fourth and fifth samples were taken 10 and 20 minutes post-film, respectively. Saliva samples were stored at −20°C until analysis. Cortisol mean-change in response to the stressor was used as a main predictor in these analyses. This variable was calculated by subtracting the mean baseline cortisol measure from the 20-minute post-stimulus measure. Notably, there were no differences between the control and depressive-symptom group in baseline cortisol.
Diurnal cortisol. Participants were asked to collect 6 saliva samples at home over 2 working days (3 per day) within 2 weeks of the test session. On each day samples were collected immediately after awakening, 30 minutes and 12 hours post-awakening. Participants were provided with a stamped addressed envelope to return the samples, which were stored at −20°C until analysis. The area under the curve of the diurnal cortisol data was skewed to the left, and was therefore log transformed (log AUC), and log AUC was used as a main predictor in this study.
Cortisol assay
Salivary cortisol analysis was carried out by a direct double-antibody radioimmunoassay (RIA) with utilization of 125I-cortisol as the ligand, in accordance with the manufacturer's instructions (Salimetrics Inc., UK).
Infant NR3C1 1F and BDNF IV methylation
Buccal swab collection
Infants were on average 53.6 days old (SD = 9.99, range = 26-98 days) when the buccal swabs were collected. The buccal swabs were obtained using Catch-All soft sample swabs (Epicentre Ltd), which were firmly bushed across the inside of the infant's cheek, and stored in sterilized 2 ml tubes. Samples were stored at −20°C at the Department of Psychiatry, Oxford, before being shipped to the Department of Psychology at Columbia University on dry ice (World Courier Ltd), where they were stored at −80°C until analysis.
DNA isolation
Buccal cell DNA isolation was performed using a QIAamp DNA mini kit (Qiagen Ltd, USA) in accordance with the manufacturer's protocol, and the buccal DNA was quantified using a Nanodrop 1000 spectrophotometer (Thermo Scientific Ltd, USA). The extracted DNA was in a range of 6-31 ng/μl.
Bisulfite pyrosequencing
DNA methylation at specific CpG sites was analyzed using the quantitative bisulfite-pyrosequencing method. The extracted buccal DNA (125 ng) was bisulfite converted using an EpiTect Bisulphite kit (Qiagen Ltd, USA) according to the manufacturer's instructions, and stored at −20°C until further analysis. A PyroMark PCR kit (Qiagen Ltd, USA) and PCR primers specific for NR3C1 and BDNF were used to obtain biotinylated PCR products and a specific sequencing primer was then used to determine CpG methylation in the regions of interest. Two separate assays were used to assess 10 CpG sites within NR3C1 exon 1F, and one assay was used to assess 5 CpG sites within BDNF promoter IV (Fig. 3). Details of the primers used are available in Table 3. The PCR and pyrosequencing primers were designed using PyroMark Assay Design Software 2.0. The regions of interest within NR3C1 and BDNF were chosen based on previous research that has identified these areas as susceptible to epigenetic regulation following early exposure to adverse environmental influences.23,26,28,33 Pyrosequencing was performed using a PyroMark Q24 pyrosequencer (Qiagen Ltd, USA) with specific pyrosequencing primers. Before sample analysis, each pyrosequencing assay was validated using standard curves by analyzing 0, 20, 40, 60, 80, and 100% methylated human genomic DNA standards (Fig. S1). Those standards were generated by mixing commercially available unmethylated and hypermethylated DNA standards (EpiTect PCR Control DNA Set, Qiagen) in the following ratios: 0:5; 1:4; 2:3; 3:2; 4:1; and 5:0 (10 ng of total bisulfite-converted DNA). Following assay validation, 10 ng of each bisulfite-converted sample DNA was run in a 25-μL PCR reaction; 3 μL of a PCR product was run on a gel to confirm the band size and 20 μL was run on a pyrosequencer. The average DNA methylation levels of specific CpG sites were quantified using PyroMark Q24 2.0.4 software (Qiagen Ltd, USA).
Figure 3.
