Abstract
Cellular resistance in advanced gastric cancer (GC) might be related to function of multidrug resistance (MDR) proteins. The adaptor protein NHERF1 (Na+/H+ exchanger regulatory factor) is an important player in cancer progression for a number of solid malignancies, even if its role to develop drug resistance remains uncertain. Herein, we aimed to analyze the potential association between NHERF1 expression and P-gp, sorcin and HIF-1α MDR-related proteins in advanced GC patients treated with epirubicin/oxaliplatin/capecitabine (EOX) chemotherapy regimen, and its relation to response. Total number of 28 untreated patients were included into the study. Expression and subcellular localization of all proteins were assessed by immunohistochemistry on formalin-fixed paraffin embedded tumor samples. We did not found significant association between NHERF1 expression and the MDR-related proteins. A trend was observed between positive cytoplasmic NHERF1 (cNHERF1) expression and negative nuclear HIF-1α (nHIF-1α) expression (68.8% versus 31.3% respectively, P = 0.054). However, cytoplasmic P-gp (cP-gp) expression was positively correlated with both cHIF-1α and sorcin expression (P = 0.011; P = 0.002, respectively). Interestingly, nuclear NHERF1 (nNHERF1) staining was statistically associated with clinical response. In detail, 66.7% of patients with high nNHERF1 expression had a disease control rate, while 84.6% of subjects with negative nuclear expression of the protein showed progressive disease (P = 0.009). Multivariate analysis confirmed a significant correlation between nNHERF1 and clinical response (OR 0.06, P = 0.019). These results suggest that nuclear NHERF1 could be related to resistance to the EOX regimen in advanced GC patients, identifying this marker as a possible independent predictive factor.
Keywords: chemotherapy, gastric cancer, immunohistochemistry, multi-drug resistance, NHERF1/EBP50, predictive factor
Abbreviations
- DCR
disease control rate
- PD
progression disease
- cNHERF1
cytoplasmic NHERF1
- nNHERF1
nuclear NHERF1
- cP-gp
cytoplasmic P-gp
- mP-gp
membranous P-gp
- cSR1
cytoplasmic SR1
- cHIF-1α
cytoplasmic HIF-1α
- nHIF-1α
nuclear HIF-1α
- OR
odds ratio
- Cl
confidence interval
Introduction
Gastric cancer (GC) is the second leading cause of mortality worldwide.1 Approximately 65% of patients with GC present a locally advanced or metastatic stage at diagnosis.2 In this stage of the cancer, Her2-neu positive GC patients receiving regimens containing Trastuzumab showed a median overall survival of 13.8 months, whereas Her2-neu negative patients who can be treated with chemotherapy alone have a survival of less than 12 months.3 Currently for this latter group of patients the gold standard includes treatment with the triplet formed by epirubicin, oxaliplatin, and capecitabine (EOX). The median survival of patients treated with EOX is 11.2 months and is higher than other triplets consisting of epirubicin, cisplatin and 5-FU (ECF), epirubicin, cisplatin and capecitabine (ECX), or epirubicin, oxaliplatin and 5-FU (EOF), whose median survival times are 9.9 months for ECF and ECX and 9.3 months for EOF.4,5 Despite the search for new therapeutic protocols in GC, there is much evidence of progression under one year of diagnosis due to resistance to chemotherapy, which remains the major obstacle to treatment success.6
