Abstract
In the current work, we investigated the effects of dopamine, an neurotransmitter found in several plant species on antioxidant enzyme activities and ROS in soybean (Glycine max L. Merrill) roots. The effects of dopamine on SOD, CAT and POD activities, as well as H2O2, O2•−, melanin contents and lipid peroxidation were evaluated. Three-day-old seedlings were cultivated in half-strength Hoagland nutrient solution (pH 6.0), without or with 0.1 to 1.0 mM dopamine, in a growth chamber (25°C, 12 h photoperiod, irradiance of 280 μmol m−2 s−1) for 24 h. Significant increases in melanin content were observed. The levels of ROS and lipid peroxidation decreased at all concentrations of dopamine tested. The SOD activity increased significantly under the action of dopamine, while CT activity was inhibited and POD activity was unaffected. The results suggest a close relationship between a possible antioxidant activity of dopamine and melanin and activation of SOD, reducing the levels of ROS and damage on membranes of soybean roots.
Keywords: antioxidant enzymes, dopamine, melanin, plants, reactive oxygen species, soybean
Abbreviations
- SOD
Superoxide dismutase
- CAT
catalase
- POD
peroxidase
- ROS
reactive oxygen species
- H2O2
hydrogen peroxide
- O2•−
superoxide anion
- HO−
hydroxyl radical
- L-DOPA
L-3,4-dihydroxyphenylalanine
Introduction
Dopamine is a well known neurotransmitter in animals and it is present in many plant species, such as mucuna (Mucuna pruriens), potato (Solanum tuberosum), banana (Musa acuminata, Musa sapientum), plantain (Plantago major) and avocado (Persea americana).1-4 Mucuna is a legume widely used in consortia with sorghum, maize and millet and it contributes to the suppression of nematodes and weeds and helps control erosion and nitrogen supply. Dopamine is found in mucuna leaves, representing 1–2% of the dry weight. In plants with 2–3 weeks of growth, the content of dopamine, in the leaves, exceeded the content of L-DOPA, the most abundant allelochemical found in mucuna. However, no dopamine was detected in seeds, stems and roots at any stage of development.5
Some studies have reported the effects of dopamine on different plant species. These effects are associated with nitrogen fixation, flowering, preventing oxidation of auxins, regulation of ion permeability, photophosphorylation in chloroplasts and defense against herbivores. Exogenous dopamine applied at concentrations from 5 to 100 μM stimulated the biosynthesis of ethylene in beet (Beta vulgaris L.) leaves and can still influence flowering plants, relieving the inhibition of flowering caused by other agents such as sucrose and ammonium ions in Lemnaa pausicostata.6,7
In plants, dopamine is synthesized from the amino acid tyrosine using L-DOPA or tyramine. Tyrosine may either undergo hydroxylation to L-DOPA and then decarboxylation to dopamine, or undergo decarboxylation producing tyramine, which is hydroxylated to form dopamine.8 Dopamine is also a precursor of melanin. Tyrosinases are involved in this mechanism, and oxidize dopamine-generating dopaminoquinones that can undergo polymerization, forming melanin.9 This process can also occur by auto-oxidation, without the action of enzymes. There are reports that during these oxidation reactions to form melanin, ROS such as H2O2, O2•− and HO− can be formed in different cellular compartments.10-12 Under optimal growth conditions, ROS are produced at a very low level in the cell organelles, but their production is dramatically enhanced by the effects of biotic and abiotic stresses. Increased production of ROS causes oxidative damage and lipid peroxidation, damaging macromolecules such as pigments, proteins, nucleic acids and lipids.13 To avoid cell disintegration by ROS, some antioxidant compounds (ascorbic acid and glutathione) and enzymes such as POD, CT and SOD detoxify ROS, which are converted in less reactive compounds. Indeed, oxidative stress leads to an increase of ROS and causes, in addition to the cellular damage, inhibition of plant growth. This increase in free radicals has been considered as one of the mechanisms of action of allelochemicals.14
There is also evidence that dopamine can act as an antioxidant.4,15,16 Yen and Hsieh16 suggested that dopamine is an effective ROS scavenger. Furthermore, Kanazawa and Sakakibara4 showed that dopamine had greater antioxidant capacity than glutathione and food additives, and more resembled stronger antioxidants such as ascorbic acid. In view of this apparent contradiction concerning the oxidative or antioxidant properties of dopamine, the purpose of this study was to investigate the mode of action of dopamine on soybean, evaluating its effects on several issues related to the condition of oxidative stress parameters. The production of O2•- and H2O2 and the level of lipid peroxidation, as well as the activities of certain enzymes in antioxidant defense systems, such as POD, SOD and CAT, were evaluated. The production of melanin from dopamine was also quantified.
