Abstract
AIM: To investigate the association of TNF polymorphisms with chronic atrophic gastritis (CAG) and gastric adenocarcin-oma in Chinese Han patients.
METHODS: The TNFa-e 5 microsatellites and 3 RFLP sites were typed using PCR technique, followed by high-voltage denaturing PAGE with silver staining and restriction enzyme digestion respectively in specimens from 53 patients with CAG and 56 patients with gastric adenocarcinoma and 164 healthy controls. The PCR products were cloned and sequenced.
RESULTS: The frequency of TNF-β Ncol*1/2 genotype was higher in patients with chronic atrophic gastritis than in healthy controls, but no significant difference was observed (60.38% vs 46.34%, P = 0.076). The frequency of TNa10 allele was significantly higher in patients with chronic atrophic gastritis than in healthy controls (19.81% vs 11.89%, P = 0.04). However, it did not relate to age, gender, atrophic degree or intestinal metaplasia in patients with chronic atrophic gastritis. The frequency of TNF-β Ncol*1/2 and d2/d6 genotypes were significantly higher in patients with gastric adenocarcinoma than in healthy individuals (P > 0.05). However, TNF-β Ncol*1/2 and d2/d6 genotypes did not relate to age, gender, grade of differentiation and clinicopathologic stage in patients with gastric adenocarcinoma. The frequency of TNFa6b5c1 haplotype homozygote was significantly lower in patients with gastric adenocarcinoma than in healthy controls (1.79% vs 15.85%, P = 0.006).
CONCLUSION: TNFa10 allele may be a risk factor for chronic atrophic gastritis. TNF-β Ncol*1/2 and d2/d6 genotypes are associated with the susceptibility to gastric adenocarcinoma, whereas TNFa6b5c1 haplotype homozygote may contribute to the resistance against gastric adenocarcinoma.
INTRODUCTION
Gastric adenocarcinoma is one of the most frequent malignant diseases in the world, but the causes of gastric cancer remains unclear. The crude mortality rate of stomach cancer in China was 25.2 per 105 (32.8 per 105 for males and 17.0 per 105 for females), which comprised 23.2% of the total death of cancer from 1990 to 1992, making stomach cancer the leading cause of the death among cancers[1].
Tumor necrosis factor (TNF) is a multifunctional cytokine and its anti-tumor effect has attracted particular attention. TNF was found at a higher concentration in patients with malignant tumor. Forones et al[2] reported increased TNF-α expression in the sera of patients with advanced gastric cancer. TNF-α mRNA was markedly increased in gastric carcinoma tissue[3]. Recombinant TNF has been demonstrated to have considerable treatment effect on gastric cancer in vitro[4,5].
The gene for TNF-α and lymphotoxin-α (TNF-β), referred to as the TNF locus, are tandemly arranged within a 7-kilobase region in the HLA on the short arm of chromosome 6. HLA has recently been found to contribute to cancer development[6-9]. Several RFLP sites and 5 microsatellites within the TNF gene have been identified. Some studies have shown that TNF individual alleles were correlated to secretion from activated monocytes[10-13]. Furthermore, some experiments found that TNF secretion was also associated with TNF haplotypes, not only individual alleles[14]. These findings suggest that TNF polymorphisms may play a role in the pathogenesis of several autoimmune, infectious and neoplastic diseases.
Chronic atrophic gastritis (CAG) is believed to be the precancerous lesion of gastric adenocarcinoma. In the present study, we examined whether TNF genetic polymorphisms were associated with CAG and gastric adenocarcinoma in Chinese Han population. Additionally, we determined whether the associations between TNF genetic polymorphisms and CAG and gastric adenocarcinoma varied with clinicopathologic features of the 2 diseases.
MATERIALS AND METHODS
Patients and genomic DNA extraction
The subjects of this study included 56 patients with gastric adenocarcinoma (43 males, 13 females; mean age 55.6 ± 12.2 years), 53 patients with CAG (32 males, 21 females; mean age 53.5 ± 11.4 years), and 164 unrelated healthy individuals (113 males, 51 females; mean age 52.5 ± 11.3 years) from Chinese Han population in Hubei province of China. The diagnoses of gastric adenocarcinoma and CAG were confirmed by histopathology examinations. Genomic DNA was extracted from venous blood by a salting out procedure with minor modifications.
TNF polymorphism typing
Five microsatellites were amplified using a single step PCR reaction with primers described by Pociot et al[15]. TNF microsatellite alleles were typed by a 60 g/L polyacrylamide, 0.4-mm sequencing gel, followed by silver nitrate staining. Fragments were sized using DNA markers and simultaneously typed with known alleles derived from the cloned PGEM-T vector.
