Abstract
Tomato fruits (Solanum lycopersicum L.) accumulate flavonoids in their cuticle and epidermal cells during ripening. These flavonoids come from de novo biosynthesis due to a significant increase in chalcone synthase (CHS) activity during ripening. Virus-induced gene silencing (VIGS) of tomato fruits have been used to down-regulate SlCHS expression during ripening and analyze the effects at the epidermal and cuticle level. Besides the expected change in fruit color due to a lack of flavonoids incorporated to the cuticle, several other modifications such as a decrease in the amount of cutin and polysaccharides were observed. These indicate a role for either flavonoids or CHS in the alteration of the expression levels of some genes involved in cuticle biosynthesis. Moreover, a negative interaction between the 2 cuticle components, flavonoids and waxes, suggests a relationship between these 2 metabolic pathways.
Keywords: cutin, flavonoids, plant cuticle, tomato, waxes
The plant cuticle is an extracellular membrane that covers the outer epidermal cell wall of aerials organs such as leaves, flowers and fruits.1 It is chiefly composed of a lipid matrix of interesterified polyhydroxy fatty acids named cutin that is intertwined with cell wall polysaccharides. Other components are waxes and phenolics. In tomato, phenolics accumulated in the fruit cuticle vary with the developmental stage, being the main compounds coumaric and benzoic acid during fruit growth and flavonoids such as naringenin chalcone and naringenin flavanone during ripening.2 Incorporation of flavonoids was postulated to modify the cuticle's mechanical properties by increasing its resistance to deformation.3 Thus, during the period of organ expansion, the cuticle can be deformed with little stress applied.2 CHALCONE SYNTHASE (CHS) is the enzyme responsible for the synthesis of chalconaringenin, the flavonoid mainly accumulated in tomato fruit cuticle and responsible for its orange-yellow color. Flavonoids are being shown to interact with several metabolic pathways sometimes by altering hormone levels. In this sense, constitutive SlCHS suppression in tomato led to parthenocarpy, probably as a response to the observed pollen growth impairment.4
In a recent publication we analyzed how flavonoid accumulation during ripening affected epidermal and cuticle properties. In this work, we studied the results of down-regulating the expression of SlCHS during ripening by means of virus-induced gene silencing (VIGS) in different tomato genotypes. Firstly, we analyzed the epidermal expression level of the 2 CHS genes (SlCHS1 Solyc09g091510.2 and SlCHS2 Solyc05g053550) known to be expressed in the fruit during ripening. Both CHS showed a very low expression at mature green but peaked at breaker, with SlCHS2 showing a higher expression level than SlCHS1. Silencing of flavonoid synthesis during ripening rendered tomatoes with differentially colored regions: red (non-silenced) and pink (silenced) which allowed their physical isolation and ulterior analysis. Silenced regions showed an alteration in the final step of cell expansion since the epidermal cells of pink sectors were similar in size and shape to those of mature green fruits. Thus, cells were more rounded due to a decreased in tangential width. These results clearly support a role of flavonoids in modifying cell expansion possibly mediated by auxins. Flavonols, such as quercetin, have been shown to modify auxin transport thus altering tissue growth.5 More recently, naringenin has also been involved in retarding cotton cell development.6 Despite the results observed in the epidermal cells suggested that mature silenced regions were similar to the mature green stage, this was not the case for the cuticle. Cuticles of the pink regions were significantly different from those of the red sectors but also from the mature green stage already reported in the literature.2,4 In tomato, flavonoids are synthetized during fruit development7,8 and most of them are assumed to accumulate in the vacuole.9,10 Inhibition of flavonoid synthesis during ripening clearly indicated that flavonoids incorporated to the cuticle come from de novo synthesis and not from vacuole stored flavonoids. This lack of flavonoids decreased the amount of cuticle accumulated mostly due to a reduction of its main components cutin and polysaccharides (Table 1). Although a decrease in cuticle polysaccharides during fruit ripening has been reported,11 this association of flavonoid biosynthesis and cuticle deposition has not been described before. Ripening has been shown to cause a reduction in the number of ester bonds in the cutin matrix.2 This reduction was shown to be associated with flavonoid incorporation to the cuticle and not ripening since the pink sectors ripened but had a higher number of ester bonds than the red sectors. Clearly, flavonoids seem to affect the expression of several genes involved in the last stages of cuticle deposition and/or remodeling.