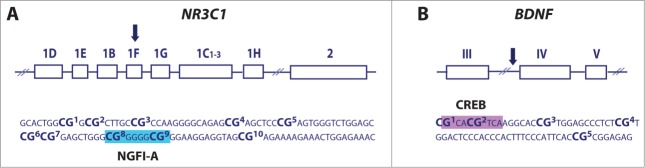
Schematic and the analyzed sequence of the NR3C1 (A) and BDNF (B) gene. Shown are specific CpG sites analyzed using bisulfite-pyrosequencing assays as well as NGFI-A (blue box) and CREB (purple box) binding sites within NR3C1 exon 1F and BDNF promoter IV, respectively.
Table 3.
PCR and pyrosequencing primers
| NR3C1 1F Assay 2 – CpG sites 5–1 - chr5:143,404,013–143,404,147* | |
| PCR primer forward- Biotinylated | /5Biosg/ GTTGTTATTAGTAGGGGTATTGG |
| PCR primer reverse | AACCACCCAATTTCTCCAATTTCTTTTC |
| Pyrosequencing primer (reverse) | CAACTCCCCCACTCCAAACCC |
| NR3C1 1F Assay 1 – CpG sites 6–10 - chr5:143,404,011–143,404,097* | |
| PCR primer forward | AGTTTTAGAGTGGGTTTGGAG |
| PCR primer reverse-Biotinylated | /5Biosg/ AAAACCACCCAATTTCTCCAATTTCTT |
| Pyrosequencing primer (forward) | GAGTGGGTTTGGAGT |
| BDNF IV – CpG sites 1–5 - chr11:27,701,519–27,701,826* | |
| PCR primer forward | GGGTTGGAAGTGAAAATATTTGTAAA |
| PCR primer reverse-Biotinylated | /5Biosg/CCCCATCAACCAAAAACTCCATTTAATCTC |
| Pyrosequencing primer (forward) | GGTAGAGGAGGTATTATATGATAG |
Genomic coordinates show genomic regions amplified by PCR and are based on the UCSC Genome Browser Human Dec. 2013 (GRCh38/hg38) Assembly.
Statistical analysis
For analysis, participants were split into 2 groups based on maternal EPDS score: those scoring 9 or below were the control group, whereas those scoring 10 or above were the ‘depressive symptom’ group. Independent samples t-tests and Pearson chi-squared tests were used to determine group differences in demographic characteristics and maternal prenatal mood. Pearson's correlations were used to assess the relationship between variables and DNA methylation at independent CpG sites, and multiple regression models were constructed to examine predictive models. Data were entered into each model in a stepwise fashion: batch number for DNA methylation analysis was entered at step 1 as a covariate, as was postnatal depression. The postnatal depression group (control vs. depressive-symptoms) was defined using the same criteria as the prenatal depression group. Main predictor variables were entered at step 2. Either prenatal depression or maternal cortisol was used as a main predictor, as was infant gender. Where prenatal cortisol was a main predictor, days of gestation at the point of cortisol collection was entered as a covariate. Interaction variables between infant gender and prenatal depression or cortisol were created using standardized or centered variables, and entered into step 3 of the model.
Disclosure of Potential Conflicts of Interest
Dr. Susannah E Murphy has received consultancy payments from p1Vital and has participated in paid speaking engagements for Lilly UK. Dr. Braithwaite, Dr. Kundakovic, Dr. Ramchandani and Professor Champagne report no financial disclosures or conflicts of interest.
Acknowledgments
We would like to thank all of the mothers and their infants who volunteered their time to take part in this study. We would also like to acknowledge Li Chen at the Department of Psychiatry, University of Oxford, for assaying the maternal saliva samples for cortisol.
Funding
This research was funded by a UK Medical Research Council Studentship awarded to Dr. Elizabeth Braithwaite (grant number MR/J500501/1). Dr. Susannah E Murphy is supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Center Program. Dr. Frances Champagne is supported by the National Institute of Mental Health (grant number 1R01MH092580-01A1).