The sites in the first intron of TNF-β marked by AspH1 and Nco1 were analyzed using PCR and endonuclease digestion. BSIHKA1, an isoschizomer of AspH1 (New England Biolabs, Beverley, MA) was used instead of AspH1. The digested products were electrophoresed in 1 g/L ethidium bromide-stained 15 g/L agarose gels. Similarly, the TNF-308 polymorphism was examined by PCR amplification and Nco1 digestion. The digested products were separated on a 100 g/L non-denaturing polyacrylamide gel. Alleles were visualized as described for TNF microsatellite typing.
Cloning and sequencing
The PCR products of 5 microsatellites were purified and ligated with PGEM-T vector. High efficiency JM 109 competent cells were used in the process of transformation. Needed transformants were obtained by blue/white color screening and standard ampicillin selection. Recombinant plasmid DNA were isolated and identified. Sequencing was done on ABI 377 DNA sequencer.
Statistical analysis
Allele frequencies in patients and control groups were calculated by direct counting. The χ2 test and Fisher’s exact test were used for statistical analysis. Hardy-Weiberg equilibrium was tested in a 2×n analysis using χ2.
RESULTS
The frequencies of the various alleles at 8 TNF polymorphic sites in 3 groups are shown in Table 1. The frequency of TNFa10 allele was significantly higher in patients with CAG than in healthy controls (19.81% vs 11.89%, P = 0.04, OR = 1.83, 95%CI: 1.02-3.27). However, it was not related to age, gender, atrophic degree and intestinal metaplasia in patients with CAG (Table 2). The frequency of TNF-β Ncol*1/2 genotype was higher in patients with chronic atrophic gastritis than in healthy controls (Table 3). However no significant difference was observed (60.38% vs 46.34%, P = 0.076).
Table 1.
Distribution frequency of TNF alleles at 8 polymorphic loci in CAG, gastric adenocarcinoma and healthy controls
| TNF locus | Allele | Size (bp) | Controls (n = 164) | CAG (n = 53) | Gastric adenocarcinoma (n = 56) | P value |
| TNFa | a1 | 97 | 0.0183 | 0.0094 | 0.0089 | |
| a2 | 99 | 0.1583 | 0.1698 | 0.2054 | ||
| a3 | 101 | 0 | 0.0094 | 0.0268 | ||
| a4 | 103 | 0.0061 | 0.0094 | 0.0178 | ||
| a5 | 105 | 0.0396 | 0.0378 | 0.0089 | ||
| a6 | 107 | 0.3811 | 0.3585 | 0.3928 | ||
| a7 | 109 | 0.0793 | 0.0378 | 0.0536 | ||
| a8 | 111 | 0.0061 | 0.0094 | 0 | ||
| a9 | 113 | 0.0335 | 0.0566 | 0.0152 | ||
| a10 | 115 | 0.1189 | 0.1981* | 0.1161 | 0.04 | |
| a11 | 117 | 0.0945 | 0.066 | 0.0803 | ||
| a12 | 119 | 0.0091 | 0 | 0 | ||
| a13 | 121 | 0.0549 | 0.0378 | 0.0152 | ||
| TNFb | b1 | 125 | 0.1006 | 0.0755 | 0.1339 | |
| b2 | 126 | 0 | 0 | 0 | ||
| b3 | 127 | 0.1128 | 0.0943 | 0.0536 | ||
| b4 | 128 | 0.3293 | 0.3585 | 0.3482 | ||
| b5 | 129 | 0.4512 | 0.4717 | 0.4643 | ||
| b6 | 130 | 0 | 0 | 0 | ||
| b7 | 131 | 0.0061 | 0 | 0 | ||
| TNFc | c1 | 159 | 0.2104 | 0.7264 | 0.7679 | |
| c2 | 161 | 0.7896 | 0.2736 | 0.2321 | ||
| TNFd | d1 | 124 | 0.0061 | 0.0094 | 0 | |
| d2 | 126 | 0.0548 | 0.0755 | 0.0536 | ||
| d3 | 128 | 0.0457 | 0.0566 | 0.0446 | ||
| d4 | 130 | 0.3537 | 0.3774 | 0.3393 | ||
| d5 | 132 | 0.1341 | 0.1226 | 0.1786 | ||
| d6 | 134 | 0.3018 | 0.2925 | 0.2857 | ||
| d7 | 136 | 0 | 0 | 0 | ||
| d8 | 138 | 0.1037 | 0.066 | 0.0982 | ||
| TNFe | e1 | 99 | 0.064 | 0.0755 | 0 | |
| e3 | 103 | 0.811 | 0.8302 | 0.7768 | ||
| e4 | 105 | 0.1159 | 0.066 | 0.125 | ||
| TNF-α 308 | 1 | 0.9329 | 0.9434 | 0.9732 | ||
| 2 | 0.0671 | 0.0566 | 0.0268 | |||
| TNF-β Nco1 | 1 | 0.4817 | 0.4528 | 0.4464 | ||
| 2 | 0.5183 | 0.5472 | 0.5536 | |||
| TNF- β AspH1 | 1 | 0.3079 | 0.3302 | 0.3571 | ||
| 2 | 0.6921 | 0.6698 | 0.6429 |
Table 2.
