Abstract
We previously suggested that At-FLA4 and ABA signaling act in synergy. Reactive oxygen species generated from the NADPH oxidases At-RBOHD and At-RBOHF play an important role in cell wall integrity control and ABA signaling and here we investigate their role for the At-FLA4 pathway. We find that in the At-fla4 At-rbohD At-rbohF triple mutant the root phenotype of At-fla4 is enhanced. Moreover, the abnormally high level of reactive oxygen species in At-fla4 mutant does not depend on AtRBOHD and -F. Likewise, suppression of the At-fla4 phenotype by ABA does not depend on the 2 oxidases. Consistent with their lack of effect on ROS level in At-fla4, transcript level of AtRBOHD and -F is reduced in the At-fla4 mutant background. Taken together, our findings suggest that neither At-RBOHD nor At-RBOHF is involved in the synergism between ABA and At-FLA4. Consistently, the oxidases and At-FLA4 act independently of each other in ROS control.
Keywords: ABA, At-FLA4, At-RBOHD, At-rbohF, ROS, qRT-PCR
Abbreviations
- ROS
reactive oxygen species
- NADPH
nicotinamide adenine dinucleotide phosphate-oxidase
- RBOH
respiratory burst oxidase homolog
- FLAs
fasciclin-like arabinogalactan-proteins
- AGP
arabinogalactan protein
- ABA
abscisic acid
Fasciclin-like arabinogalactan-proteins (FLAs) form a sub-group of arabinogalactan proteins (AGP), 1 which were previously implicated in cell wall polymer biosynthesis, cell wall remodeling, and signaling.2 The At-FLA4 locus of A. thaliana plays a non-redundant role for root growth and salt tolerance.3-5 The root of At-fla4 shows a short and fat phenotype, which is caused by abnormal expansion of epidermal, cortical, and endodermal cells.5 Externally applied ABA suppresses the At-fla4 phenotype, both its salt-oversensitivity and its root elongation defect under salt-free conditions. However, the mechanistic role of At-FLA4 in ABA response is unclear.3
Arabidopsis contains 10 RESPIRATORY BURST OXIDASE HOMOLOG (RBOH) genes and RBOH-dependent Reactive oxygen species (ROS) have now been established as an important second messenger that regulates expression of hundreds of genes in response to stress.6 Reactive oxygen species generated by the partially redundant RBOH isoforms D and F play an important role in abscisic acid (ABA) signaling in stomatal guard cells and roots.7 ROS derived from At-RBOHF are involved in the regulation of osmosensitive metabolic changes8 and both At-RBOHD and At-RBOHF play crucial roles in modulating ABA-inhibited root growth via production of ROS.9 On the other hand, At-RBOHD and At-RBOHF are required for lignin deposition caused by cellulose biosynthesis inhibition in Arabidopsis roots implicating these loci with cell wall integrity control.10,11
Because of the involvement of At-RBOHD and -F in ABA signaling, salt tolerance and cell wall integrity control and the proposed role of At-FLA4 as a link between cell walls and ABA signaling we tested the possibility that At-FLA4 and At-RBOHD and -F might act in the same genetic pathway by isolating At-fla4 At-rbohD At-rbohF triple mutants.5,7 The At-rbohD AtrbohF and the At-fla4 At-rbohD At-rbohF mutant combinations are hitherto referred to as double and triple mutant, respectively.
On NaCl-free medium (MS0) the At-fla4 mutant and the double mutant show a significant reduction of root length (Fig. 1A), as previously reported.3,7 Moreover, the triple mutant shows shorter root length compared to the At-fla4 mutant and the double mutant (Fig. 1A). Transfer to and growth on 100 mM NaCl for 48 hrs leads to a moderate suppression of root growth in the wild type and the double mutant. The At-fla4 single mutant and the triple mutant are both dramatically shorter (P < 0.001) than the wild type (Fig. 1A) and show root swelling to a comparable degree (Fig. 1B). The data indicate an additive effect of At-FLA4 and the 2 oxidases on root growth.
Figure 2.
