Abstract
Root apical meristem (RAM) is central for indeterminate growth of plant roots and in sensing environmental stimuli, such as water status. Recently, we reported that PEG8000-simulated mild and moderate osmotic stress induces premature differentiation of RAM, which is a conserved adaptive mechanism in higher plants to cope with water stress. Microarray data analysis revealed that the ABA signaling pathway may be involved in water stress-induced RAM premature differentiation. Here we showed that in wheat, ABA contents increased under water stress with the highest level of ABA in the RAM. Exogenous ABA also induces RAM premature differentiation in both wheat and Arabidopsis plants. Further genetic analysis revealed that loss of function mutations in ABA2 and ABA receptors significantly reduced the level of root tip swelling and RAM premature differentiation in response to PEG-simulated water stress. Together, the results suggest that ABA participates in regulation of PEG-mediated premature differentiation of RAM.
Keywords: ABA, premature differentiation, root apical meristem, water stress
Abbreviations
- PEG8000
polyethylene glycol
- RAM
root apical meristem
- ABA
abscisic acid
Introduction
Mild and moderate water stresses appear frequently during the whole life of plants. To cope with the changing water status in the growing environment, plants have evolved various adaptive mechanisms by which plants can modify root allocation and root system architecture to obtain more water. For example, plant roots can sense soil moisture gradients and grow toward to a source of water (referred as hydrotropism).1 Plants also can modify their root system architecture to adapt themselves to the water stress conditions. We have previously shown that plants are able to stimulate lateral root development with enhanced stress tolerance through programmed cell death of the root apical meristem (RAM) of the primary roots under severe water stress.2 Recently, we reported that under mild and moderate water stresses, plant primary roots undergo root tip swelling and RAM premature differentiation resulting in cessation of the primary root growth.3 We demonstrated that the RAM premature differentiation of primary roots and subsequent lateral formation are directly correlated to plant stress tolerance. Importantly, we provide evidence that the RAM premature differentiation and remodeling of root system architecture induced by mild and moderate water stresses simulated by PEG8000 is a conserved adaptive mechanism that is well adopted by various plants to cope with water stress. Genome wide gene expression analysis of wheat root tips undergoing premature differentiation under mild stress conditions suggests that histone modification plays a key role in the regulation of gene expression and the developmental reprogramming that determine RAM premature differentiation in response to PEG-mediated mild water stress; and altered expression levels of ABA related genes suggest that ABA participates PEG-mediated RAM premature differentiation and subsequent root system remodeling.
Results
ABA contents in RAM increased under water stress
To examine whether ABA modulates the morphological changes of root tips and RAM premature differentiation, we first analyzed the ABA contents of wheat root tips under water stress conditions using enzyme linked immunosorbent assay (ELISA) method.4 6-day-old wheat seedlings grown under normal conditions were treated with 5% PEG 8000 solution (-0.047 MPa), and ABA contents of the RAM region (2 mm in length) and the region above the RAM (2 mm in length) were measured. As shown in Fig. 1A, ABA contents in both regions were markedly increased starting at 8 h after stress treatment. ABA in the RAM region increasingly accumulated over a prolonged period of time (72 h), whereas the ABA content in the upper region of the stress plants remained relatively unchanged over 72 h. The result indicates that ABA might be involved in the morphological changes including root tip cell swelling and root premature differentiation.
Figure 1.

ABA promotes root meristem premature differentiation. (A) ABA contents in root RAM region (RAM, 2 mm in length) and the region above the RAM (ARAM, 2 mm in length) were measured. Six-day-old K199 variety of wheat root tips were collected at 0, 8 and 72 h after 5% PEG8000 treatment. (B) Six-day-old K199 variety of wheat seedlings were treated with 20 μM ABA solution at 0 and 72 h and stained with 0.005% Tetrazolium Violet (reddish color). Bar = 0.5 mm. (C) Quantification of length of stained root tips (reddish color) shown in (B). (D) Six-day-old Arabidopsis seedlings (Col-0) were treated with 2 μM ABA solution at 0 and 72 h. The red arrowheads show the transition zone. Bar = 100 μm. (E) Quantification of meristem size shown in (D). The meristem sizes of root tips are defined by measuring the length from the initial cortical cell adjacent to the quiescence center to the first enlarged cortex cell. Data are presented as means ± SD, and columns marked with asterisks indicate significant differences in each treatment using Student's t-test analysis (P < 0.05).