Association between TNFa10 allele and clinical features of CAG
| Groups | Age |
Gender(yr) |
Atrophic degree |
Intestinal metaplasia |
|||
| M | F | Mild | Moderate | Severe | Positive | ||
| TNFa10+ | 53.9 ± 9.6 | 13 | 8 | 3 | 12 | 6 | 14 |
| TNFa10- | 53.2 ± 12.5 | 19 | 13 | 6 | 18 | 8 | 22 |
Table 3.
Distribution frequency of TNF TNF-β Ncol*1/2 and TNFd2/d6 genotypes in CAG, gastric adenocarcinoma and healthy controls
| Group |
TNF-β Ncol*1/2 |
TNFd2/d6 |
||
| + | - | + | - | |
| Controls | 76 | 88 | 4 | 160 |
| CAG | 32 | 21 | 2 | 51 |
| Gastric adenocarcinoma | 36a | 20 | 6c | 50 |
P < 0.05 vs compared with control group of TNF-β Ncol*1/2,
P < 0.05 vs compared with control group of TNFd2/d6.
The frequency of TNF-β Ncol*1/2 genotype was significantly higher in patients with gastric adenocarcinoma than in healthy controls (64.29% vs 46.34%, P = 0.020, OR = 2.08, 95%CI: 1.12-3.86). The frequency of d2/d6 genotype was also significantly higher in patients with gastric adenocarcinoma than in healthy individuals (10.71% vs 2.44%, P = 0.028, OR = 4.8, 95%CI: 1.18-19.47). However, TNF-β Ncol*1/2 and d2/d6 genotypes were not related to age, gender, grade of differentiation and clinicopathologic stage in patients with gastric adenocarcinoma (Table 4).
Table 4.
Association between d2/d6 and TNF-β Ncol*1/2 genotypes and clinical features of gastric adenocarcinoma
| Group | Age (yr) |
Gender |
Grade of differentiation |
Clinicopathologic stage |
|||
| M | F | High and moderate | Low | I-II | III-IV | ||
| d2/d6+ | 59.7±9.5 | 5 | 1 | 0 | 6 | 4 | 2 |
| d2/d6- | 55.1±12.5 | 38 | 12 | 13 | 37 | 21 | 29 |
| 1/2+ | 56.7±12.2 | 26 | 10 | 9 | 27 | 15 | 21 |
| 1/2- | 53.6±11.5 | 17 | 3 | 4 | 16 | 10 | 10 |
Based on maximum likelihood estimate, 4 most frequent 3-locus haplotypes have been described in 4 European populations: TNFa11b4c1, TNFa2b1c2, TNFa6b5c1, TNFa10b4c1. These haplotypes have also been observed in our study. By analysis of 2 locus association, we established 5 extended haplotypes which integrated alleles across the TNF locus in our population: TNFa6b5c1d8e4TNF308-1TNF β Nco1-1TNFAspH1-2, TNFa2b1c2d5e1TNF308-1TNF β Nco1-2TNFAspH1-2, TNFa11b4c1d4e3TNF308-1TNF β Nco1-2TNFAspH1-1, TNFa10b4c1d4e3TNF308-1TNF β Nco1-2TNFAspH1-1, TNFa2b3c1d2e3TNF308-2TNFAspH1-2. There were no significant differences in haplotype frequencies between CAG, gastric adenocarcinoma, and control groups for these haplotypes. However the frequency of TNFa6b5c1 haplotype homozygote was significantly lower in patients with gastric adenocarcinoma than in healthy individuals (1.79% vs 15.85%, P = 0.006). It was not related to age, gender, grade of differentiation and clinicopathologic stage in patients with gastric adenocarcinoma.