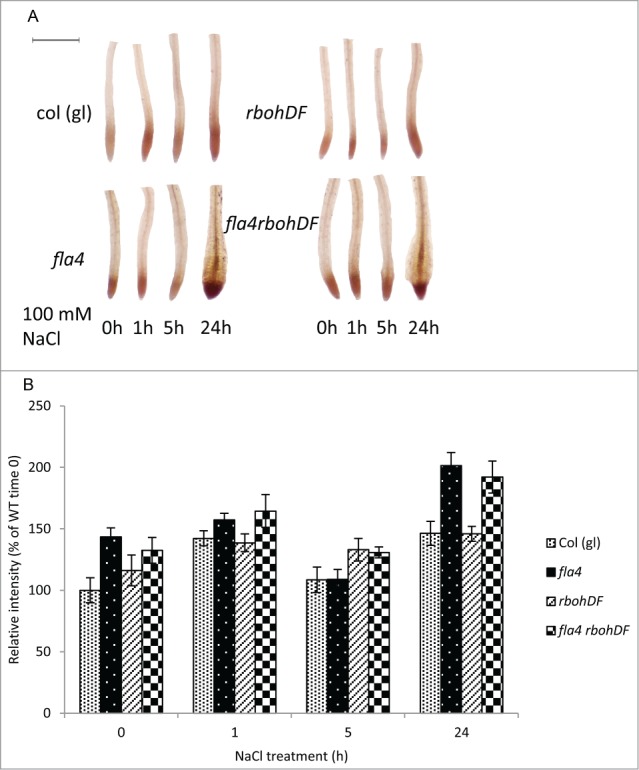
Effect of At-FLA4, At-RBOHD, -F and NaCl treatment on ROS levels. (A) DAB staining for ROS (brown) of seedlings grow on NaCl medium. The six-day-old seedlings were transferred to 100 mM NaCl medium for different time period. ROS was detected by DAB (Sigma) staining according to manufacturers’ instructions. Pictures were taken by a dissecting microscope (Leica EZ4 HD). Scale bar = 0.5 mm. (B) Quantification of ROS after transfer to NaCl medium. The data showed is the inverted gray value in the meristem minus background measured by Image J (n ≥ 8, ± confidence interval, α = 0.05). Normalized to Col at time 0.
Figure 3.
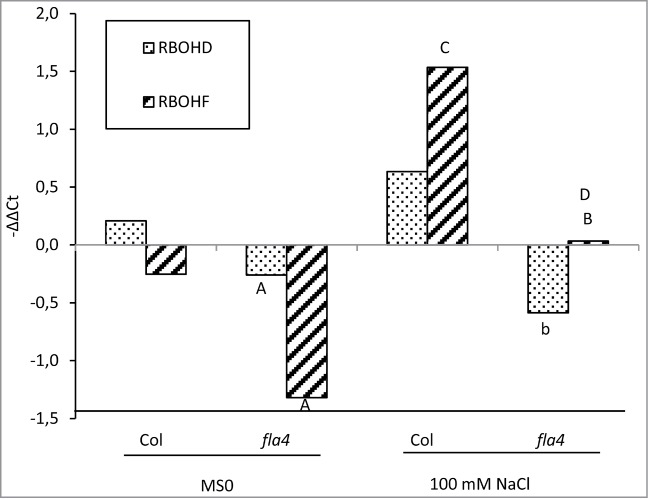
QRT-PCR-based analysis of the effects of NaCl treatment on At-RBOHD and At-RBOHF gene expression. 150 seedlings of Col-0 and At-fla4 were grown on a nylon mesh (20 mm mesh size; Prosep, Belgium) for 5 d and were transferred to standard medium with or without 100 mM NaCl and incubated for 40 min. Roots were removed from the seedlings for RNA extraction. Samples were treated in biological triplicates. Detailed data analysis was described previously.3 The indicated pairs were tested for statistically significant differences, and t-test values ≤0.05 and values ≤0.01 are indicated with lower case and uppercase letters, respectively, in the figures. (A) Col vs. At-fla4 on standard medium (MS0), (B) Col vs. At-fla4 on 100 mM NaCl, C: Col MS0 vs. Col NaCl, D: At-fla4 MS0 vs. At-fla4 NaCl.
Figure 1.