Results
Figure 1 shows that melanin was gradually produced from dopamine. After 0.1 to 1.0 mM dopamine treatments, the melanin level reached from 2.5 to 4.9 μg g−1 fresh biomass. Regarding ROS, dopamine significantly reduced levels of O2•- to undetectable levels, and decreased the H2O2 content by up to 91% compared to the control (Fig. 2A and B). These results were corroborated by inhibition of lipid peroxidation by up to 90% after exposure to 1.0 mM dopamine, in comparison with the control (Fig. 3). Antioxidant enzyme activities in the roots subjected to dopamine were significantly diverse from controls (Fig. 4), except for POD (Fig. 4B). The SOD activity was affected by the action of the neurotransmitter. Dopamine at 0.1 to 1.0 mM increased enzyme activity by 70% on average (Fig. 4A). Finally, CT activity was reduced by up to 61% after exposure to dopamine (Fig. 4C).
Figure 1.
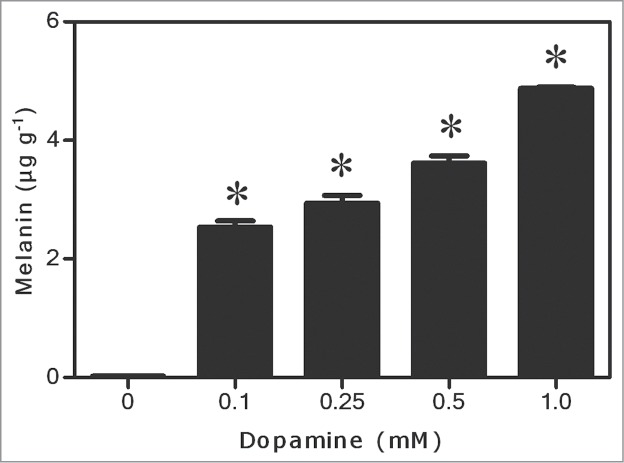
Effects of dopamine on melanin contents. *Values (N = 3 ± SE) differ statistically (Dunnett´s multiple comparison test) from control (P < 0.05).
Figure 2.
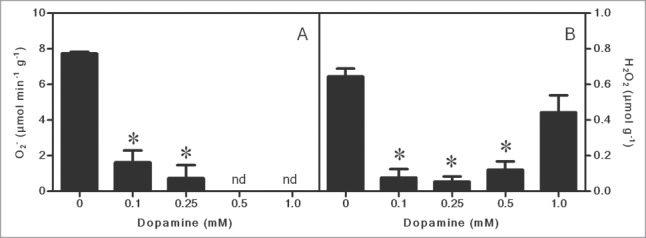
Effects of different concentrations of dopamine on the contents of superoxide anion (O2•−) and hydrogen peroxide (H2O2). *Values (N = 3 ± SE) differ statistically (Dunnett´s multiple comparison test) from control (P < 0.05). nd = not detected.
Figure 3.
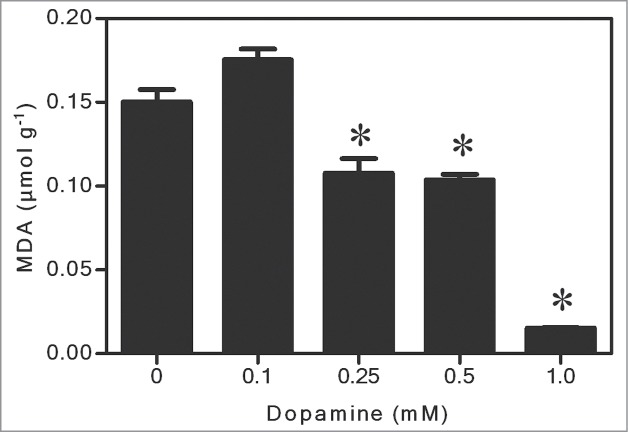
Effects of dopamine on the levels of lipid peroxidation. *Values (N = 3 ± SE) differ statistically (Dunnett´s multiple comparison test) from control (P < 0.05).
Figure 4.
