Abstract
Evidence is presented for linkage of opioid receptors directly to the stimulatory G protein (guanine nucleotide-binding protein), Gs, in addition to the generally accepted linkage to the inhibitory and "other" G proteins, gi and Go, in F-11 (neuroblastoma-dorsal root ganglion neuron) hybrid cells. Treatment of intact F-11 cells with cholera toxin decreased specific binding of the opioid agonist [D-Ala2,D-Leu5]enkephalin to F-11 cell membranes by 35%, with the remaining binding retaining high affinity for agonist. Under these conditions cholera toxin influenced the alpha subunit of Gs (Gs alpha) but had no effect on the alpha subunit of Gi/o (Gi/o alpha), based on ADP-ribosylation studies. Pertussis toxin treatment decreased high-affinity opioid agonist binding by about 50%; remaining binding was also of high affinity, even though pertussis toxin had inactivated Gi/o alpha selectively and essentially completely. Simultaneous treatment with both toxins had an additive effect, reducing specific binding by about 80%. While opioid agonists inhibited forskolin-stimulated adenylate cyclase activity of F-11 cells as expected, opioids also stimulated basal adenylate cyclase activity, indicative of interaction with Gs as well as Gi. Cholera toxin treatment attenuated opioid-stimulation of basal adenylate cyclase, whereas pertussis toxin treatment enhanced stimulation. In contrast, inhibition by opioid of forskolin-stimulated activity was attenuated by pertussis toxin but not by cholera toxin. It is concluded that a subset of opioid receptors may be linked directly to Gs and thereby mediate stimulation of adenylate cyclase. This Gs-adenylate cyclase interaction is postulated to be responsible for the novel excitatory electrophysiologic responses to opioids found in our previous studies of sensory neurons and F-11 cells.
Full text
PDF
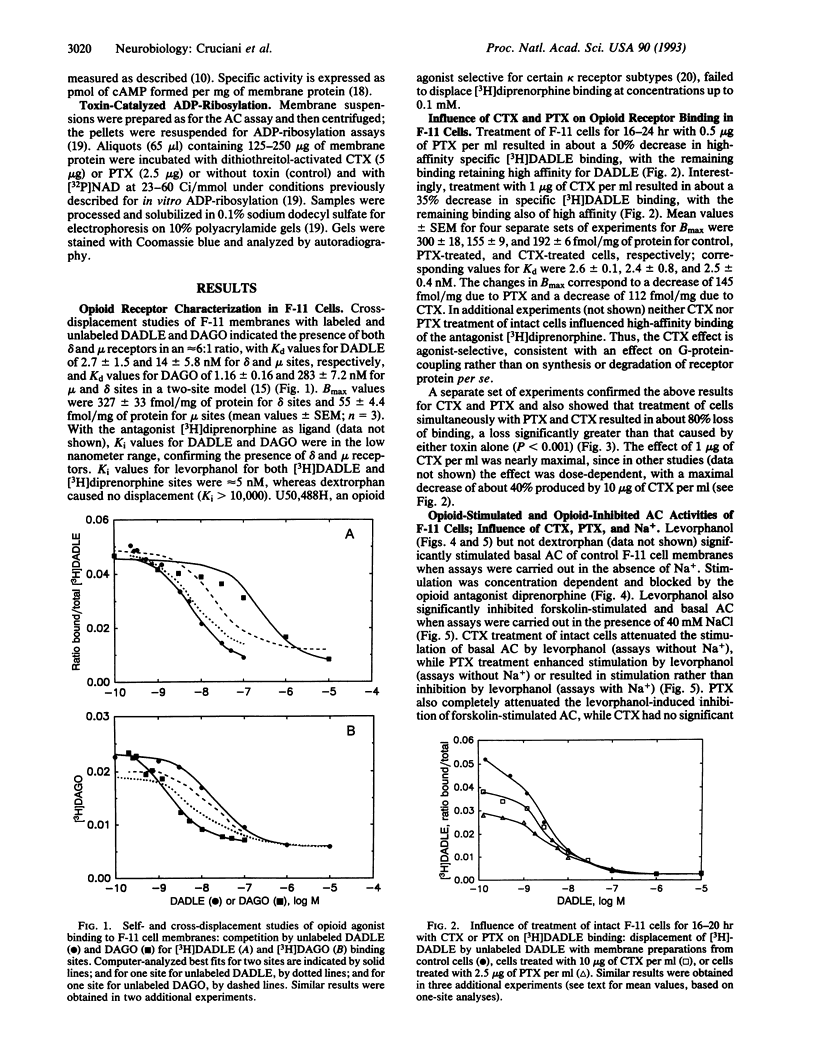
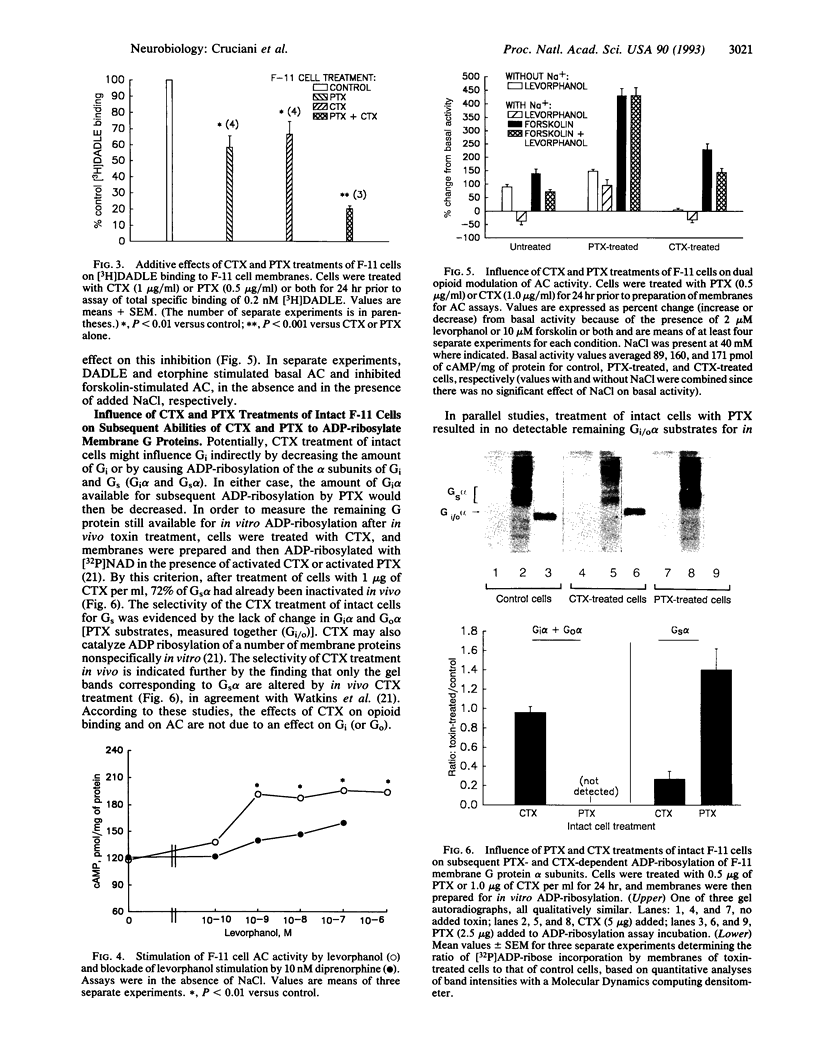
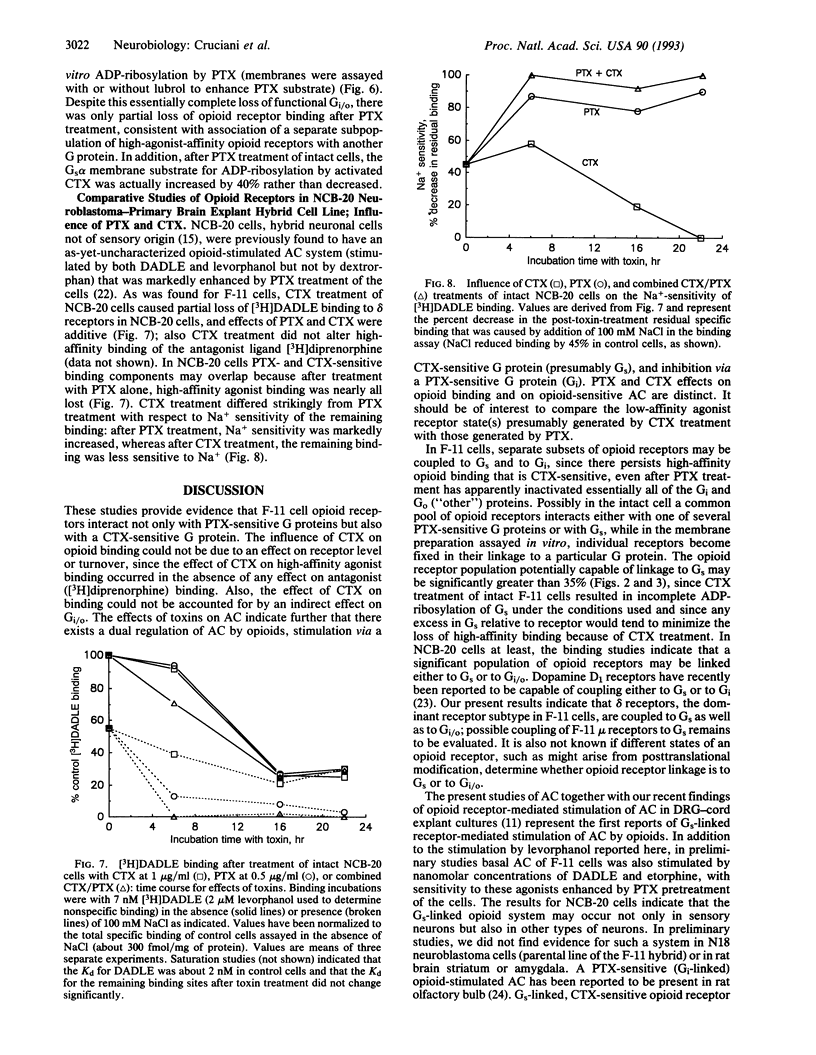

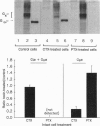