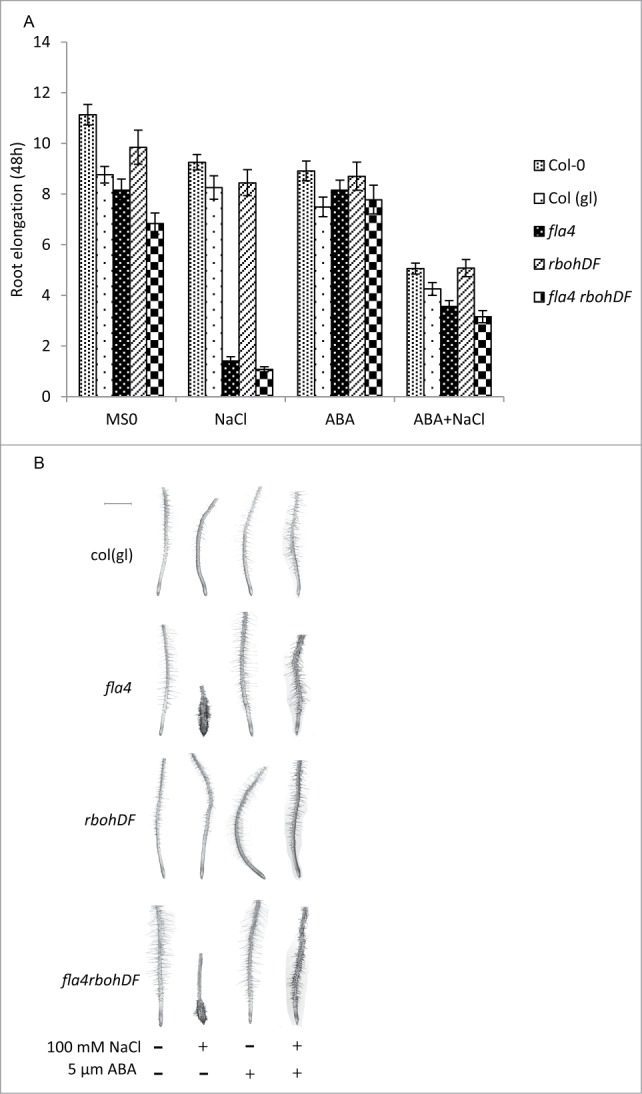
Interaction between At-FLA4 and ABA is independent on At-RBOHD and At-RBOHF. (A) The effect of ABA on root length requires At-FLA4. Six-day-old WT, At-fla4, double and triple mutant seedlings were transferred to MS medium, or MS medium supplemented with 100 mM NaCl (NaCl), 5 μm ABA (ABA), or 100 mM NaCl +5 μm ABA (NaCl+ABA), respectively. Root length was measured after 48 h. Data are mean ± confidence interval (n ≥ 50, α = 0.05). (B) Salt tolerance in mutant in presence and absence of ABA. Six-day-old seedlings were transferred to different medium as described previously. Pictures were taken by a dissecting microscope (Leica EZ4 HD). Scale bar = 1 mm.
As previously reported, the At-fla4 phenotype is suppressed by 5 μM ABA both on salt free medium and on 100 mM NaCl (Fig. 1).3 We also confirmed the recent observation that the double mutant is shorter and less responsive to ABA than wild type (Fig. 1A).7 However, triple mutant roots are fully responsive to 5 μM ABA with respect to the suppression of the At-fla4 mutant phenotype (Fig. 1). Taken together, this means that the ABA effect on At-fla4 does not depend on the function of At-RBOHD and At-RBOHF.
Next, we analyzed salt stress-induced ROS in roots with 3, 3’-diaminobenzidine tetrahydrochloride (DAB).12,13 Salt-stimulated ROS production show a biphasic profile with an elevation after 1 h NaCl treatment followed by a drop after 5 hrs and a second maximum at 24 hrs (Fig. 2A). Surprisingly, the double mutant shows the same profile and no significant differences to wild type. By contrast, in At-fla4 and triple mutant roots the initial control level and final level of ROS are significantly (P < 0.001) higher compared to the wild type and the double mutant (Fig. 2B). This experiment suggests that loss of At-FLA4 function triggers a signaling events leading to increased ROS production. However, the effect of At-RBOHD and -F in this process might either be negligible or too subtle to detect with DAB staining.
It was previously shown that NaCl medium leads to increased transcript levels of At-RBOHD and -F.14 Consistently, transfer to and growth on 100 mM NaCl for 40 min leads to an increase of At-RBOHF mRNA level in both wild type and mutant (Fig. 3). However, despite the increase in DAB staining, RNA levels of both At-RBOHD and -F are significantly (P < 0.05) lower in At-fla4 compared to wild type.
In this study, all the evidence suggests that the interaction between At-fla4 and ABA signaling is independent of At-RBOHD and At-RBOHF. ABA suppresses At-fla4 and the triple mutant, both the salt-oversensitive phenotype and its root elongation phenotype, under salt-free conditions. ROS generated by At-RBOHD and At-RBOHF are not required for the At-fla4 phenotype. The unchanged level of ROS in the double mutant compared to the wild type and in the triple mutant compared to the At-fla4 single mutants in the absence and presence of NaCl, indicates that other cellular mechanism may contribute to ROS generation in Arabidopsis roots.15 Our results do not exclude the possibility that other NADPH oxidase isoforms may be involved in the At-FLA4 pathway. Apart from elevated ROS levels, the increase of DAB staining in At-fla4 might also be due to altered peroxidase levels in mutant roots. The mechanism behind ABA suppressing At-fla4 phenotype is presently unknown, however our data indicate that this process acts independently of At-RBOHD and -F.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Funding
This work was supported by the Austrian Science Fund (FWF - grant numbers P21782-B12, I1182-B22). H.X. was supported by the China Scholarship Council.