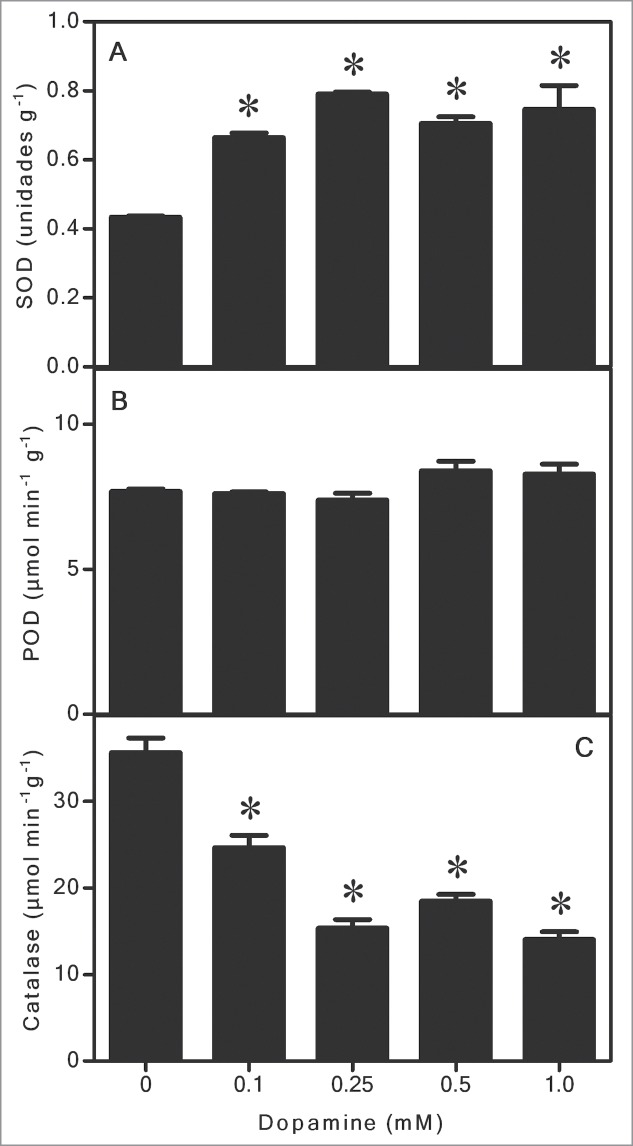
The effects of different concentrations of dopamine on the activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CT) in soybean roots. *Values (N = 3 ± SE) differ statistically (Dunnett´s multiple comparison test) from control (P < 0.05).
Discussion
In general, our studies show that dopamine increased melanin contents at all of the concentrations tested (Fig. 1). The O2•- (Fig. 2A) and H2O2 (Fig. 2B) contents decreased to a great extent, and these results also correlated with a significant diminution of lipid peroxidation (Fig. 3). The activity of SOD (Fig. 4A) increased, and it was followed by a reduction in the O2•- content. The POD activity showed no changes (Fig. 4B), while CAT activity was considerably lesser after dopamine treatment (Fig. 4C).
Increases in the melanin content of plants by treatment with catecholamines has been reported in earlier studies.14,17 Melanin can be produced by either enzymatic (tyrosinases and other enzymes) or non-enzymatic oxidation of dopamine and other catecholamines. With oxygen as the electron acceptor, these reactions generate ROS, such as O2•−, and radical semiquinones and quinones during metabolism into the pigment.1,9,11,12,18,19
Some research has shown that the toxicity of dopamine, in both animal and plant cells, is related to the synthesis of melanin and it is ROS dependent.20,21 Overall, ROS can attack cellular components, denaturing and inhibiting proteins, causing damage to DNA and membranes, blocking the absorption of nutrients and preventing root growth.13 By evaluating seed germination by different plant species on a filter paper in the presence of the L-DOPA, precursor of dopamine, Nishihara et al.22 observed a correspondence between alterations in the color of the paper and reduction of root. Yamato et al.20 using X-band electron spin resonance (ESR), provided evidence for ROS generation during dopamine metabolism in neurons. In addition, Guidotti et al.21 suggested that the increase in SOD activity and the decrease in cell viability in soybean root could be associated to augmented contents of ROS as a consequence of dopamine-induced oxidative stress, due its transformation into melanin.