Supplemental Material
References
- 1.Glover V. Annual research review: prenatal stress and the origins of psychopathology: an evolutionary perspective. J Child Psychol Psychiatry 2011; 52:356-67; PMID:21250994; http://dx.doi.org/ 10.1111/j.1469-7610.2011.02371.x [DOI] [PubMed] [Google Scholar]
- 2.Gitau R, Fisk NM, Glover V.. Maternal stress in pregnancy and its effect on the human foetus: an overview of research findings. Stress 2001; 4:195-203; PMID:22432140[http://dx.doi.org/ 10.3109/10253890109035018 [DOI] [PubMed] [Google Scholar]
- 3.Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ.. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry 2010; 67:1012-24; PMID:20921117; http://dx.doi.org/ 10.1001/archgenpsychiatry.2010.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talge NM, Neal C, Glover V.. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry 2007; 48:245-61; PMID:17355398; http://dx.doi.org/ 10.1111/j.1469-7610.2006.01714.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Connor TG, Heron J, Glover V.. Antenatal anxiety predicts child behavioral/emotional problems independently of postnatal depression. J Am Acad Child Adolesc Psychiatry 2002; 41:1470-7; PMID:12447034; http://dx.doi.org/ 10.1097/00004583-200212000-00019 [DOI] [PubMed] [Google Scholar]
- 6.Van den Bergh BR, Mulder EJ, Mennes M, Glover V.. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev 2005; 29:237-58; PMID:15811496; http://dx.doi.org/ 10.1016/j.neubiorev.2004.10.007 [DOI] [PubMed] [Google Scholar]
- 7.Pearson RM, Evans J, Koundali D, Lewis G, Ramchandani PG, O'Connor TG, Stein A.. Maternal depression during pregnancy and the postnatal period: risks and possible mechanisms for offspring depression at 18 years. JAMA Psychiatry 2013; 70(12):1312-9; PMID:24108418; http://dx.doi.org/ 10.1001/jamapsychiatry.2013.2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buitelaar JK, Huizink AC, Mulder EJ, de Medina PGR, Visser GHA.. Prenatal stress and cognitive development and temperament in infants. Neurobiol Aging 2003; 24(Supplement 1):S53-S60; PMID:12829109; http://dx.doi.org/ 10.1016/S0197-4580(03)00050-2 [DOI] [PubMed] [Google Scholar]
- 9.Buss C, Davis EP, Muftuler LT, Head K, Sandman CA.. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6-9-year-old children. Psychoneuroendocrinology 2010; 35:141-53; PMID:19674845; http://dx.doi.org/ 10.1016/j.psyneuen.2009.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straub H, Adams M, Kim JJ, Silver RK.. Antenatal depressive symptoms increase the likelihood of preterm birth. Am J Obstet Gynecol 2012; 207:329 e1-4; PMID:22789523; http://dx.doi.org/ 10.1016/j.ajog.2012.06.033 [DOI] [PubMed] [Google Scholar]
- 11.Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L.. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology 2008; 33:536-45; PMID:17507916; http://dx.doi.org/ 10.1038/sj.npp.1301450 [DOI] [PubMed] [Google Scholar]
- 12.O'Connor TG, Tang W, Gilchrist MA, Moynihan JA, Pressman EK, Blackmore ER.. Diurnal cortisol patterns and psychiatric symptoms in pregnancy: Short-term longitudinal study. Biol Psychol 2013; 96:35-41; PMID:24239618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy SE, Braithwaite EC, Hubbard I, Williams K, Tindall E, Holmes E, Ramchandani PG.. Salivary cortisol response to infant distress in pregnant women with symptoms of depression. Arch Women Ment Health 2014; 18(2); 247-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braithwaite EC, Murphy SE, Ramchandani PG.. Prenatal risk factors for depression: a critical review of the evidence and potential mechanisms. J Dev Orig Health Dis 2014; 19:1-12; PMID:25081923 [DOI] [PubMed] [Google Scholar]