Table 1.
Resume of changes observed in the epidermis and cuticle of tomato fruits agroinoculated to silence SlCHS. Comparisons were established between the control red sectors and the SlCHS-silenced pink sectors
| Traits affected by SlCHS silencing | |
|---|---|
| Amount (μg cm−2) | |
| cuticle | decreased |
| cutin | decreased |
| polysaccharides | decreased |
| phenolics | decreased |
| Cuticle biomechanical properties | |
| stiffness | lower |
| deformation | increased |
| Cuticle water permeability | lower |
| Cuticle invagination | decreased |
| Cutin esterification index | higher |
| Epidermal cell shape | more rounded |
Waxes, on the other hand, did not seem to be affected by the inhibition of SlCHS expression since their amount did not change between the silenced and non-silenced sectors. Hence, their relative contribution to the overall cuticle (% of waxes) was increased in the pink sectors due to the decrease in the amount of cuticle (Fig. 1A). Results obtained from 2 of the genotypes studied supported this explanation. However, results from the VIGS-Ready genotype did not support it. VIGS-Ready is a tomato that accumulates anthocyanins in the pericarp due to the ripening specific expression of 2 Antirrhinum majus transcription factors: ROSEA1 and DELILA.12 VIGS silencing of these transcription factors rendered ripe tomato with purple (non-silenced) and red (silenced) sectors. In both cases, cuticles were very similar except for a significant increase in the amount of waxes present in the purple sectors. Thus, another explanation for the results would be a negative cross-talk between the flavonoid and wax pathways. Indeed, comparison of the percentages of waxes and phenolics showed an inverse relationship between these 2 minor cuticle components (Fig. 1A) which seems controlled by the phenolic compounds. Waxes and flavonoids are minor compounds present in the cuticle of the tomato fruit (Fig. 1B). As such, they have been shown to modify the physical properties of the cuticle, especially the biomechanics.2,13 Waxes are complex mixtures of very long chain aliphatic molecules (aldehydes, alcohols, fatty acids, alkanes) and cyclic compounds (sterols, triterpenoids)14 which are the product of different metabolic pathways.15 Our results indicate a negative interaction between the flavonoid and wax pathways; however, it remains to be determined which type or types of waxes are modified. This change in the relative contribution of waxes associated with flavonoids could also be responsible for the decreased water permeability observed in the cuticles of the silenced regions (Table 1). In tomato, cuticle water permeability has been negatively correlated with the amount of fruit waxes, mostly with the very-long-chain aliphatic fraction.16
Figure 1.
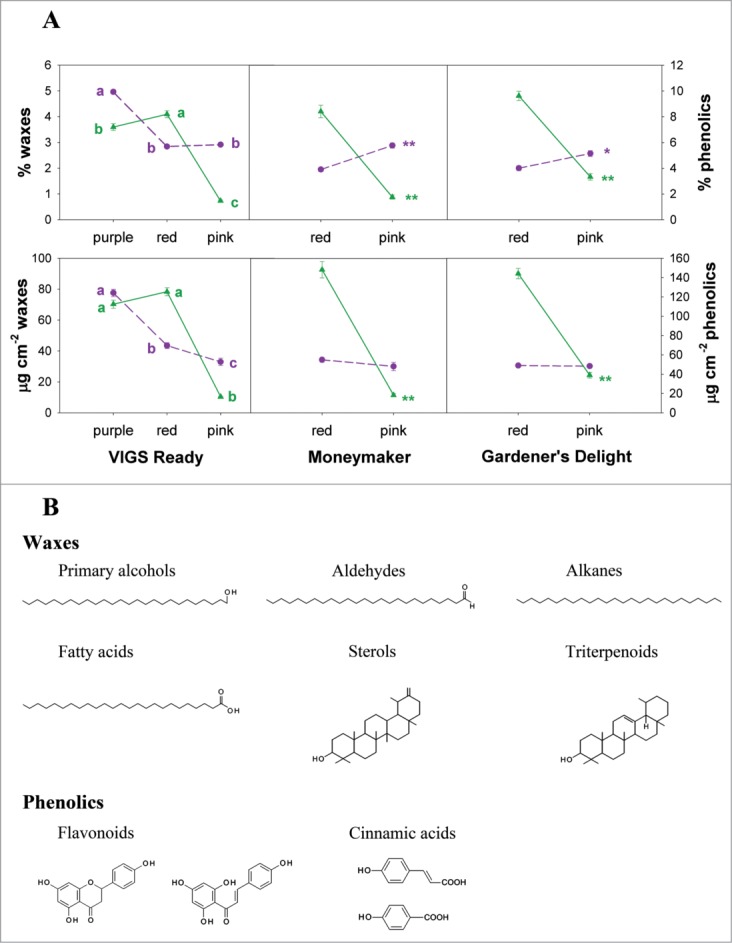
(A) Changes in the percentages and amount (expressed as μg cm−2) of phenolics (solid green lines) and waxes (dashed purple lines) in the cuticles isolated from the different sectors obtained after fruits were agroinoculated to silence SlCHS. Letters indicate significant differences according to one-way ANOVA with P < 0.05 or with asterisks according to t-tests with P < 0.01 (**). (B) Main chemical components of the wax and phenolic fractions of tomato fruit cuticles.