Multiple drug resistance (MDR) is defined as the capability of cancer cells to survive after exposure to antineoplastic drugs, causing a reduction in effectiveness and leading to useless treatment. Several distinct mechanisms of MDR have been identified. Multidrug transporters such as P-glycoprotein (P-gp) are the major mechanisms involved in the MDR phenotype by decreasing drug concentration within the cell or modifying its distribution to intracellular compartments. The overexpression of P-gp encoded by the MDR1 gene is associated with resistance to chemotherapeutic agents or poor survival in several neoplasms, including GC.7-12 Furthermore, the soluble resistance-related calcium binding protein (sorcin) is involved in the up-regulation of P-gp in GC cell lines and thus in the development of the MDR phenotype.13-16 Hypoxia, a common characteristic of many tumors, has also been described as a crucial player in the drug resistance mechanisms.17-19 In particular, hypoxia-inducible factor-1α (HIF-1α) is involved in the tumor response to cellular hypoxia through the transcriptional regulation of a large number of hypoxia-related genes, including MDR genes.20,21
Some data support the hypothesis of a possible involvement of Na+/H+ exchanger regulatory factor 1 (NHERF1)/ezrin-radixin-moesin (ERM) binding phosphoprotein 50 (EBP50), in the drug resistance of neoplastic cells. NHERF1, a member of a scaffold protein family, is characterized by the presence of 2 tandem PDZ domains and a C-terminal ERM-binding region. It is expressed in the luminal membrane of many epithelia and has a key role within various signaling complexes.22 Moreover, it binds to the cytoplasmic part of different types of transmembrane receptors and to members of the ERM family of actin-binding proteins.22,23 Hegedus T et al.24 and later Hoque T. et al.25 showed a physical interaction of NHERF1 with MDR protein 2 and with MDR protein 4 through PDZ motifs.
In previous studies, we have observed a strong correlation between HIF-1α and NHERF1. In breast and colorectal cancers, we found that NHERF1 expression is increased by low oxygen tension, providing a direct functional link between the hypoxic and acidic tumor microenvironment.26,27 The strong correlation of NHERF1 with HIF-1α also showed an association with increased tumor cell aggressiveness.28 In addition, Karn and coworkers29 demonstrated that NHERF1 expressing breast cancers have a greater risk of developing resistance to endocrine therapy. This evidence led us to explore the involvement of NHERF1 in drug resistance in GC patients.
The aim of the present study was to analyze for the first time in the literature the potential association between NHERF1 expression and MDR-related proteins (P-gp, sorcin and HIF-1α) in advanced GC patients, and its relation to chemotherapy response in these patients.
Results
Sixteen patients (57%) were male and 12 (43%) were female, with a median age of 62 years (range: 31–75 years). 64% of all patients had an intestinal phenotype in Lauren classification while the others (32%) had a diffused or mixed phenotype; the majority of patients had a poorly differentiated tumor (85%) and in 62% of patients vascular invasion was present. Sixteen patients (57%) had a PD (progressive disease), while 12 (43%) had disease control rate (DCR), a PR (partial response) or a SD (stable disease) (Table 1).
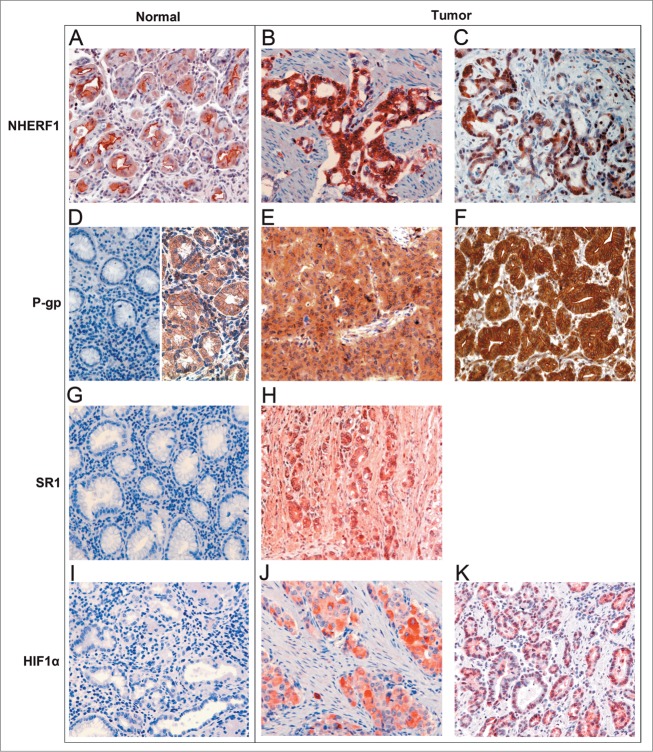