The sequences of five TNF microsatellites (Figure 1, Figure 2, Figure 3 and Figure 4) were consistent with that from a GeneBank database. However, several base exchanges were noted as following: TNFa at position 218 T→G, TNFc at 129 G→C, 170 A→G, TNFd at 87 G→A, 91 A→G, and TNFe at 81 C→T
Figure 1.
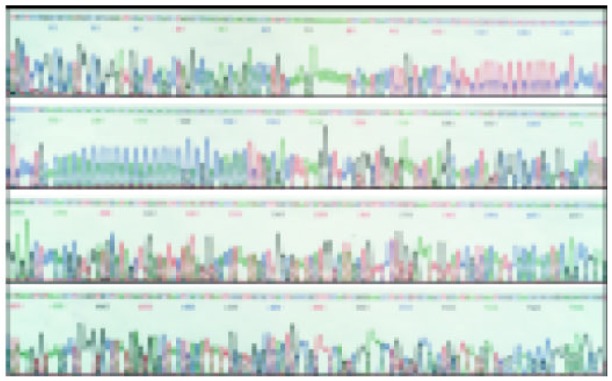
Nucleotide sequence of the cloned PGEM-T vector comprising TNFa6 allele (135→241) and TNFb5 allele (29→157), 150→179 fragment is 15 (AC/GT) repeats, 107→127 fragment is 10.5 (TC/GA) repeats.
Figure 2.
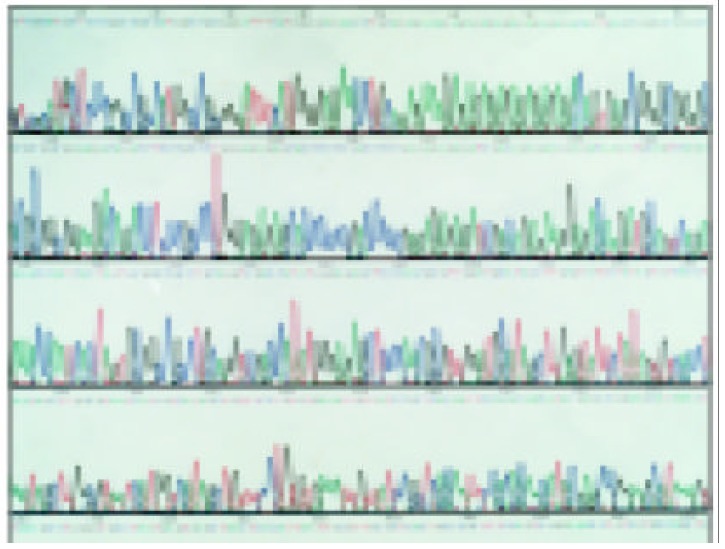
Nucleotide sequence of the cloned PGEM-T vector comprising TNFc1 allele (35→193), 59→76 fragment is 9 (TC/GA) repeats.
Figure 3.
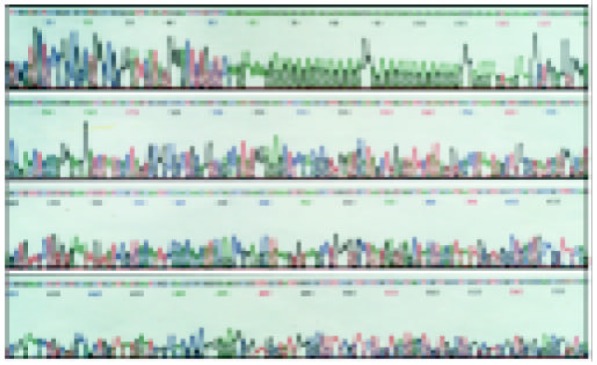
Nucleotide sequence of the cloned PGEM-T vector comprising TNFd4 allele (34→163), 64→85 fragment is 11 (TC/GA) repeats.
Figure 4.
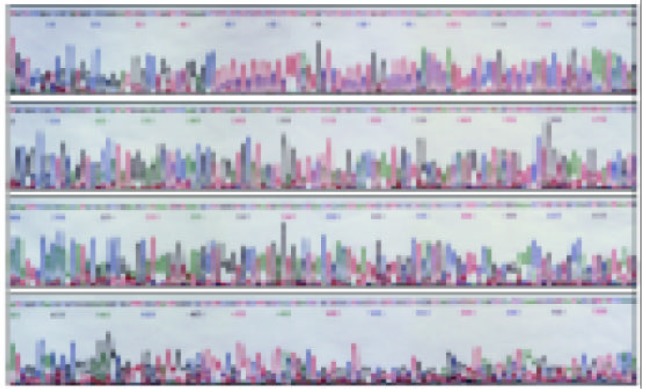
Nucleotide sequence of the cloned PGEM-T vector comprising TNFe3 allele (29→131), 47→62 fragment is 8 (TC/GA) repeats.