As mentioned above, we observed an increase in the melanin content and, in contrast to the mentioned studies, dopamine decreased ROS levels (Fig. 2). In this context, some studies have reported contradictory effects with respect to the formation of ROS, suggesting a possible antioxidant activity of dopamine.15,16 The apparent contradiction seems to be determined by the conditions of the test and the dopamine concentration used. Kanazawa and Sakakibara4 tested the effect of dopamine on the peroxidation of linoleic acid and the elimination of the difenilpicrilidrazil radical ((DPPH). They noted that dopamine had superior antioxidant activities than food preservatives such as hydroxytoluene and butylated hydroxyanisole and a comparable power to strong antioxidants such as ascorbic acid. Yen and Hsieh16 observed that the antioxidant effects of dopamine and correlated molecules on the peroxidation of fat acid (linoleic), were in the sequence: dopamine > α-tocopherol = tyramine > tyrosine > noradrenaline. Herein, the results showed significant reductions in the contents of O2•- and H2O2 (Fig. 2A and B) and, in agreement with a decreased ROS levels, we also observed an inhibition of lipid peroxidation (Fig. 3), after dopamine treatments. Therefore, it is reasonable to suggest that, under the conditions tested, dopamine can act as an antioxidant compound. In fact, Spencer et al.15 reported that dopamine inhibited peroxidation of phospholipids in the brain; however, the compound appears to possess both antioxidant and pro-oxidant activity, depending on the concentration used.
Some studies also suggest a role as an antioxidant for melanin.23-25 Hoogduijn et al26 reported that melanin protects melanocytes from DNA rupture by H2O2, which suggests that the pigment has an significant antioxidant function. Tada et al.27 evaluated the effects of melanin in reaction with ROS generated by xanthine oxidase and hypoxanthine. The authors reported that melanin potently interacts with O2•- and singlet oxygen, eliminating these ROS. As noted herein, the melanin content increased after exposure to dopamine (Fig. 1), suggesting a possible role of this pigment in the elimination of ROS.
As aforementioned, SOD, POD and CT are antioxidant enzymes essential for cells. SOD acts by dismutating O2•- to H2O2. After the action of SOD, CAT and POD also protect the cells against ROS by detoxifying H2O2 to H2O.13 Soares et al.14 reported that soybean seedlings treated with L-DOPA, increased the SOD activity in roots. Takano et al.28 reported that SOD activity in astrocytic glial cells was augmented in a dose-dependent manner, in rats after 24 h of dopamine treatments. Herein, SOD activity also increased significantly after exposure to dopamine (Fig. 4A). Activation of SOD could lead to marked alterations in the levels of O2•- and thus contribute to explaining, at least in part, the observed reduction. In addition, we found that POD did not change significantly (Fig. 4B) and CT was reduced at all concentrations tested (Fig. 4C). We believe that both O2•- (Fig. 2A) and H2O2 (Fig. 2B) were eliminated mainly by the antioxidant activity of dopamine absorbed by the roots, and also by the melanin synthesized. These results are supported by the inhibition of lipid peroxidation (Fig. 3), showing that the effects observed with CAT and POD (Fig. 4B and C) are in agreement with the levels of ROS quantified in soybean roots treated with dopamine. Thus, it is possible that the lack of change in POD and the reduction in CT activity are due to the low contents of H2O2 in seedling roots subjected to dopamine.
The surprising information revealed in the present work is that the toxicity of dopamine seems not due purely to ROS species produced over its metabolism to melanin, as conjectured by some researchers aforementioned. Regarding the latter, a study exploring the gene products of soybean treated to dopamine would be remarkable. This advance may direct to a greater comprehension of the mechanism of action of dopamine in plants.
Materials and Methods
General procedures
Soybean (Glycine max (L.) Merr. cv. BRS-232) seeds, surface-sterilized with 2% sodium hypochlorite for 5 min and rinsed extensively with deionized water, were dark-germinated (at 25°C) on 3 sheets of moistened filter paper. Twenty-5 3-day-old seedlings of uniform size were supported on an adjustable acrylic plate and transferred into a glass container (10 × 16 cm) filled with 200 ml of half-strength Hoagland's solution (pH 6.0), without or with 0.25 to 1.0 mM dopamine. The container was kept in a growth chamber (25°C, 12 h photoperiod, irradiance of 280 μmol m−2 s−1). Roots were measured at the start and at the end of experiments (24 h). Fresh root weight was determined immediately after incubation and dry weight estimated after oven-drying at 80°C, for 24 h. Dopamine was purchased from Sigma Chemical Co. (St Louis, USA) and all other reagents used were of the purest grade available or chromatographic grade.