ABA and signaling pathway affect RAM premature differentiation under water stress
To verify the role of ABA in root tip swelling and RAM premature differentiation under PEG-mediated water stress, we performed a phenotypic analysis of the wild type seedlings of wheat (Kn199) and Arabidopsis (Col-0) in response to ABA. Six day-old young seedlings grown under normal conditions were transferred to the solution supplemented with different concentrations of ABA. As shown in Fig. 1B–E, the meristem sizes of the ABA-treated wheat and Arabidoposis seedlings were much shorter than the untreated controls. Tetrazolium Violet staining of ABA-treated wheat roots also revealed that the vitality of the upper region of the treated root tips was substantially reduced compared with that of the untreated roots (Fig. 1B and C). Based on the above results, we proposed that ABA is involved in adaptive morphological changes of root tips including root tip swelling and root meristem premature differentiation under drought stress.
To confirm our prediction, we conducted a genetic study of the ABA related mutants in Arabidopsis. Six day-old mutants defective in ABA biosynthesis (aba2–1) and the signaling pathway (the triple mutant of ABA receptors pyr1/pyl1pyl4) were subjected to 5% PEG 8000 simulated water stress as described previously.3 As shown in, Fig. 2A root tips of the aba2-1 and pyr1/pyl1pyl4 mutants were morphologically comparable to the wild type under normal conditions. When they were subjected to water stress simulated by 5% PEG8000, root tips of both mutants were swollen, but the degrees of root tip swelling of the mutants were significant smaller than that of wild type. Importantly, RAMs of both mutants were also prematurely differentiated under PEG-simulated water stress, but the rates of cell differentiation of both mutant roots were lower than the wild type (Fig. 2B and C). The results demonstrate that ABA and the ABA signaling pathway participate in PEG-mediated adaptive morphological response of root tips.
Figure 2.
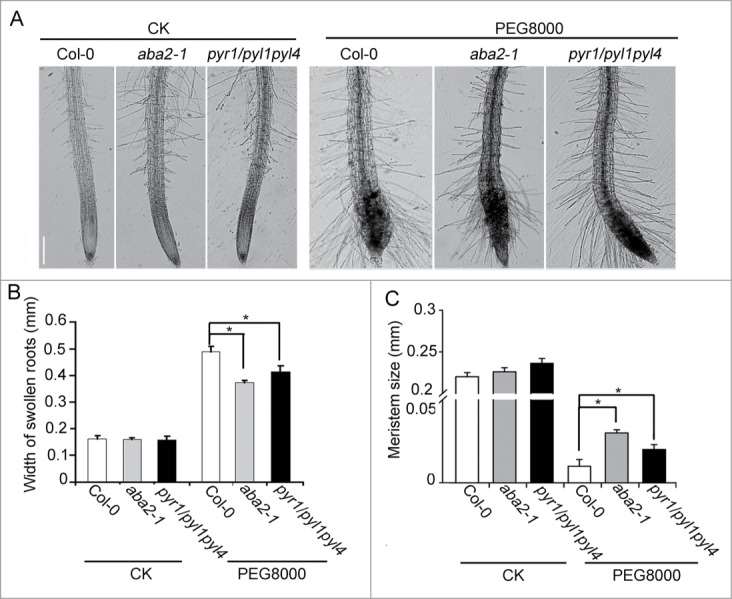
ABA involves in root swollen and premature differentiation under water stress. (A) Six-day-old seedlings of Col-0, aba2–1 and pyr1/pyl1pyl4 mutants were treated with (PEG8000) or without (CK) 5% PEG8000 solution for 3 d Bar = 0.2 mm. (B) Quantification of root width shown in (A). (C) Quantification of root meristem size shown in (A). Data are presented as means ± SD, and columns marked with asterisks indicate significant differences in each treatment using Student's t-test analysis (P < 0.05).