DISCUSSION
Genetic predisposition appears to be important in the pathogenesis of gastric adenocarcinoma. Genetic factors determining cancer risk have been postulated for the last decades and seem to be more apparent for gastric adenocarcinoma[16-21]. The relevant genes mediating the risk of gastric adenocarcinoma have not been identified until now. Progress of CAG towards gastric adenocarcinoma seems to be influenced by genetic factors[22,23].
A diallelic TNF-β polymorphism detected using the enzyme Ncol influences the TNF-α and/or -β secretion of peripheral blood mononuclear cells[24]. Park et al[25] found that TNF-β Ncol*1/1 genotypes showed an increased risk for colorectal cancer, and that TNF-β*1 allele played some role in the initial step of tumorigenesis or activation of dormant tumor cells, whereas TNF-β*2 allele mediated some functions associated with cytotoxicity of tumor cells. Our result showed that the frequency of TNF-β Ncol*1/2 genotype was significantly higher in patients with gastric adenocarcinoma than in healthy individuals. It suggested that TNF-β Ncol*1/2 genotype was related to the pathogenesis of gastric cancer. At the same time we found the frequency of TNF-β Ncol*1/2 genotype was also high in patients with CAG. However, the association between TNF-β Ncol*1/2 genotype and CAG did not show a significant difference. Further studies are necessary to elucidate If TNF-β Ncol*1/2 genotype is a risk factor for CAG. The frequency of d2/d6 genotype was also significantly higher in patients with gastric adenocarcinoma than in healthy individuals. It indicated that d2/d6 genotype might have some effect on pathogenesis of gastric adenocarcinoma.
Azuma et al[23] reported that the DQA1*0102 might contribute to resistance against Helicobacter pylori (H. pylori) associated gastric atrophy and immunogenetic factors were important in the etiology of H. pylori associated gastric atrophy. Proinflammatory IL-1beta polymorphisms are associated with hypochlorhydria and atrophic gastritis in Japan[24]. The presence of the IL-1*C allele may also indicate a risk of mucosal atrophy of the stomach in the Japanese population[26]. Our study has shown an association between TNFa10 allele and CAG, but not between TNFa10 allele and gastric adenocarcinoma. It suggested that TNFa10 allele might be a host risk factor for CAG. However, it was not related to age, gender, atrophic degree and intestinal metaplasia in patients with CAG.
A significant reduction in high expressing haplotypes was found in patients with follicular lymphoma[27]. Hajeer et al[28] reported the TNF a2-b4-d5 haplotype was significantly associated with the number of basal cell carcinoma (BCC) lesions. It provides the evidence that TNF microsatellite haplotype may influence the pathogenesis of multiple BCC. We found negative association between TNFa6b5c1 haplotype homozygote and gastric adenocarcinoma. The frequency of TNFa6b5c1 haplotype homozygote was lower in patients with gastric adenocarcinoma. It suggested that TNFa6b5c1 haplotype homozygote might contribute to the resistance against gastric adenocarcinoma. The absence of TNFa6b5c1 haplotype homozygote might increase the risk of gastric adenocarcinoma.
The TNF-α 308A allele has been associated with enhanced TNF-α expression[10]. Machado et al[29] proposed that individuals carrying TNF-α 308A allele had an increased risk for gastric cancer with an OR of 1.9. However our results showed that there was no association between TNF-α 308 allele and gastric adenocarcinoma. Similarly, Wu et al[30] reported no association was noted between gastric cancer and controls in the distribution of TNF-α genotypes in Taiwanese Chinese population. The discrepancy may be attributable to ethnic difference.
Onishi et al[31] demonstrated a significantly favorable prognosis in the renal carcinoma patients with TNF-β1/1 homozygote compared with other zygotes of TNF-beta polymorphism. The patients with TNF-β1/1 homozygote showed much lower stage and/or grade than those of other zygotes. TNF polymorphisms TNF + 488A and TNF-859T were associated with grade of tumour in patients with bladder cancer[32]. However, in our study TNF-β Ncol*1/2 and d2/d6 genotypes and TNFa6b5c1 haplotype were not related to age, gender, grade of differentiation and clinicopathologic stage in patients with gastric adenocarcinoma. Patients with d2/d6 genotype were present with a lowly differentiated tumor, however the difference did not reach statistical significance. Larger sample studies are needed.