NHERF1, P-gp, sorcin (SR1) and HIF-1α expression in GC samples are shown in Table 2. In normal epithelial cells, NHERF1 immunoreactivity showed mostly an apical membranous reactivity, (Fig. 1A). NHERF1 expression was present both in the cytoplasm (Fig. 1B) and nucleus (Fig. 1C) of tumor cells with varying intensity. Cytoplasmic NHERF1 (cNHERF1) expression was positive (≥70% of tumor cells) in 57% of the samples and nuclear NHERF1 (nNHERF1) expression was positive (≥32% of tumor cells) in 54% of tumor tissues. In normal gastric mucosa, negative (Fig. 1D, left) or low cytoplasmic and membranous staining (Fig. 1D, right) of P-gp were observed. Expression pattern of the P-gp protein was predominantly localized in the cytoplasm (Fig. 1E), and a membranous immunoreactivity (Fig. 1F) was also observed in tumor samples. Diffuse and positive (≥40% of tumor cells) cytoplasmic P-gp (cP-gp) was present in 71% of tumors. Positive membrane staining (> 0% of tumor cells) was observed in 39% of tumor tissues. In normal gastric tissues staining immunoreactivity of SR1 was negative (Fig. 1G), while positive cytoplasmic immunohistochemical staining was identified in malignant cells (Fig. 1H). Only 4 of the 28 cases analyzed also had a low nuclear expression, but this was not considered. Cytoplasmic SR1 (cSR1) overexpression (≥45% of tumor cells) was present in 50% of GC tumors. HIF-1α immunostaining was absent in normal gastric tissues (Fig. 1I) and was heterogeneous with immunoreactivity localized in the cytoplasm (Fig. 1J) and in the nucleus (Fig. 1K) of gastric cancer cells. Positive cytoplasmic HIF-1α (cHIF-1α) and nuclear HIF-1α (nHIF-1α) expression were observed in 54% (≥70% of tumor cells) and 50% (≥8% of tumor cells) of tissues, respectively. Representative examples of immunostaining distribution and intensity observed for NHERF1, P-gp, SR1 and HIF-1α in normal and tumor tissues are shown in Figure 1. Associations between tumor markers and pathological characteristics are shown in Table 3. No statistically significant association was found between cNHERF1 expressions and any clinicopathological characteristics. Interestingly, nNHERF1 staining was statistically associated with clinical response. In detail, 10/15 patients (66.7%) with high nNHERF1 expression had a DCR, while 11/13 (84.6%) of patients with negative nuclear expression of the protein showed PD (P = 0.009). Membrane P-gp positive expression was more frequent in patients with intestinal tumor type (90.9% vs. 9.1% in those with diffuse tumor type, P = 0.042). Cytoplasmic P-gp positive staining was observed in the tumors with present vascular invasion (75%, P = 0.018), while mP-gp positive staining was observed in the tumors with absent vascular invasion (63, 6%, P = 0.043). Cytoplasmic SR1 positive expression was present in the tumor tissues with vascular invasion (92.9%, P = 0.001). Cytoplasmic HIF-1α positive expression was frequent in patients with intestinal tumor type (92.9% versus 7.1% in those with diffuse tumor type, P = 0.004). No significant differences of SR1, HIF-1α and P-gp expression in age, gender, tumor differentiation or clinical response were found. In addition, we did not find significant association between NHERF1 expression and the MDR-related proteins, only a trend was observed between positive cNHERF1 expression and negative nHIF-1α expression (68.8% vs. 31.3% respectively, P = 0.054, data not shown). Moreover, cP-gp expression was correlated positively with both cHIF-1α and cSR1 expression (P = 0.011; P = 0.002, respectively, Table 4). Lastly, we evaluated the correlation of NHERF1 and other biomarkers with the EOX toxicity profile, but our data did not show a significant variation of the expression of these immunohistochemical markers and haematological, skin, and gastrointestinal toxicities. Multivariate analysis revealed a significant correlation between nNHERF1 and clinical response (OR 0.06 (0.01–0.63), P = 0.019; Table 5). The other proteins were not found to be correlated to clinical response.