Discussion
ABA is a most important hormone mediating plant response to water stress.5-7 It has shown that high levels of ABA at micromolar inhibit root growth, whereas lower levels of ABA stimulate root growth. A recent study showed that root growth stimulation by low concentration of ABA is caused by enhanced QC maintenance and inhibited stem cell differentiation.8 However, the mechanism underlying root growth inhibition at high levels of ABA remains unknown. Here, we provide evidence that inhibition of root growth by high levels of ABA at micromolar is due to premature differentiation of RAM and reduced activity of RAM. It is possible that the degrees of premature differentiation of RAM and root growth dormancy are regulated by the levels of ABA. When ABA accumulation in root tips exceeds the threshold, root tips may become totally differentiated and completely lose the RAM, resulting in growth cessation of roots. It is well-known that ABA content remains low under normal conditions and ABA is highly accumulated under water stress.5 Therefore, we proposed that water stress-induced morphological response of root tips and root growth rates are regulated by the levels of ABA. Moreover, ABA regulation of the RAM activity and root growth rates is a general adaptive mechanism of high plants. This hypothesis is supported by the data that exogenous application of ABA also induces root tip swelling and RSA premature differentiation in rice.9
Root growth is regulated by multiple plant hormones.10-11 In particular, auxin plays a key role in RAM maintenance.12 In our study, we indeed observed that the degrees of root tip swelling and RAM premature differentiation under ABA treatments (Fig. 2A) were much less than that under PEG8000.3 Thus, despite the critical role of ABA in RAM activity maintenance, it is conceivable that RAM activity and root growth under water stress are coordinated by crosstalk of several hormonal signaling pathways, especially the ABA and auxin signaling pathways. It is likely that the ratio of ABA and auxin fine tunes activity of RAM and growth rates of roots under water stress. Further identification of the mediators of crosstalk between ABA and auxin in controlling RAM activity will help us to uncover the underlying molecular mechanism.
Materials and Methods
Plant materials and growth conditions
Seeds of Arabidopsis thaliana (ecotype Columbia, Col-0) were cold stratified for 72 h at 4°C and then germinated on Murashige and Skoog (MS) medium containing 1% sucrose (w/v, pH 5.7) at 22°C under 16-h-light/8-h-dark cycles. The following mutants were used: aba2-1,13 pyr1/pyl1pyll4 mutants.14 Wheat seeds of variety Kn199 were germinated and grown on filter papers wetted with water for 6 d under a 16-h-light/8-h-dark photoperiod at 22°C before PEG 8000 treatment.
PEG 8000 and ABA treatments
For PEG 8000 treatment, wheat and Arabidopsis seedlings were transferred to liquid solutions containing 5% PEG 8000 (PEG 8000/water, w/v) for 72 h. For ABA treatment, wheat and Arabidopsis seedlings were transferred to different concentrations of ABA solution for 72 h. In all treatments, only the root system was immersed into the solution. For Tetrazolium Violet staining, a 20 times diluted 0.1% phosphate-buffered saline (PBS) stock solution was used.
Microscopic analysis
For measurement of Arabidopsis root meristem size, the roots of seedlings were mounted in water and the images were taken by Leica DM5000B microscope. The meristem sizes of root tips were obtained by measuring the length from the initial cortical cell adjacent to the quiescence center to the first enlarged cortex cell using Photoshop 7.0 software.
Measurements of ABA content
ABA extraction and ABA contents in root tips were quantified by enzyme-linked immunosorbent assay (ELISA) as described in Yang et al.4
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by the National Program on Key Basic Research Project (2012CB114300), the National Transgenic Key Project of the Ministry of Agriculture of China (2011ZX08009-003-002), and the State Key Laboratory of Plant Cell & Chromosome Engineering (PCCE-2008-TD-02).