The association between TNF genetic polymorphisms and CAG and gastric adenocarcinoma is not clear. However, 2 distinct potential mechanisms can be proposed. First, TNF alleles may be involved in genetically controlled variations in TNF production as previously described. Another possible explanation is that these genes are not responsible for the pathophysiological mechanisms but are linked closely to other responsible genes. TNF has been reported to be in linkage disequilibrium with HLA genes[33-35]. The negative association of TNFa3-e1 with rheumatoid arthritis may be secondary to the negative linkage disequilibrium between TNFa3-e1 and HLA-DR4[36]. Additional studies will be necessary to investigate whether TNF genes are independent of other linked genes to play a role.
With regard to several base exchanges in our sequencing result, more studies should be done to verify whether they are caused by ethnic difference or other factors.
Footnotes
Supported by Grant from provincial public health bureau, Hubei Province, No. 97420
Edited by Zhu LH, Xu FM
References
- 1.Sun X, Mu R, Zhou Y, Dai X, Qiao Y, Zhang S, Huangfu X, Sun J, Li L, Lu F. [1990-1992 mortality of stomach cancer in China] Zhonghua Zhong Liu ZaZhi. 2002;24:4–8. [PubMed] [Google Scholar]
- 2.Forones NM, Mandowsky SV, Lourenço LG. Serum levels of interleukin-2 and tumor necrosis factor-alpha correlate to tumor progression in gastric cancer. Hepatogastroenterology. 2001;48:1199–1201. [PubMed] [Google Scholar]
- 3.Izutani R, Katoh M, Asano S, Ohyanagi H, Hirose K. Enhanced expression of manganese superoxide dismutase mRNA and increased TNFalpha mRNA expression by gastric mucosa in gastric cancer. World J Surg. 1996;20:228–233. doi: 10.1007/s002689900035. [DOI] [PubMed] [Google Scholar]
- 4.Wei XC, Wang XJ, Chen K, Zhang L, Liang Y, Lin XL. Killing effect of TNF-related apoptosis inducing ligand regulated by tetracycline on gastric cancer cell line NCI-N87. World J Gastroenterol. 2001;7:559–562. doi: 10.3748/wjg.v7.i4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buell JF, Reed E, Lee KB, Parker RJ, Venzon DJ, Amikura K, Arnold S, Fraker DL, Alexander HR. Synergistic effect and possible mechanisms of tumor necrosis factor and cisplatin cytotoxicity under moderate hyperthermia against gastric cancer cells. Ann Surg Oncol. 1997;4:141–148. doi: 10.1007/BF02303797. [DOI] [PubMed] [Google Scholar]
- 6.Ghaderi M, Nikitina Zake L, Wallin K, Wiklund F, Hallmans G, Lenner P, Dillner J, Sanjeevi CB. Tumor necrosis factor A and MHC class I chain related gene A (MIC-A) polymorphisms in Swedish patients with cervical cancer. Hum Immunol. 2001;62:1153–1158. doi: 10.1016/s0198-8859(01)00306-8. [DOI] [PubMed] [Google Scholar]
- 7.Juszczynski P, Kalinka E, Bienvenu J, Woszczek G, Borowiec M, Robak T, Kowalski M, Lech-Maranda E, Baseggio L, Coiffier B, et al. Human leukocyte antigens class II and tumor necrosis factor genetic polymorphisms are independent predictors of non-Hodgkin lymphoma outcome. Blood. 2002;100:3037–3040. doi: 10.1182/blood-2002-02-0654. [DOI] [PubMed] [Google Scholar]
- 8.Gelder CM, Williams OM, Hart KW, Wall S, Williams G, Ingrams D, Bull P, Bunce M, Welsh K, Marshall SE, et al. HLA class II polymorphisms and susceptibility to recurrent respiratory papillomatosis. J Virol. 2003;77:1927–1939. doi: 10.1128/JVI.77.3.1927-1939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto K, Yasugi T, Nakagawa S, Okubo M, Hirata R, Maeda H, Yoshikawa H, Taketani Y. Human papillomavirus type 16 E6 variants and HLA class II alleles among Japanese women with cervical cancer. Int J Cancer. 2003;106:919–922. doi: 10.1002/ijc.11332. [DOI] [PubMed] [Google Scholar]