Table 2.
Protein expression in 28 advanced gastric cancer patients
| Proteins expression | n. of patients | (%) |
|---|---|---|
| cNHERF1 | ||
| Negative | 12 | (43) |
| Positive | 16 | (57) |
| nNHERF1 | ||
| Negative | 13 | (46) |
| Positive | 15 | (54) |
| cP-gp | ||
| Negative | 8 | (29) |
| Positive | 20 | (71) |
| mP-gp | ||
| Negative | 17 | (61) |
| Positive | 11 | (39) |
| cSR1 | ||
| Negative | 14 | (50) |
| Positive | 14 | (50) |
| cHIF-1α | ||
| Negative | 13 | (46) |
| Positive | 15 | (54) |
| nHIF-1α | ||
| Negative | 14 | (50) |
| Positive | 14 | (50) |
Abbreviations: cNHERF1, cytoplasmic NHERF1; nNHERF1, nuclear NHERF1; cP-gp, cytoplasmic P-gp; mP-gp, membranous P-gp; cSR1, cytoplasmic SR1; cHIF-1α, cytoplasmic HIF-1α; nHIF-1α, nuclear HIF-1α.
Figure 1.
Normal epithelial cells with apical membranous reactivity of NHERF1 (A), NHERF1 expression in the cytoplasm (B) and nucleus (C) of tumor cells; normal gastric mucosa with negative (D, left) or low cytoplasmic and membranous staining of P-gp (D, right), tumor samples with positive cytoplasmic expression (E) and with strong membranous staining of P-gp (F); normal gastric tissues with negative expression of SR1 (G) and cytoplasmic immunohistochemical staining of SR1 in malignant cells (H); HIF-1α immunostaining was absent in normal gastric tissues (I), and in gastric cancer cells immunoreactivity was localized in the cytoplasm (J) and in the nucleus (K). Original magnification 20×.
Table 3.
Association of protein expressions with clinicopathological characteristics
| cNHERF1 |
nNHERF1 |
cP-gp |
mP-gp |
cSR1 |
cHIF-1α |
nHIF-1α |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | ||||||||
| Characteristics | neg. | pos. | neg. | pos. | neg. | pos. | neg. | pos. | neg. | pos. | neg. | pos. | neg. | pos. |
| Age (years) | ||||||||||||||
| < 62 | 41.7 | 56.3 | 53.8 | 46.7 | 25 | 60 | 47.1 | 54.5 | 50 | 50 | 38.5 | 60 | 57.1 | 42.9 |
| ≥ 62 | 58.3 | 43.8 | 46.2 | 53.3 | 75 | 40 | 52.9 | 45.5 | 50 | 50 | 61.5 | 40 | 42.9 | 57.1 |
| P value | ns | ns | ns | ns | ns | ns | ns | |||||||
| Gender | ||||||||||||||
| Male | 66.7 | 50 | 46.2 | 66.7 | 62.5 | 55 | 47.1 | 72.7 | 71.4 | 42.9 | 53.8 | 60 | 57.1 | 57.1 |
| Female | 33.3 | 50 | 53.8 | 33.3 | 37.5 | 45 | 52.9 | 27.3 | 28.6 | 57.1 | 46.2 | 40 | 42.9 | 42.9 |
| P value | ns | ns | ns | ns | ns | ns | ns | |||||||
| Lauren type | ||||||||||||||