Quantification of melanin
Fresh roots (50 mg) were incubated with 1 ml of 1 M NaOH at 100°C. After 1 h, the absorbance was recorded at 470 nm. Melanin content was calculated by using a standard curve prepared with known concentrations of melanin.29 The results are expressed as μg melanin g−1 fresh weight.
Reactive oxygen species (ROS)
Superoxide anion (O2•−) was determined colorimetrically, according to the method of Wu and von Tiedemann.30 Roots (0.5 g) were homogenized with 2.7 ml of 50 mM phosphate buffer (pH 7.8). The homogenate (900 μl) was incubated with 100 μl of hydroxylamine (15 min, 30°C), and then 350 μl of the mixture were mixed with 350 μl of 17 mM sulfanilamide (in 30% acetic acid) and 350 μl of 7 mM N-(1-naphthyl) ethylenediamine dihydrochloride. Absorbance was measured at 540 nm. Results are expressed as μmol O2•− g−1 fresh weight. The H2O2 content was determined colorimetrically, according to the method of Hsu and Kao,31 with modifications. Roots (2 g) were homogenized in 3 ml of 50 mM phosphate buffer (pH 6.8). The homogenate was centrifuged (2,200 × g, 20 min), and then 3 ml of the extracted solution were mixed with 1 ml of 0.1% titanium chloride in 20% (v/v) H2SO4. The mixture was centrifuged at 2,200 × g for 15 min. Absorbance of the supernatant was measured at 410 nm by using the reaction mixture without the tissue extract as a blank. The H2O2 content was calculated from the extinction coefficient (0.25 mM−1 cm−1). Results are expressed as μmol H2O2 g−1 fresh weight.
Lipid peroxidation
After 24 h of incubation, seedlings were removed, and the roots were detached for the determination of the level of lipid peroxidation in terms of malondialdehyde (MDA). Fresh roots (0.25 g) were extracted with 2.5 ml of 67 mM phosphate buffer (pH 7.0). The extract was centrifuged (10,000 × g, 15 min, 4°C), and the supernatant was used to determine the content of MDA.32 An aliquot of 0.5 ml of the supernatant was added to 4 ml of 0.5% of thiobarbituric acid (prepared in 20% of trichloroacetic acid). The mixture was heated in boiling water for 10 min and then quickly cooled in an ice-bath; samples were then centrifuged (3,500 × g, 5 min). Absorbance of the supernatant was recorded at 532 nm, and this value was subtracted from the nonspecific absorbance (at 600 nm) value. The quantity of MDA was calculated from the extinction coefficient (155 mM−1 cm−1). The level of lipid peroxidation is expressed as nmol MDA g−1 fresh weight.
Enzymatic assays
After incubation, all treated or untreated seedling roots were detached and enzymes were extracted. SOD activity was assayed by using the photochemical nitroblue tetrazolium (NBT) method.33 Fresh roots (0.5 g) were ground in a mortar with 0.01 g of PVPP and 2 ml of 50 mM potassium phosphate buffer (pH 7.8) containing 1.0 mM EDTA. After centrifugation (2,200g, 20 min, 4°C), the supernatant was used for the determination of the enzyme activity. The reaction mixture (1.5 ml) contained 75 μM NBT, 4 μM riboflavin, 13 μM methionine, 50 mM phosphate buffer (pH 7.8) containing 1.0 mM EDTA, and 20 μl of enzyme extract. To exclude eventual interference on SOD activity, parallel controls with dopamine added in the reaction mixture without enzyme preparation were undertaken under the same experimental conditions. The reaction was initiated by placing the reaction-mixture tubes under 15-W fluorescent lamps (56 μmol m−2 s−1) for 10 min. The reaction was stopped by keeping the tubes in the dark for 10 min. The photoreduction of NBT (formation of purple formazan) was measured at 560 nm. One unit of SOD enzyme activity was defined as the amount of enzyme required to produce a 50% inhibition of the reduction of NBT. SOD activity is expressed as unit g−1 fresh weight.