It remains an unanswered question whether the 2 SlCHS genes play a redundant role or a differential one. Both shared the same epidermal expression profile during ripening, with SlCHS2 showing the highest expression level. Nuclear localization of CHS and flavonoids has been found in some species and clearly suggests a key function in the control of transcription of specific genes.17,18 Considering the significant number of effects found to be regulated by CHS at the epidermal level, both SlCHS might be playing different roles with one more related to the regulation of gene expression and the other one to flavonoid biosynthesis. This is however speculative since the high degree of similarity of both genes prevents an efficient down regulation of one of them without affecting the expression level of the other.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grant AGL2012-32613 from the Plan Nacional de I+D, Ministry of Science and Innovation (MICINN), Spain, co-funded by EU. FEDER. José A. Heredia-Guerrero is supported by a Marie Curie Intra-European Fellowship (BIOPROTO project), financed by the EU's 7th Framework Program for Research (FP7).
References
- 1.Domínguez E, Heredia-Guerrero JA, Heredia A. The biophysical design of plant cuticles: an overview. New Phytol 2011; 189: 938–49; PMID:21374891; http://dx.doi.org/ 10.1111/j.1469-8137.2010.03553.x [DOI] [PubMed] [Google Scholar]
- 2.España L, Heredia-Guerrero JA, Segado P, Benítez JJ, Heredia A, Domínguez E. Biomechanical properties of tomato fruit cuticle during development are modulated by changes in the relative amount of their components. New Phytol 2014; 202: 790–802; PMID:24571168; http://dx.doi.org/ 10.1111/nph.12727 [DOI] [PubMed] [Google Scholar]
- 3.Domínguez E, España L, López-Casado G, Cuartero J, Heredia A. Biomechanics of isolated tomato fruit cuticles during ripening: the role of flavonoids. Func Plant Biol 2009; 36: 613–20; http://dx.doi.org/ 10.1071/FP09039 [DOI] [PubMed] [Google Scholar]
- 4.Schijlen EGWM, de Vos RCH, Martens S, Jonker HH, Rosin FM, Molthoff JW, Tikunov YM, Angement GC, van Tunen AJ, Bovy AG. RNA interference silencing of chalcone synthase, the first step in the flavonoid biosynthesis pathway, leads to parthenocarpic tomato fruits. Plant Physiol 2007; 144: 1520–30; PMID:17478633; http://dx.doi.org/ 10.1104/pp.107.100305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buer CS, Kordbacheh F, Truong TT, Hocart CH, Djordjevic MA. Alteration of flavonoid accumulation patterns in transparent testa mutants disturbs auxin transport, gravity responses, and imparts long-term effects on root and shoot architecture. Planta 2013; 238: 171–89; PMID:23624937; http://dx.doi.org/ 10.1007/s00425-013-1883-3 [DOI] [PubMed] [Google Scholar]