| Intestinal | 50 | 80 | 50 | 80 | 62.5 | 68.4 | 50 | 90.9 | 64.3 | 69.2 | 38.5 | 92.9 | 69.2 | 64.3 |
| Diffuse | 50 | 20 | 50 | 20 | 37.5 | 31.6 | 50 | 9.1 | 35.7 | 30.8 | 61.5 | 7.1 | 30.8 | 35.7 |
| P value | ns | ns | ns | 0.042 | ns | 0.004 | ns | |||||||
| Tumor differentiation | ||||||||||||||
| Moderate | 9.1 | 20 | 23.1 | 7.7 | 0 | 20 | 13.3 | 18.2 | 0 | 28.6 | 0 | 26.7 | 7.1 | 25 |
| Poor | 90.9 | 80 | 76.9 | 92.3 | 100 | 80 | 86.7 | 81.8 | 100 | 71.4 | 100 | 73.3 | 92.9 | 75 |
| P value | ns | ns | ns | ns | ns | ns | ns | |||||||
| Vascular invasion | ||||||||||||||
| Present | 45.5 | 73.3 | 69.2 | 53.8 | 16.7 | 75 | 80 | 36.4 | 25 | 92.9 | 45.5 | 73.3 | 64.3 | 58.3 |
| Absent | 54.5 | 26.7 | 30.8 | 46.2 | 83.3 | 25 | 20 | 63.6 | 75 | 7.1 | 54.5 | 26.7 | 35.7 | 41.7 |
| P value | ns | ns | 0.018 | 0.043 | 0.001 | ns | ns | |||||||
| Clinical response | ||||||||||||||
| DCR | 50 | 37.5 | 15.4 | 66.7 | 62.5 | 35 | 47.1 | 36.4 | 57.1 | 28.6 | 53.8 | 33.3 | 28.6 | 57.1 |
| PD | 50 | 62.5 | 84.6 | 33.3 | 37.5 | 65 | 52.9 | 63.6 | 42.9 | 71.4 | 46.2 | 66.7 | 71.4 | 42.9 |
| P value | ns | 0.009 | ns | ns | ns | ns | ns | |||||||
Abbreviations: cNHERF1, cytoplasmic NHERF1; nNHERF1, nuclear NHERF1; cP-gp, cytoplasmic P-gp; mP-gp, membranous P-gp; cSR1, cytoplasmic SR1; cHIF-1α, cytoplasmic HIF-1α; nHIF-1α, nuclear HIF-1α; DCR, disease control rate; PD, progression diseas.
P-values were calculated with the Fisher's exact test.
Two patients out 28 had missing values for Tumor differentiation and Vascular invasion; one patient, classified as other histological type, was not included in the statistical analyses.
Table 4.
Correlation between P-gp, HIF-1α and SR1 protein expression
| cP-gp |
mP-gp |
|||||
|---|---|---|---|---|---|---|
| n (%) |
n (%) |
|||||
| Characteristics | negative | positive | P value | negative | positive | P value |
| cHIF-1α | ||||||
| negative | 7(87.5) | 6(30) | 0.011 | 9(52.9) | 4(36.4) | 0.460 |
| positive | 1(12.5) | 14(70) | 8(47.1) | 7(63.6) | ||
| nHIF-1α | ||||||
| negative | 2(25) | 12(60) | 0.209 | 9(52.9) | 5(45.5) | 1.000 |
| positive | 6(75) | 8(40) | 8(47.1) | 6(54.5) | ||
| cSR1 | ||||||
| negative | 8(100) | 6(30) | 0.002 | 7(41.2) | 7(63.6) | 0.440 |
| positive | 0(0) | 14(70) | 10(58.8) | 4(36.4) | ||
Abbreviations: cP-gp, cytoplasmic P-gp; mP-gp, membranous P-gp; cHIF-1α, cytoplasmic HIF-1α; nHIF-1α, nuclear HIF-1α; cSR1, cytoplasmic SR1. P values were calculated with the Fisher's exact test.
Table 5.