For the POD assay, fresh roots (0.5 g) were ground in a mortar with 0.01 g of polyvinyl polypyrrolidone (PVPP) and 5 ml of 67 mM phosphate buffer (pH 7.0). The extract was centrifuged (2,200 × g, 5 min, 4°C), and the supernatant was used to determine the activity of POD. The enzyme activity was determined according to the method of Cakmak and Horst,34 with modifications. The reaction mixture (3 ml) contained 25 mM sodium phosphate buffer (pH 6.8), 2.58 mM guaiacol, and 10 mM H2O2. The reaction was initiated by the addition of the enzyme extract. The oxidation of guaiacol was followed for 5 min at 470 nm, and the enzyme activity was calculated from the extinction coefficient (25.5 mM−1 cm−1). POD activity is expressed as μmol tetraguaiacol min−1 g−1 fresh weight.
For the CAT assay, fresh roots (1.0 g) were ground in a mortar with 0.01 g of PVPP and 1 ml of 0.1 M potassium phosphate buffer (pH 7.0). The extract was centrifuged (2,200 × g, 10 min, 4°C), and the supernatant was used to determine the activity of CT. The enzyme activity was determined according to method of Tománkóva et al.35 The reaction mixture (0.5 ml) contained 60 mM H2O2 in 60 mM sodium phosphate buffer (pH 7.4). The reaction was initiated by the addition of 0.1 ml of enzyme extract. The mixture was incubated for 4 min at 37°C, and the reaction was stopped by the addition of 0.5 ml of 32.4 mM ammonium molybdate. The yellow complex of molybdate and H2O2 was measured at 405 nm, and the enzyme activity was calculated from the extinction coefficient (0.0655 mM−1 cm−1). CAT activity is expressed as μmol H2O2 consumed min−1 g−1 fresh weight.
Statistical design
The experimental design was completely randomized and each plot was represented by one glass container with 2five seedlings. Data are expressed as mean of 3 independent experiments ±SE. Significance of differences was undertaken by one-way variance analysis with GraphPad Prism® package (Version 2.0, GraphPad Software Inc.., USA, 1995). Difference between parameters was evaluated by Dunnett´s multiple comparison test and P values <0 .05 were considered statistically significant.
Funding Statement
Research was financially supported by the Brazilian Council for Scientific and Technological Development (CNPq).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors kindly thank Aparecida M. D. Ramos and Fabiano Rodrigo de Assis for their technical assistance.
References
- 1. Kulma A, Szopa J. Catecholamines are active compounds in plants. Plant Science 2007; 172:433-40; http://dx.doi.org/ 10.1016/j.plantsci.2006.10.013 [DOI] [Google Scholar]
- 2. Ponchet M, Martin-Tanguy J, Marais A, Martin C. Hydroxycinnamoyl acid amides and aromatic amines in the inflorescences of some Araceae species. Phytochemistry 1982; 21:2865-9; http://dx.doi.org/ 10.1016/0031-9422(80)85057-6 [DOI] [Google Scholar]
- 3. Lundström J, Agurell S. Biosynthesis of mescaline and tetrahydroisoquinoline alkaloids in Lophophora williamsii (Lem.) Coult. Acta Pharmaceutica Suecica 1971; 8:261-74; PMID:5560271 [PubMed] [Google Scholar]
- 4. Kanazawa K, Sakakibara H. High content of dopamine, a strong antioxidant, in cavendish banana. J Agricul Food chem 2000; 48:844-8; http://dx.doi.org/ 10.1021/jf9909860 [DOI] [PubMed] [Google Scholar]
- 5. Wichers HJ, Visser JF, Huizing HJ, Pras N. Occurrence of L-DOPA and dopamine in plants and cell cultures of Mucuna pruriens and effects of 2,4-D and NaCI on these compounds. Plant Cell, Tissue and Organ Culture 1993; 33:259-64; http://dx.doi.org/ 10.1007/BF02319010 [DOI] [Google Scholar]