- 15.Mueller BR, Bale TL.. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci 2008; 28:9055-65; PMID:18768700; http://dx.doi.org/ 10.1523/JNEUROSCI.1424-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kundakovic M, Champagne FA.. Early-life experience, epigenetics, and the developing brain. Neuropsychopharmacology 2015; 40:141-53; PMID:24917200; http://dx.doi.org/ 10.1038/npp.2014.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meaney MJ, Szyf M.. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci 2005; 7:103-23; PMID:16262207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szyf M, Weaver IC, Champagne FA, Diorio J, Meaney MJ.. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrinol 2005; 26:139-62; PMID:16303171; http://dx.doi.org/ 10.1016/j.yfrne.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 19.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ.. Epigenetic programming by maternal behavior. Nat Neurosci 2004; 7:847-54; PMID:15220929; http://dx.doi.org/ 10.1038/nn1276 [DOI] [PubMed] [Google Scholar]
- 20.Francis D, Diorio J, Liu D, Meaney MJ.. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 1999; 286:1155-8; PMID:10550053; http://dx.doi.org/ 10.1126/science.286.5442.1155 [DOI] [PubMed] [Google Scholar]
- 21.Zhang TY, Hellstrom IC, Bagot RC, Wen X, Diorio J, Meaney MJ.. Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. J Neurosci 2010; 30:13130-7; PMID:20881131; http://dx.doi.org/ 10.1523/JNEUROSCI.1039-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ.. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology 2006; 147:2909-15; PMID:16513834; http://dx.doi.org/ 10.1210/en.2005-1119 [DOI] [PubMed] [Google Scholar]
- 23.McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ.. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 2009; 12:342-8; PMID:19234457; http://dx.doi.org/ 10.1038/nn.2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romens SE, McDonald J, Svaren J, Pollak SD.. Associations between early life stress and gene methylation in children. Child Dev 2014; 86(1):303-9; PMID:25056599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Knaap LJ, Riese H, Hudziak JJ, Verbiest MM, Verhulst FC, Oldehinkel AJ, van Oort FV.. Glucocorticoid receptor gene (NR3C1) methylation following stressful events between birth and adolescence. The TRAILS study. Transl Psychiatry 2014; 4:e381; PMID:24713862; http://dx.doi.org/ 10.1038/tp.2014.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM.. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 2008; 3:97-106; PMID:18536531; http://dx.doi.org/ 10.4161/epi.3.2.6034 [DOI] [PubMed] [Google Scholar]
- 27.Radtke KM, Ruf M, Gunter HM, Dohrmann K, Schauer M, Meyer A, Elbert T.. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl Psychiatry 2011; 1:e21; PMID:22832523; http://dx.doi.org/ 10.1038/tp.2011.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boulle F, van den Hove DLA, Jakob SB, Rutten BP, Hamon M, van Os J, Lesch KP, Lanfumey L, Steinbusch HW, Kenis G.. Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Mol Psychiatry 2012; 17:584-96; PMID:21894152; http://dx.doi.org/ 10.1038/mp.2011.107 [DOI] [PubMed] [Google Scholar]
- 29.Boersma GJ, Lee RS, Cordner ZA, Ewald ER, Purcell RH, Moghadam AA, Tamashiro KL.. Prenatal stress decreases Bdnf expression and increases methylation of Bdnf exon IV in rats. Epigenetics 2014; 9:437-47; PMID:24365909; http://dx.doi.org/ 10.4161/epi.27558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fumagalli F, Molteni R, Racagni G, Riva MA.. Stress during development: Impact on neuroplasticity and relevance to psychopathology. Prog Neurobiol 2007; 81:197-217; PMID:17350153; http://dx.doi.org/ 10.1016/j.pneurobio.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 31.Branchi I, Francia N, Alleva E.. Epigenetic control of neurobehavioural plasticity: the role of neurotrophins. Behav Pharmacol 2004; 15:353-62; PMID:15343058; http://dx.doi.org/ 10.1097/00008877-200409000-00006 [DOI] [PubMed] [Google Scholar]
- 32.Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM.. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci 2007; 25:3091-8; PMID:17561822; http://dx.doi.org/ 10.1111/j.1460-9568.2007.05522.x [DOI] [PubMed] [Google Scholar]