Multivariate analysis with respect to clinical response in advanced gastric cancers patients
| Clinical response |
||
|---|---|---|
| Characteristics | OR (95% Cl) | P value |
| cNHERF1 | 1.04 (0.10–10.76) | 0.975 |
| nNHERF1 | 0.06 (0.01–0.63) | 0.019 |
| cP-gp | 1.25 (0.06–24.34) | 0.882 |
| mP-gp | 3.94 (0.42–37.39) | 0.232 |
| cSR1 | 6.39 (0.44–93.57) | 0.175 |
| cHIF-1α | 0.71 (0.07–7.52) | 0.775 |
| nHIF-1α | 0.34 (0.03–3.73) | 0.381 |
Abbreviations: OR, odds ratio; CI, confidence interval; cNHERF1, cytoplasmic NHERF1; nNHERF1, nuclear NHERF1; cP-gp, cytoplasmic P-gp; mP-gp, membranous P-gp; cHIF-1α, cytoplasmic HIF-1α; nHIF-1α, nuclear HIF-1α; cSR1, cytoplasmic SR1.
Discussion
The combination chemotherapy regimen EOX represents one of the best therapeutic choices in advanced GC patients. There have been other attempts in the literature to obtain better results with other combinations of different chemotherapeutic schedules. In an Italian phase II study to gemcitabine 1000 mg/m2 was added to FOLFOX obtaining similar results in terms of time to progression and overall survival (OS) to EOX.30 In another phase II study, performed in elderly patients over 70, it was demonstrated that the weekly OXALF (oxaliplatin, 5-fluorouracil and folinic acid) regimen can lead to less toxicity at the expense of a lower OS, common in the elderly. Nonetheless, EOX currently remains the standard in HER2 negative GC.31 However, the identification of new factors that affect the efficacy of these drugs represents a significant challenge in these patients. To the best of our knowledge this is the first study to investigate the relationship between NHERF1 expression and drug resistance in GC patients treated with the EOX chemotherapy regimen through the evaluation of its association with MDR-related proteins. Moreover, the association between the expression and subcellular localization of NHERF1, P-gp, SR1 and HIF-1α proteins and clinicopathological status was investigated, hypothesizing that the localization in different cell compartments of these proteins may be of importance for their function.
In this study, we observed both nuclear and cytoplasmic NHERF1 staining patterns. Previous reports demonstrated that the switch from apical membranous to cytoplasmic expression is compatible with a dual role for NHERF1. Membranous or apical distribution would support a tumor suppressor function, while a cytoplasmic or nuclear subcellular localization might attribute oncogene properties.22,33,34 In addition, examining the expression of P-gp and HIF-1α proteins two distinct patterns were observed, in agreement with other studies.35,36 Our data showed P-gp immunoreactivity was mainly localized in the cytoplasm of the cells and HIF-1α immunoreactivity was localized in the cytoplasm and nuclei of GC cells.
With regard to the histological type and Lauren classification, both mP-gp and cHIF-1α are frequently expressed in intestinal type GC as demonstrated, and mP-gp differently from cP-gp expression was also significantly correlated with absence of vascular invasion.11,37 These data may suggest a possible link between membranous P-gp localization and better biological behavior.
In this study, we verified the existence of possible correlations among these different protein expressions. In accordance with other Authors, we found an association between HIF-1α and P-gp expression. In detail, cHIF-1α was positively correlated with cP-gp.8,38 Moreover, the expression of cP-gp and sorcin were significantly correlated.13 Regarding NHERF1 and multidrug resistance proteins, we demonstrated only an inverse trend between positive cNHERF1 expression and negative nHIF-1α expression. In previous studies, we showed an upregulation of this protein by hypoxia in breast cancer and a significant association between NHERF1 and HIF-1α in colorectal cancer.26-28 It is possible that these differences derived from different histological subtypes or the small sample size.