- 6. Elstner EF, Konze JR, Selman BR, Stoffer C. Ethylene formation in sugar beet leaves - Evidence for the involvement of 3-hydroxytyramine and phenoloxidase after wounding. Plant Physiol 1976; 58:163-8; PMID:16659639; http://dx.doi.org/ 10.1104/pp.58.2.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ives MJ, Posner HB. Epinephrine, propanolol and the sucrose-ammonium inhibition of flowering in Lemnaa pausicostata. Plant Physiol 1982; 70:311-2; PMID:16662468; http://dx.doi.org/ 10.1104/pp.70.1.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagatsu I, Sudo Y, Nagatsu T. Tyrosine hydroxylation in the banana plant. Enzymologia 1972; 43:25-31; PMID:4403227 [PubMed] [Google Scholar]
- 9. Rosei MA, Blarzino C, Foppoli C, Mosca L, Coccia R. Lipoxygenase-catalyzed oxidation of catecholamines. Biochem Biophys Res Commun 1994; 200:344-50; PMID:8166703; http://dx.doi.org/ 10.1006/bbrc.1994.1454 [DOI] [PubMed] [Google Scholar]
- 10. Matsumoto H. The mechanisms of phytotoxic action and selectivity of non-protein aromatic amino acids L-DOPA and m-tyrosine. J Pesticide Sci 2011; 36:1-8; http://dx.doi.org/ 10.1584/jpestics.R10-15 [DOI] [Google Scholar]
- 11. Klegeris A, Korkina LG, Greenfield SA. Autoxidation of dopamine: A comparison of luminescent and spectrophotometric detection in basic solutions. Free Radical Biol Med 1995; 18:215-22; PMID:7744304; http://dx.doi.org/ 10.1016/0891-5849(94)00141-6 [DOI] [PubMed] [Google Scholar]
- 12. Pattison DI, Dean RT, Davies MJ. Oxidation of DNA, proteins and lipids by DOPA, protein-bound DOPA, and related catechol(amine)s. Toxicology 2002; 177:23-37; PMID:12126793; http://dx.doi.org/ 10.1016/S0300-483X(02)00193-2 [DOI] [PubMed] [Google Scholar]
- 13. Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Ann Rev Plant Biol 2004; 55:373-99; PMID:15377225; http://dx.doi.org/ 10.1146/annurev.arplant.55.031903.141701 [DOI] [PubMed] [Google Scholar]
- 14. Soares AR, Lucio Ferrarese MdL, Siqueira-Soares RdC, Marchiosi R, Finger-Teixeira A, Ferrarese-Filho O. The allelochemical L-DOPA increases melanin production and reduces reactive oxygen species in soybean roots. J Chem Ecol 2011; 37:891-8; PMID:21710366; http://dx.doi.org/ 10.1007/s10886-011-9988-2 [DOI] [PubMed] [Google Scholar]
- 15. Spencer J, Jenner A, Butler J, Aruoma O, Dexter D, Jenner P. Evaluation of the pro-oxidant and antioxidant actions of L-DOPA and dopamine in vitro: implications for Parkinson disease. Free Radical Res 1996; 24:95-105; PMID:8845917; http://dx.doi.org/ 10.3109/10715769609088005 [DOI] [PubMed] [Google Scholar]
- 16. Yen GC, Hsieh CL. Antioxidant effects of dopamine and related compounds. Biosci Biotechnol Biochem 1997; 61:1646-9; PMID:10336274; http://dx.doi.org/ 10.1271/bbb.61.1646 [DOI] [PubMed] [Google Scholar]
- 17. Hachinohe M, Matsumoto H. Involvement of melanin synthesis and reactive oxygen species in phytotoxic action of l-DOPA in carrot cells. Crop Protection 2007; 26:294-8; http://dx.doi.org/ 10.1016/j.cropro.2005.06.011 [DOI] [Google Scholar]
- 18. Khaldy H, Escames G, León J, Vives F, Luna JD, Acuña-Castroviejo D. Comparative effects of melatonin, L-deprenyl, Trolox and ascorbate in the suppression of hydroxyl radical formation during dopamine autoxidation in vitro. J Pineal Res 2000; 29:100-7; PMID:10981823; http://dx.doi.org/ 10.1034/j.1600-079X.2000.290206.x [DOI] [PubMed] [Google Scholar]
- 19. Meiser J, Weindl D, Hiller K. Complexity of dopamine metabolism. Cell Commun Signal 2013; 11; PMID:23683503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamato M, Kudo W, Shiba T, Yamada KI, Watanabe T, Utsumi H. Determination of reactive oxygen species associated with the degeneration of dopaminergic neurons during dopamine metabolism. Free Radical Res 2010; 44:249-57; PMID:20014978; http://dx.doi.org/ 10.3109/10715760903456084 [DOI] [PubMed] [Google Scholar]