References
- 1. Cassab GI, Eapen D, Campos ME. Root hydrotropism: an update. Am J Bot 2013; 100:14-24; PMID:23258371; http://dx.doi.org/ 10.3732/ajb.1200306 [DOI] [PubMed] [Google Scholar]
- 2. Duan Y, Zhang W, Li B, Wang Y, Li K, Sodmergen, Han C, Zhang Y, Li X. An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress in Arabidopsis. New Phytol 2010; 186:681-95; PMID:20298483; http://dx.doi.org/ 10.1111/j.1469-8137.2010.03207.x [DOI] [PubMed] [Google Scholar]
- 3. Ji H, Liu L, Li K, Xie Q, Wang Z, Zhao X, Li X. PEG-mediated osmotic stress induces premature differentiation of the root apical meristem and outgrowth of lateral roots in wheat. J Exp Bot 2014; 65:4863-72; pii:eru255; PMID:24935621; http://dx.doi.org/ 10.1093/jxb/eru255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang J, Zhang J, Wang Z,. Zhu Q, Wang W. Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol 2001; 127:315-23; PMID:11553759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saab IN, Sharp RE, Pritchard J, Voetberg GS. Increased endogenous abscisic Acid maintains primary root growth and inhibits shoot growth of maize seedlings at low water potentials. Plant Physiol 1990; 93:1329-36; PMID:16667621; http://dx.doi.org/ 10.1104/pp.93.4.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seiler C, Harshavardhan VT, Rajesh K, Reddy PS, Strickert M, Rolletschek H, Scholz U, Wobus U, Sreenivasulu N, ABA biosynthesis and degradation contributing to ABA homeostasis during barley seed development under control and terminal drought-stress conditions. J Exp Bot 2011; 62:2615-32; PMID:21289079; http://dx.doi.org/ 10.1093/jxb/erq446 [DOI] [PubMed] [Google Scholar]
- 7. Planchet E, Verdu I, Delahaie J, Cukier C, Girard C, Morere-Le Paven MC, Limami AM. Abscisic acid-induced nitric oxide and proline accumulation in independent pathways under water-deficit stress during seedling establishment in Medicago truncatula. J Exp Bot 2014; 65:2161-70; PMID:24604737; http://dx.doi.org/ 10.1093/jxb/eru088 [DOI] [PubMed] [Google Scholar]
- 8. Zhang H, Han W, De Smet I, Talboys P, Loya R, Hassan A, Rong H, Jürgens G, Paul Knox J, Wang MH. ABA promotes quiescence of the quiescent centre and suppresses stem cell differentiation in the Arabidopsis primary root meristem. Plant J 2010; 64: 764-74; PMID:21105924; http://dx.doi.org/ 10.1111/j.1365-313X.2010.04367.x [DOI] [PubMed] [Google Scholar]
- 9. Chen CW, Yang YW, Lur HS, Tsai YG, Chang MC. A novel function of abscisic acid in the regulation of rice (Oryza sativa L.) root growth and development. Plant Cell Physiol 2006; 47:1-13; PMID:16299003; http://dx.doi.org/ 10.1093/pcp/pci216 [DOI] [PubMed] [Google Scholar]
- 10. Liu J, Rowe J, Lindsey K. Hormonal crosstalk for root development:a combined experimental and modeling perspective. Front Plant Sci 2014; 5:116; PMID:24734037; http://dx.doi.org/ 10.3389/fpls.2014.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo X, Chen Z, Gao J, Gong Z. Abscisic acid inhibits root growth in Arabidopsis through ethylene biosynthesis. Plant J 2014; 79:44-55; PMID: 24738778; http://dx.doi.org/ 10.1111/tpj.12534 [DOI] [PubMed] [Google Scholar]
- 12. Saini S, Sharma I, Kaur N, Pati PK. Auxin: a master regulator in plant root development. Plant Cell Rep 2013; 32:741-57; PMID:23553556; http://dx.doi.org/ 10.1007/s00299-013-1430-5 [DOI] [PubMed] [Google Scholar]
- 13. Léon-Kloosterziel KM, Gil MA, Ruijs GJ, Jacobsen SE, Olszewski NE, Schwartz SH, Zeevaart JA, Koornneef M. Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J 1996; 10:655-61; PMID: 8893542 [DOI] [PubMed] [Google Scholar]
- 14. Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009; 324:1068-71; PMID: 19407142; http://dx.doi.org/ 10.1126/science.1173041 [DOI] [PMC free article] [PubMed] [Google Scholar]