It has been reported that the NHERF1 protein is able to induce an invasive phenotype in an in vitro model of cancer, implicating this protein as a new and important enhancer of cancer progression.26,39,40 More importantly, it was demonstrated that its overexpression is significantly correlated with poor prognosis in particular in breast and colon cancers, and could be helpful in predicting the aggressiveness of the disease.26,27,40-42 Previous studies have shown that NHERF1 is involved in various human cancers, including those of the breast, hepatocellular, colon and gastric carcinomas.33,34,40,43,44 In breast cancer, increased NHERF1 cytoplasmic expression correlated to tumor progression. In fact, this protein significantly changes from normal to in situ to invasive breast cancer tissues.33 A preclinical study on hepatocellular carcinoma demonstrated that NHERF1/EBP50 can up-regulated the Wnt/β-catenin pathway, improving proliferation and the malignant phenotype.34 In colon cancer Hayashi Y et al. showed that NHERF1 alterations induced a malignant phenotype, implicating this protein as a potential malignant progression marker.40 In gastric cancer NHERF1 expression was associated with malignant clinicopathological characteristics but it was not correlated with survival.44 Furthermore, previously we investigated the significance of NHERF1 subcellular localization in breast carcinomas, showing that the loss of nNHERF1 expression was associated with poor survival.41 Interestingly, in our series of advanced GCs, lower nNHERF1 correlated with resistance to chemotherapy and a worse outcome, suggesting a potential involvement of this protein in mechanisms of drug resistance. These patients also showed a high, although not significant, expression of P-gp, sorcin and HIF-1α, challenging for the first time the hypothesis that lower nNHERF1 expression could induce increased expression of the multidrug resistance proteins in GC patients. It would be interesting to know how this localization of NHERF1 may cause changes in expression in the other proteins. Further investigations into NHERF1 expression compared to the expression of the other MDR proteins will be important to clarify the role of nNHERF1 in multidrug resistance in GC.
The absence of correlation among clinical response and the expression of P-gp, sorcin and HIF-1α expression indicates that there might be other mechanisms responsible for MDR in GC cells, such as the loss of nNHERF1. This hypothesis could be supported by the results of multivariate analysis, identifying nuclear NHERF1 expression as an independent predictive factor in this cohort of advanced GC patients.
In conclusion, this result suggests that nNHERF1 could be related to a resistance to the EOX regimen in advanced GC patients. Clearly, these data need to be confirmed in larger and prospective clinical series.
Materials and Methods
Patients and tissue samples
A total of 28 untreated advanced GC patients were retrospectively analyzed. All patients had HER2 negative disease and received an intravenous bolus of epirubicin at a dose of 50 mg/m2 (day 1) in combination with oxaliplatin at a dose of 130 mg/m2 (day 1) and capecitabine at a twice daily dose of 625 mg/m2 (days 121) (EOX) in a cycle scheduled every 3 weeks. According to the Response Evaluation Criteria in Solid Tumors (RECIST) definition 1.1, all patients were evaluated after 3 cycles of treatment.45 All patients were reviewed and the following data were collected (Table 1): gender (male versus female), median age, Lauren type (intestinal type vs. diffuse type), Tumor differentiation (moderate versus poor differentiation), Vascular invasion (present vs. absent) and response to chemotherapy (CR: complete response; PR: partial response; ORR, objective response rate: CR + PR; SD: stable disease; DCR, disease control rate: ORR + SD, PD: progressive disease).
Table 1.
Patient characteristics
| Characteristics | n. of patients | (%) |
|---|---|---|
| Age (years) | ||
| < 62years | 14 | (50) |
| ≥ 62years | 14 | (50) |
| Gender | ||
| Male | 16 | (57) |
| Female | 12 | (43) |
| Lauren type | ||
| Intestinal | 18 | (64) |
| Diffuse | 9 | (32) |
| Mixed | 1 | (4) |
| Tumor differentiation | ||
| Moderate | 4 | (15) |
| Poor | 22 | (85) |
| Vascular invasion | ||
| Present | 16 | (62) |
| Absent | 10 | (38) |
| Clinical response | ||
| DCR | 12 | (43) |
| PD | 16 | (57) |
Abbreviations: DCR, disease control rate; PD, progression disease.