- 21. Guidotti BB, Gomes BR, Siqueira-Soares RdC, Soares AR, Ferrarese-Filho O. The effects of dopamine on root growth and enzyme activity in soybean seedlings. Plant Signal Behav 2013; 8:e25477; PMID:23838960; http://dx.doi.org/ 10.4161/psb.25477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nishihara E, Parvez MM, Araya H, Fujii Y. Germination growth response of different species to the allelochemical L-3,4-dihydroxyphelalanine (L-DOPA). Plant Growth Regulation 2004; 42:181-9; http://dx.doi.org/ 10.1023/B:GROW.0000017483.76365.27 [DOI] [Google Scholar]
- 23. Sava VM, Yang SM, Hong MY, Yang PC, Huang GS. Isolation and characterization of melanic pigments derived from tea and tea polyphenols. Food Chem 2001; 73:177-84; http://dx.doi.org/ 10.1016/S0308-8146(00)00258-2 [DOI] [Google Scholar]
- 24. Sarna T, Wielgus A. Antioxidant and photoprotective properties of human iridial Melanin. Pigment Cell Res 2004; 17:434-; http://dx.doi.org/ 10.1111/j.1600-0749.2004.00175_23.x [DOI] [Google Scholar]
- 25. Wang Z, Dillon J, Gaillard ER. Antioxidant properties of melanin in retinal pigment epithelial cells. Photochem Photobiol 2006; 82:474-9; PMID:16613501; http://dx.doi.org/ 10.1562/2005-10-21-RA-725 [DOI] [PubMed] [Google Scholar]
- 26. Hoogduijn MJ, Cemeli E, Ross K, Anderson D, Thody AJ, Wood J. Melanin protects melanocytes and keratinocytes against H2O2-induced DNA strand breaks through its ability to bind Ca2+. Exp Cell Res 2004; 294:60-7; PMID:14980501; http://dx.doi.org/ 10.1016/j.yexcr.2003.11.007 [DOI] [PubMed] [Google Scholar]
- 27. Tada M, Kohno M, Niwano Y. Scavenging or quenching effect of melanin on superoxide anion and singlet oxygen. J Clin Biochem Nutr 2010; 46:224-8; PMID:20490317; http://dx.doi.org/ 10.3164/jcbn.09-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takano K, Tanaka N, Kawabe K, Moriyama M, Nakamura Y. Extracellular superoxide dismutase induced by dopamine in cultured astrocytes. Neurochem Res 2012; 38:32-41; PMID:22983620; http://dx.doi.org/ 10.1007/s11064-012-0882-2 [DOI] [PubMed] [Google Scholar]
- 29. Abdel-Naser MB, Krasagakis K, Garbe C, Eberle J. Direct effects on proliferation, antigen expression and melanin synthesis of cultured normal human melanocytes in response to UVB and UVA light. Photodermatol Photoimmunol Photomed 2003; 19:122-7; http://dx.doi.org/ 10.1034/j.1600-0781.2003.00034.x [DOI] [PubMed] [Google Scholar]
- 30. Wu YX, Von Tiedemann A. Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ Poll 2002; 116:37-47; PMID:11808554; http://dx.doi.org/ 10.1016/S0269-7491(01)00174-9 [DOI] [PubMed] [Google Scholar]
- 31. Hsu YT, Kao CH. Heat shock-mediated H2O2 accumulation and protection against Cd toxicity in rice seedlings. Plant Soil 2007; 300:137-47; http://dx.doi.org/ 10.1007/s11104-007-9396-0 [DOI] [Google Scholar]
- 32. Doblinski PMF, Lucio Ferrarese MdL, Huber DA, Scapim CA, Ferrarese-Filho O. Peroxidase and lipid peroxidation of soybean roots in response to p-coumaric and p-hydroxibenzoic acids. Brazilian Archives of Biol Technol 2003; 46:193-8; http://dx.doi.org/ 10.1590/S1516-89132003000200009 [DOI] [Google Scholar]
- 33. Giannopolitis CN, Ries SK. Superoxide dismutases occurrence in higher plants. Plant Physiol 1977; 59:309-14; PMID:16659839; http://dx.doi.org/ 10.1104/pp.59.2.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cakmak I, Horst W. Effect of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plantarum 1991; 83:463-8; http://dx.doi.org/ 10.1111/j.1399-3054.1991.tb00121.x [DOI] [Google Scholar]
- 35. Tománkóva K, Luhová L, Petrvalsky M, Pec P, Lebeda A. Biochemical aspects of reactive oxygen species formation in the interaction between Lycopersicon spp. and Oidium neolycopersici. Physiol Mol Plant Pathol 2006; 68:22-32; http://dx.doi.org/ 10.1016/j.pmpp.2006.05.005 [DOI] [Google Scholar]