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abood M. E., Lee N. M., Loh H. H. Modification of opioid agonist binding by pertussis toxin. Brain Res. 1987 Aug 4;417(1):70–74. doi: 10.1016/0006-8993(87)90180-6. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E., Dawson G. Evidence for [D-Ala2,D-Leu5]enkephalin-induced supersensitivity to 5-hydroxytryptamine in a neurotumor x brain hybrid cell line (NCB-20). J Neurochem. 1985 Dec;45(6):1731–1738. doi: 10.1111/j.1471-4159.1985.tb10528.x. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. G proteins in signal transduction. Annu Rev Pharmacol Toxicol. 1990;30:675–705. doi: 10.1146/annurev.pa.30.040190.003331. [DOI] [PubMed] [Google Scholar]
- Blume A. J., Lichtshtein D., Boone G. Coupling of opiate receptors to adenylate cyclase: requirement for Na+ and GTP. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5626–5630. doi: 10.1073/pnas.76.11.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers S. R., Snyder S. H. Differential regulation by guanine nucleotides or opiate agonist and antagonist receptor interactions. J Neurochem. 1980 Mar;34(3):583–593. doi: 10.1111/j.1471-4159.1980.tb11184.x. [DOI] [PubMed] [Google Scholar]
- Clark J. A., Liu L., Price M., Hersh B., Edelson M., Pasternak G. W. Kappa opiate receptor multiplicity: evidence for two U50,488-sensitive kappa 1 subtypes and a novel kappa 3 subtype. J Pharmacol Exp Ther. 1989 Nov;251(2):461–468. [PubMed] [Google Scholar]
- Crain S. M., Crain B., Makman M. H. Pertussis toxin blocks depressant effects of opioid, monoaminergic and muscarinic agonists on dorsal-horn network responses in spinal cord-ganglion cultures. Brain Res. 1987 Jan 1;400(1):185–190. doi: 10.1016/0006-8993(87)90670-6. [DOI] [PubMed] [Google Scholar]
- Crain S. M., Shen K. F. Opioids can evoke direct receptor-mediated excitatory effects on sensory neurons. Trends Pharmacol Sci. 1990 Feb;11(2):77–81. doi: 10.1016/0165-6147(90)90322-y. [DOI] [PubMed] [Google Scholar]
- Cruciani R. A., Lutz R. A., Munson P. J., Rodbard D. Naloxonazine effects on the interaction of enkephalin analogs with mu-1, mu and delta opioid binding sites in rat brain membranes. J Pharmacol Exp Ther. 1987 Jul;242(1):15–20. [PubMed] [Google Scholar]