References
- 1. Johnson KL, Jones BJ, Bacic A, Schultz CJ. The fasciclin-like arabinogalactan proteins of Arabidopsis. A multigene family of putative cell adhesion molecules. Plant Physiol 2003; 133:1911-25; PMID:14645732; http://dx.doi.org/ 10.1104/pp.103.031237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seifert GJ, Roberts K. The biology of arabinogalactan proteins. Ann Rev Plant Biol 2007; 58:137-61; PMID:17201686; http://dx.doi.org/ 10.1146/annurev.arplant.58.032806.103801 [DOI] [PubMed] [Google Scholar]
- 3. Seifert GJ, Xue H, Acet T. The Arabidopsis thaliana FASCICLIN LIKE ARABINOGALACTAN PROTEIN 4 gene acts synergistically with abscisic acid signalling to control root growth. Ann Bot 2014; PMID:24603604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu SL, Rahman A, Baskin TI, Kieber JJ. Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell 2008; 20:3065-79; PMID:19017745; http://dx.doi.org/ 10.1105/tpc.108.063354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi H, Kim Y, Guo Y, Stevenson B, Zhu JK. The Arabidopsis SOS5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. Plant Cell 2003; 15:19-32; PMID:12509519; http://dx.doi.org/ 10.1105/tpc.007872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li S, Ge FR, Xu M, Zhao XY, Huang GQ, Zhou LZ, Wang JG, Kombrink A, McCormick S, Zhang XS, et al. Arabidopsis COBRA-LIKE 10, a GPI-anchored protein, mediates directional growth of pollen tubes. Plant J 2013; 74:486-97; PMID:23384085; http://dx.doi.org/ 10.1111/tpj.12139 [DOI] [PubMed] [Google Scholar]
- 7. Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 2003; 22:2623-33; PMID:12773379; http://dx.doi.org/ 10.1093/emboj/cdg277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wormit A, Butt SM, Chairam I, McKenna JF, Nunes-Nesi A, Kjaer L, O'Donnelly K, Fernie AR, Woscholski R, Barter MC, et al. Osmosensitive changes of carbohydrate metabolism in response to cellulose biosynthesis inhibition. Plant Physiol 2012; 159:105-17; PMID:22422940; http://dx.doi.org/ 10.1104/pp.112.195198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiao Y, Sun L, Song Y, Wang L, Liu L, Zhang L, Liu B, Li N, Miao C, Hao F. AtrbohD and AtrbohF positively regulate abscisic acid-inhibited primary root growth by affecting Ca2+ signalling and auxin response of roots in Arabidopsis. J Exp Bot 2013; 64:4183-92; PMID:23963673; http://dx.doi.org/ 10.1093/jxb/ert228 [DOI] [PubMed] [Google Scholar]
- 10. Hamann T, Bennett M, Mansfield J, Somerville C. Identification of cell-wall stress as a hexose-dependent and osmosensitive regulator of plant responses. Plant J 2009; 57:1015-26; PMID:19036034; http://dx.doi.org/ 10.1111/j.1365-313X.2008.03744.x [DOI] [PubMed] [Google Scholar]
- 11. Denness L, McKenna JF, Segonzac C, Wormit A, Madhou P, Bennett M, Mansfield J, Zipfel C, Hamann T. Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol 2011; 156:1364-74; PMID:21546454; http://dx.doi.org/ 10.1104/pp.111.175737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daudi A, Cheng Z, O'Brien JA, Mammarella N, Khan S, Ausubel FM, Bolwell GP. The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 2012; 24:275-87; PMID:22247251; http://dx.doi.org/ 10.1105/tpc.111.093039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bindschedler LV, Dewdney J, Blee KA, Stone JM, Asai T, Plotnikov J, Denoux C, Hayes T, Gerrish C, Davies DR, et al. Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J 2006; 47:851-63; PMID:16889645; http://dx.doi.org/ 10.1111/j.1365-313X.2006.02837.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ma L, Zhang H, Sun L, Jiao Y, Zhang G, Miao C, Hao F. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na(+)/K(+)homeostasis in Arabidopsis under salt stress. J Ex Bot 2012; 63:305-17; PMID:21984648; http://dx.doi.org/ 10.1093/jxb/err280] [DOI] [PubMed] [Google Scholar]
- 15. Marino D, Dunand C, Puppo A, Pauly N. A burst of plant NADPH oxidases. Trends Plant Sci 2012; 17:9-15; PMID:22037416; http://dx.doi.org/ 10.1016/j.tplants.2011.10.001 [DOI] [PubMed] [Google Scholar]