- 10.Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci USA. 1997;94:3195–3199. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaluza W, Reuss E, Grossmann S, Hug R, Schopf RE, Galle PR, Maerker-Hermann E, Hoehler T. Different transcriptional activity and in vitro TNF-alpha production in psoriasis patients carrying the TNF-alpha 238A promoter polymorphism. J Invest Dermatol. 2000;114:1180–1183. doi: 10.1046/j.1523-1747.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 12.Fargion S, Valenti L, Dongiovanni P, Scaccabarozzi A, Fracanzani AL, Taioli E, Mattioli M, Sampietro M, Fiorelli G. Tumor necrosis factor alpha promoter polymorphisms influence the phenotypic expression of hereditary hemochromatosis. Blood. 2001;97:3707–3712. doi: 10.1182/blood.v97.12.3707. [DOI] [PubMed] [Google Scholar]
- 13.Baseggio L, Bienvenu J, Charlot C, Picollet J, Felman P, Coiffier B, Salles G. Higher LPS-stimulated TNF-alpha mRNA levels in peripheral blood mononuclear cells from non-Hodgkin's lymphoma patients. Exp Hematol. 2001;29:330–338. doi: 10.1016/s0301-472x(00)00672-x. [DOI] [PubMed] [Google Scholar]
- 14.Bouma G, Crusius JB, Oudkerk Pool M, Kolkman JJ, von Blomberg BM, Kostense PJ, Giphart MJ, Schreuder GM, Meuwissen SG, Peña AS. Secretion of tumour necrosis factor alpha and lymphotoxin alpha in relation to polymorphisms in the TNF genes and HLA-DR alleles. Relevance for inflammatory bowel disease. Scand J Immunol. 1996;43:456–463. doi: 10.1046/j.1365-3083.1996.d01-65.x. [DOI] [PubMed] [Google Scholar]
- 15.Pociot F, Briant L, Jongeneel CV, Mölvig J, Worsaae H, Abbal M, Thomsen M, Nerup J, Cambon-Thomsen A. Association of tumor necrosis factor (TNF) and class II major histocompatibility complex alleles with the secretion of TNF-alpha and TNF-beta by human mononuclear cells: a possible link to insulin-dependent diabetes mellitus. Eur J Immunol. 1993;23:224–231. doi: 10.1002/eji.1830230135. [DOI] [PubMed] [Google Scholar]
- 16.Artuñedo Pe P, Moreno Azcoita M, Alonso A, Fernández-Peralta A, González-Aguilera JJ. Prognostic significance of high microsatellite instability in a Spanish series of gastric adenocarcinomas. Anticancer Res. 2000;20:4009–4014. [PubMed] [Google Scholar]
- 17.Cai L, Yu SZ, Zhang ZF. Glutathione S-transferases M1, T1 genotypes and the risk of gastric cancer: a case-control study. World J Gastroenterol. 2001;7:506–509. doi: 10.3748/wjg.v7.i4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 19.Machado JC, Pharoah P, Sousa S, Carvalho R, Oliveira C, Figueiredo C, Amorim A, Seruca R, Caldas C, Carneiro F, et al. Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology. 2001;121:823–829. doi: 10.1053/gast.2001.28000. [DOI] [PubMed] [Google Scholar]
- 20.Oue N, Matsumura S, Nakayama H, Kitadai Y, Taniyama K, Matsusaki K, Yasui W. Reduced expression of the TSP1 gene and its association with promoter hypermethylation in gastric carcinoma. Oncology. 2003;64:423–429. doi: 10.1159/000070302. [DOI] [PubMed] [Google Scholar]
- 21.El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–1201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 22.Furuta T, El-Omar EM, Xiao F, Shirai N, Takashima M, Sugimura H. Interleukin 1beta polymorphisms increase risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology. 2002;123:92–105. doi: 10.1053/gast.2002.34156. [DOI] [PubMed] [Google Scholar]