- 6.Tan J, Tu L, Deng F, Hu H, Nie Y, Zhang X. A genetic and metabolic analysis revealed that cotton fiber cell development was retarded by flavonoid naringenin. Plant Physiol 2013; 162: 86–95; PMID:23535943; http://dx.doi.org/ 10.1104/pp.112.212142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slimestad R, Verheul M. Review of flavonoids and other phenolics from fruits of different tomato (Lycopersicon esculentum Mill.) cultivars. J Sci Food Agric 2009; 89: 1255–70; http://dx.doi.org/ 10.1002/jsfa.3605 [DOI] [Google Scholar]
- 8.Meléndez-Martínez AJ, Fraser PD, Bramley PM. Accumulation of health promoting phytochemicals in wild relatives of tomato and their contribution to in vitro antioxidant activity. Phytochemistry 2010; 71: 1104–14; PMID:20457456; http://dx.doi.org/ 10.1016/j.phytochem.2010.03.021 [DOI] [PubMed] [Google Scholar]
- 9.Koes RE, Quattrocchio F, Mol JNM. The flavonoid biosynthetic pathway in plants: function and evolution. BioEssays 1994; 16: 123–32; http://dx.doi.org/ 10.1002/bies.950160209 [DOI] [Google Scholar]
- 10.Mol JNM, Grotewold E, Koes RE. How genes paint flowers and seeds. Trends Plant Sci 1998; 3: 212–7; http://dx.doi.org/ 10.1016/S1360-1385(98)01242-4 [DOI] [Google Scholar]
- 11.Domínguez E, López-Casado G, Cuartero J, Heredia A. Development of fruit cuticle in cherry tomato (Solanum lycopersicum). Funct Plant Biol 2008; 35: 403–11; http://dx.doi.org/ 10.1071/FP08018 [DOI] [PubMed] [Google Scholar]
- 12.Butelli E, Titta L, Giorgio M, Mock HP, Matros A, Peterek S, Schijlen E, Hall RD, Bovy A, Luo J, Martin C. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotech 2008; 26: 1301–8; PMID:18953354; http://dx.doi.org/ 10.1038/nbt.1506 [DOI] [PubMed] [Google Scholar]
- 13.Khanal BP, Grimm E, Finger S, Blume A, Knoche M. Intracuticular waxes fixes and restricts strain in leaf and fruit. New Phytol 2013; 200: 134–43; PMID:23750808; http://dx.doi.org/ 10.1111/nph.12355 [DOI] [PubMed] [Google Scholar]
- 14.Leide J, Hildebrandt U, Reussing K, Riederer M, Vogg G. The developmental pattern of tomato fruit wax accumulation and its impact on cuticular transpiration barrier properties: effects of a deficiency in a b-ketoacyl-coenzyme A synthase (LeCER6). Plant Physiol 2007; 144: 1667–79; PMID:17468214; http://dx.doi.org/ 10.1104/pp.107.099481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunst L, Samuels L. Plant cuticles shine: advances in wax biosynthesis and export. Curr Opin Plant Biol 2009; 12: 721–7; PMID:19864175; http://dx.doi.org/ 10.1016/j.pbi.2009.09.009 [DOI] [PubMed] [Google Scholar]
- 16.Vogg G, Fischer S, Leide J, Emmanuel E, Jetter R, Levy AA, Riederer M. Tomato fruit cuticular waxes and their effects on transpiration barrier properties: functional characterization of a mutant deficient in a very-long-chain fatty acid b-ketoacyl-CoA synthase. J Exp Bot 2004; 55: 1401–10; PMID:15133057; http://dx.doi.org/ 10.1093/jxb/erh149 [DOI] [PubMed] [Google Scholar]
- 17.Saslowsky DE, Warek U, Winkel BSJ. Nuclear localization of flavonoid enzymes in Arabidopsis. J Biol Chem 2005; 280: 23735–40; PMID:15817473; http://dx.doi.org/ 10.1074/jbc.M413506200 [DOI] [PubMed] [Google Scholar]
- 18.Agati G, Azzarello E, Pollastri S, Tattini M. Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 2012; 196: 67–76; PMID:23017900; http://dx.doi.org/ 10.1016/j.plantsci.2012.07.014 [DOI] [PubMed] [Google Scholar]