- 33.Kundakovic M, Gudsnuk K, Herbstman J, Tang D, Frederica P, Champagne F.. DNA methylation of BDNF as a biomarker of early-life adversity. Proc Natl Acad Sci U S A 2014; [Epub ahead of print]; PMID:25385582; http://dx.doi.org/ 10.1073/pnas.1408355111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Giudice M, Ellis BJ, Shirtcliff EA.. The adaptive calibration model of stress responsivity. Neurosci Biobehav Rev 2011; 35:1562-92; PMID:21145350; http://dx.doi.org/ 10.1016/j.neubiorev.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kundakovic M, Lim S, Gudsnuk K, Champagne FA.. Sex-specific and strain-dependent effects of early life adversity on behavioral and epigenetic outcomes. Front Psychiatry 2013; 4:78; PMID:23914177; http://dx.doi.org/ 10.3389/fpsyt.2013.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Connor TG, Heron J, Golding J, Beveridge M, Glover V.. Maternal antenatal anxiety and children's behavioural/emotional problems at 4 years. Report from the avon longitudinal study of parents and children. Br J Psychiatry 2002; 180:502-8; PMID:12042228; http://dx.doi.org/ 10.1192/bjp.180.6.502 [DOI] [PubMed] [Google Scholar]
- 37.O'Connor TG, Heron J, Golding J, Glover V, Team AS.. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry 2003; 44:1025-36; PMID:14531585; http://dx.doi.org/ 10.1111/1469-7610.00187 [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez A, Bohlin G.. Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? J Child Psychol Psychiatry 2005; 46:246-54; PMID:15755301; http://dx.doi.org/ 10.1111/j.1469-7610.2004.00359.x [DOI] [PubMed] [Google Scholar]
- 39.Van den Bergh BRH, Marcoen A.. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-ilds. Child Dev 2004; 75:1085-97; PMID:15260866; http://dx.doi.org/ 10.1111/j.1467-8624.2004.00727.x [DOI] [PubMed] [Google Scholar]
- 40.Bergh BRHVd, Mennes M, Oosterlaan J, Stevens V, Stiers P, Marcoen A, Lagae L.. High antenatal maternal anxiety is related to impulsivity during performance on cognitive tasks in 14- and 15-year-olds. Neurosci Biobehav Rev 2005; 29:259-69; PMID:15811497; http://dx.doi.org/ 10.1016/j.neubiorev.2004.10.010 [DOI] [PubMed] [Google Scholar]
- 41.Zheng F, Zhou X, Moon C, Wang H.. Regulation of brain-derived neurotrophic factor expression in neurons. Int J Physiol Pathophysiol Pharmacol 2012; 4:188-200; PMID:23320132 [PMC free article] [PubMed] [Google Scholar]
- 42.Suri D, Veenit V, Sarkar A, Thiagarajan D, Kumar A, Nestler EJ, Galande S, Vaidya VA.. Early stress evokes age-dependent biphasic changes in hippocampal neurogenesis, BDNF expression, and cognition. Biol Psychiatry 2013; 73:658-66; PMID:23237316; http://dx.doi.org/ 10.1016/j.biopsych.2012.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P.. Maternal stress and preterm birth. Am J Epidemiol 2003; 157:14-24; PMID:12505886; http://dx.doi.org/ 10.1093/aje/kwf176 [DOI] [PubMed] [Google Scholar]
- 44.Pike IL. Maternal stress and fetal responses: evolutionary perspectives on preterm delivery. Am J Hum Biol 2005; 17:55-65; PMID:15611979; http://dx.doi.org/ 10.1002/ajhb.20093 [DOI] [PubMed] [Google Scholar]
- 45.Worthman CM, Kuzara J.. Life history and the early origins of health differentials. Am J Hum Biol 2005; 17:95-112; PMID:15611966; http://dx.doi.org/ 10.1002/ajhb.20096 [DOI] [PubMed] [Google Scholar]
- 46.Doyle C, Werner EA, Feng T, Lee S, Isler J, Monk C.. Pregnancy distress gets under fetal skin: maternal ambulatory assessment and sex differences in prenatal development. Dev Psychobiol accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N.. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A 2013; 110:15638-43; PMID:24019460; http://dx.doi.org/ 10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Del Giudice M, Hinnant JB, Ellis BJ, El-Sheikh M.. Adaptive patterns of stress responsivity: a preliminary investigation. Dev Psychol 2011; 28(3):775-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suglia SF, Staudenmayer J, Cohen S, Enlow MB, Rich-Edwards JW, Wright RJ.. Cumulative stress and cortisol disruption among black and hispanic pregnant women in an urban cohort. Psychol Trauma 2010; 2:326-34; PMID:21423846; http://dx.doi.org/ 10.1037/a0018953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kivlighan KT, DiPietro JA, Costigan KA, Laudenslager ML.. Diurnal rhythm of cortisol during late pregnancy: associations with maternal psychological well-being and fetal growth. Psychoneuroendocrinology 2008; 33:1225-35; PMID:18692319; http://dx.doi.org/ 10.1016/j.psyneuen.2008.