- 23.Azuma T, Ito S, Sato F, Yamazaki Y, Miyaji H, Ito Y, Suto H, Kuriyama M, Kato T, Kohli Y. The role of the HLA-DQA1 gene in resistance to atrophic gastritis and gastric adenocarcinoma induced by Helicobacter pylori infection. Cancer. 1998;82:1013–1018. doi: 10.1002/(sici)1097-0142(19980315)82:6<1013::aid-cncr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 24.Whichelow CE, Hitman GA, Raafat I, Bottazzo GF, Sachs JA. The effect of TNF*B gene polymorphism on TNF-alpha and -beta secretion levels in patients with insulin-dependent diabetes mellitus and healthy controls. Eur J Immunogenet. 1996;23:425–435. doi: 10.1111/j.1744-313x.1996.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 25.Park KS, Mok JW, Rho SA, Kim JC. Analysis of TNFB and TNFA NcoI RFLP in colorectal cancer. Mol Cells. 1998;8:246–249. [PubMed] [Google Scholar]
- 26.Kato S, Onda M, Yamada S, Matsuda N, Tokunaga A, Matsukura N. Association of the interleukin-1 beta genetic polymorphism and gastric cancer risk in Japanese. J Gastroenterol. 2001;36:696–699. doi: 10.1007/s005350170033. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgibbon J, Grenzelias D, Matthews J, Lister TA, Gupta RK. Tumour necrosis factor polymorphisms and susceptibility to follicular lymphoma. Br J Haematol. 1999;107:388–391. doi: 10.1046/j.1365-2141.1999.01704.x. [DOI] [PubMed] [Google Scholar]
- 28.Hajeer AH, Lear JT, Ollier WE, Naves M, Worthington J, Bell DA, Smith AG, Bowers WP, Jones PW, Strange RC, et al. Preliminary evidence of an association of tumour necrosis factor microsatellites with increased risk of multiple basal cell carcinomas. Br J Dermatol. 2000;142:441–445. doi: 10.1046/j.1365-2133.2000.03353.x. [DOI] [PubMed] [Google Scholar]
- 29.Machado JC, Figueiredo C, Canedo P, Pharoah P, Carvalho R, Nabais S, Castro Alves C, Campos ML, Van Doorn LJ, Caldas C, et al. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology. 2003;125:364–371. doi: 10.1016/s0016-5085(03)00899-0. [DOI] [PubMed] [Google Scholar]
- 30.Wu MS, Wu CY, Chen CJ, Lin MT, Shun CT, Lin JT. Interleukin-10 genotypes associate with the risk of gastric carcinoma in Taiwanese Chinese. Int J Cancer. 2003;104:617–623. doi: 10.1002/ijc.10987. [DOI] [PubMed] [Google Scholar]
- 31.Onishi T, Ohishi Y, Goto H, Asano K, Makino H, Hatano T, Tomita M, Abe K, Imagawa K, Kinoshita M, et al. [Study on the relationship between tumour necrosis factor gene polymorphism and prognosis in the patients with renal cell carcinoma] Nihon Hinyokika Gakkai Zasshi. 1998;89:413–420. doi: 10.5980/jpnjurol1989.89.413. [DOI] [PubMed] [Google Scholar]
- 32.Marsh HP, Haldar NA, Bunce M, Marshall SE, le Monier K, Winsey SL, Christodoulos K, Cranston D, Welsh KI, Harris AL. Polymorphisms in tumour necrosis factor (TNF) are associated with risk of bladder cancer and grade of tumour at presentation. Br J Cancer. 2003;89:1096–1101. doi: 10.1038/sj.bjc.6601165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oturai A, Larsen F, Ryder LP, Madsen HO, Hillert J, Fredrikson S, Sandberg-Wollheim M, Laaksonen M, Koch-Henriksen N, Sawcer S, et al. Linkage and association analysis of susceptibility regions on chromosomes 5 and 6 in 106 Scandinavian sibling pair families with multiple sclerosis. Ann Neurol. 1999;46:612–616. [PubMed] [Google Scholar]
- 34.Louka AS, Lie BA, Talseth B, Ascher H, Ek J, Gudjónsdóttir AH, Sollid LM. Coeliac disease patients carry conserved HLA-DR3-DQ2 haplotypes revealed by association of TNF alleles. Immunogenetics. 2003;55:339–343. doi: 10.1007/s00251-003-0586-5. [DOI] [PubMed] [Google Scholar]
- 35.Di Somma C, Charron D, Deichmann K, Buono C, Ruffilli A. Atopic asthma and TNF-308 alleles: linkage disequilibrium and association analyses. Hum Immunol. 2003;64:359–365. doi: 10.1016/s0198-8859(02)00819-4. [DOI] [PubMed] [Google Scholar]
- 36.Yen JH, Chen CJ, Tsai WC, Lin CH, Ou TT, Lin SC, Dai ZK, Liu HW. Tumor necrosis factor microsatellite alleles in patients with rheumatoid arthritis in Taiwan. Immunol Lett. 2002;81:177–182. doi: 10.1016/s0165-2478(02)00009-3. [DOI] [PubMed] [Google Scholar]