- Fan S. F., Shen K. F., Crain S. M. Opioids at low concentration decrease openings of K+ channels in sensory ganglion neurons. Brain Res. 1991 Aug 30;558(1):166–170. doi: 10.1016/0006-8993(91)90737-g. [DOI] [PubMed] [Google Scholar]
- Fan S. F., Shen K. F., Scheideler M. A., Crain S. M. F11 neuroblastoma x DRG neuron hybrid cells express inhibitory mu- and delta-opioid receptors which increase voltage-dependent K+ currents upon activation. Brain Res. 1992 Sep 11;590(1-2):329–333. doi: 10.1016/0006-8993(92)91116-v. [DOI] [PubMed] [Google Scholar]
- Francel P. C., Harris K., Smith M., Fishman M. C., Dawson G., Miller R. J. Neurochemical characteristics of a novel dorsal root ganglion X neuroblastoma hybrid cell line, F-11. J Neurochem. 1987 May;48(5):1624–1631. doi: 10.1111/j.1471-4159.1987.tb05711.x. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Hsia J. A., Moss J., Hewlett E. L., Vaughan M. ADP-ribosylation of adenylate cyclase by pertussis toxin. Effects on inhibitory agonist binding. J Biol Chem. 1984 Jan 25;259(2):1086–1090. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Makman M. H., Dvorkin B., Crain S. M. Modulation of adenylate cyclase activity of mouse spinal cord-ganglion explants by opioids, serotonin and pertussis toxin. Brain Res. 1988 Apr 5;445(2):303–313. doi: 10.1016/0006-8993(88)91193-6. [DOI] [PubMed] [Google Scholar]
- Makman M. H., Dvorkin B., Klein P. N. Sodium ion modulates D2 receptor characteristics of dopamine agonist and antagonist binding sites in striatum and retina. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4212–4216. doi: 10.1073/pnas.79.13.4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie F. R., Milligan G. Cholera toxin impairment of opioid-mediated inhibition of adenylate cyclase in neuroblastoma x glioma hybrid cells is due to a toxin-induced decrease in opioid receptor levels. Biochem J. 1991 Apr 1;275(Pt 1):175–181. doi: 10.1042/bj2750175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. A., Tanowitz H., Factor S. M., Bilezikian J. P., Wittner M. Myocardial adenylate cyclase activity in acute murine Chagas' disease. Circ Res. 1988 Apr;62(4):800–810. doi: 10.1161/01.res.62.4.800. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Onali P., Olianas M. C. Naturally occurring opioid receptor agonists stimulate adenylate cyclase activity in rat olfactory bulb. Mol Pharmacol. 1991 Apr;39(4):436–441. [PubMed] [Google Scholar]
- Platika D., Boulos M. H., Baizer L., Fishman M. C. Neuronal traits of clonal cell lines derived by fusion of dorsal root ganglia neurons with neuroblastoma cells. Proc Natl Acad Sci U S A. 1985 May;82(10):3499–3503. doi: 10.1073/pnas.82.10.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K. F., Crain S. M. Cholera toxin-A subunit blocks opioid excitatory effects on sensory neuron action potentials indicating mediation by Gs-linked opioid receptors. Brain Res. 1990 Aug 20;525(2):225–231. doi: 10.1016/0006-8993(90)90868-c. [DOI] [PubMed] [Google Scholar]
- Shen K. F., Crain S. M. Dual opioid modulation of the action potential duration of mouse dorsal root ganglion neurons in culture. Brain Res. 1989 Jul 10;491(2):227–242. doi: 10.1016/0006-8993(89)90059-0. [DOI] [PubMed] [Google Scholar]
- Sidhu A., Sullivan M., Kohout T., Balen P., Fishman P. H. D1 dopamine receptors can interact with both stimulatory and inhibitory guanine nucleotide binding proteins. J Neurochem. 1991 Oct;57(4):1445–1451. doi: 10.1111/j.1471-4159.1991.tb08312.x. [DOI] [PubMed] [Google Scholar]
- Simon E. J., Hiller J. M., Groth J., Edelman I. Further properties of stereospecific opiate binding sites in rat brain: on the nature of the sodium effect. J Pharmacol Exp Ther. 1975 Mar;192(3):531–537. [PubMed] [Google Scholar]
- Taussig R., Sanchez S., Rifo M., Gilman A. G., Belardetti F. Inhibition of the omega-conotoxin-sensitive calcium current by distinct G proteins. Neuron. 1992 Apr;8(4):799–809. doi: 10.1016/0896-6273(92)90100-r. [DOI] [PubMed] [Google Scholar]
- Watkins P. A., Moss J., Vaughan M. ADP ribosylation of membrane proteins from human fibroblasts. Effect of prior exposure of cells to choleragen. J Biol Chem. 1981 May 25;256(10):4895–4899. [PubMed] [Google Scholar]