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Keane V, Lightman S, Marsh M, Pawlby S, Papadopoulos AS, Taylor A, Moore R, Patrick K.. Increased pituitary-adrenal activation and shortened gestation in a sample of depressed pregnant women: a pilot study. J Affect Disord 2011; 130:300-5; PMID:21093926; http://dx.doi.org/ 10.1016/j.jad.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 52.Obel C, Hedegaard M, Henriksen TB, Secher NJ, Olsen J, Levine S.. Stress and salivary cortisol during pregnancy. Psychoneuroendocrinology 2005; 30:647-56; PMID:15854781; http://dx.doi.org/ 10.1016/j.psyneuen.2004.11.006 [DOI] [PubMed] [Google Scholar]
- 53.Hellgren C, Åkerud H, Skalkidou A, Sundström-Poromaa I.. Cortisol awakening response in late pregnancy in women with previous or ongoing depression. Psychoneuroendocrinology 2013; 38:3150-4; PMID:24041544; http://dx.doi.org/ 10.1016/j.psyneuen.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 54.Pluess M, Wurmser H, Buske-Kirschbaum A, Papousek M, Pirke KM, Hellhammer D, Bolten M.. Positive life events predict salivary cortisol in pregnant women. Psychoneuroendocrinology 2012; 37:1336-40; PMID:22309824; http://dx.doi.org/ 10.1016/j.psyneuen.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 55.Thompson T, Sharfi D, Lee M, Yrigollen C, Naumova O, Grigorenko E.. Comparison of whole-genome DNA methylation patterns in whole blood, saliva, and lymphoblastoid cell lines. Behav Genet 2013; 43:168-76; PMID:23269419; http://dx.doi.org/ 10.1007/s10519-012-9579-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Armstrong DA, Lesseur C, Conradt E, Lester BM, Marsit CJ.. Global and gene-specific DNA methylation across multiple tissues in early infancy: implications for children's health research. FASEB J 2014; 28:2088-97; PMID:24478308; http://dx.doi.org/ 10.1096/fj.13-238402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davies MN, Volta M, Pidsley R, Lunnon K, Dixit A, Lovestone S, Coarfa C, Harris RA, Milosavljevic A, Troakes C, et al.. Functional annotation of the human brain methylome identifies tissue-specific epigenetic variation across brain and blood. Genome Biol 2012; 13:R43; PMID:22703893; http://dx.doi.org/ 10.1186/gb-2012-13-6-r43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murray D, Cox JL.. Screening for depression during pregnancy with the edinburgh depression scale (EDDS). J Reprod Infant Psychol 1990; 8:99-107; http://dx.doi.org/ 10.1080/02646839008403615 [DOI] [Google Scholar]
- 59.Felice E, Saliba J, Grech V, Cox J.. Prevalence rates and psychosocial characteristics associated with depression in pregnancy and postpartum in Maltese women. J Affect Disord 2004; 82:297-301; PMID:15488261; http://dx.doi.org/ 10.1016/j.jad.2003.11.011 [DOI] [PubMed] [Google Scholar]
- 60.Adouard F, Glangeaud-Freudenthal NM, Golse B.. Validation of the Edinburgh postnatal depression scale (EPDS) in a sample of women with high-risk pregnancies in France. Arch Womens Ment Health 2005; 8:89-95; PMID:15883653; http://dx.doi.org/ 10.1007/s00737-005-0077-9 [DOI] [PubMed] [Google Scholar]
- 61.Adewuya AO, Ola BA, Dada AO, Fasoto OO.. Validation of the Edinburgh Postnatal Depression Scale as a screening tool for depression in late pregnancy among Nigerian women. J Psychosom Obstet Gynaecol 2006; 27:267-72; PMID:17225628; http://dx.doi.org/ 10.1080/01674820600915478 [DOI] [PubMed] [Google Scholar]
- 62.Bergink V, Kooistra L, Lambregtse-van den Berg MP, Wijnen H, Bunevicius R, van Baar A, Pop V.. Validation of the Edinburgh Depression Scale during pregnancy. J Psychosom Res 2011; 70:385-9; PMID:21414460; http://dx.doi.org/ 10.1016/j.jpsychores.2010.07.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