Two patients out 28 had missing values for Tumor differentiation and Vascular invasion.
Ethics statement
This study was approved by the Ethics Committee of the NCRC Istituto Tumori “Giovanni Paolo II,” Bari, Italy. All samples were procured from the tissue bank of the Department of Pathology of NCRC Istituto Tumori “Giovanni Paolo II,” Bari, and from the Pathology Department of G.B. Morgagni L.-Pierantoni Hospital, Forlì, Italy.
Immunohistochemical analysis
NHERF1, P-gp, SR1 and HIF-1α expressions were examined in 28 tumor samples from GC patients using standard immunoperoxidase techniques. The corresponding haematoxylin and eosin stains were assessed to confirm the diagnoses of these patients. Consecutive sections of 4-µm-thickness were cut from formalin-fixed and paraffin-embedded histological blocks, and these were immunohistochemically stained using standard immunoperoxidase techniques. The sections were deparaffinised in xylene and rehydrated through a graded ethanol series. Epitope antigen retrieval was carried out in 0.01 M sodium citrate buffer, pH 6.0 for NHERF1 (30 min.), SR1 (30 min.) and HIF-1α (45 min.), and in TRIS-EDTA (pH 9.0) for Pgp (20 min.) at 98°C in a water bath. After endogenous peroxidase activity blocking with 3% H2O2 buffer solution, sections were incubated overnight at 4°C with primary antibodies: rabbit polyclonal anti-EBP50 for NHERF1 (clone PA1-090, Affinity Bioreagents, Golden, CO, USA; 1:150 dilution), mouse monoclonal anti-P-gp (clone JSB-1, Abcam, Cambridge, UK; 1:20 dilution), rabbit polyclonal anti-SR1 (Abcam, Cambridge, UK; 1:100 dilution) and rabbit polyclonal anti-HIF-1α (clone H206, Santa Cruz Biotechnology Inc., Santa Cruz, CA USA; 1:25 dilution). After incubation, specimens were washed with phosphate-buffered saline and incubated with peroxidase-labeled secondary antibodies (EnVision+System-HRPLabelled Polymer, Dako, CA, USA) for 60 min. at RT. The bound antibody was visualized with 3-amino-9-ethylcarbazole substrate-chromogen in the dark for NHERF1, HIF-1α, SR1, and with 3,3′-diaminobenzidine (DakoCytomation, Glostrup, Denmark) for the P-gp antibody. Tissue sections were counterstained with Mayer haematoxylin. As positive control, for NHERF1 we used paraffin embedded cell pellets from the MCF-7 cell line, expressing high levels of NHERF1 protein, and human small intestinal tissue for P-gp and gastric tissues for HIF-1α and SR1. For negative controls, the primary antibody was omitted and replaced by PBS pH7.6.
The evaluation of immunohistochemical staining
For NHERF1, P-gp, SR1 and HIF-1a we examined the expression and their subcellular localization in 28 advanced GC samples and their distant normal gastric tissue. The different localization was scored separately and its significance was evaluated. For each case two consecutive sections were processed immunohistochemically. The slides were scored by two independent observers (A.M. and G.S.) blinded to both clinical and pathological data, and any discrepancies between the two observers were resolved by discussion. Protein expression analysis for antigens was quantified by counting positive cells in 3 to 5 representative areas of the tumor for each section at ×20 magnification and expressed as a percentage of positive cells/section. When a section was either uninformative or lost a case was judged as ‘not assessable’ in the statistical analysis. For NHERF1, both cytoplasmic and nuclear expression were examined in cancer cells. According to the median value, the cases were classified positive when cNHERF1 immunoreactivity was present in ≥70% of tumor cells (median value 70) and when nNHERF1 expression was present in ≥32% of tumor cells examined (median value 32). The P-gp protein was localized both in the cytoplasm and membrane. Cytoplasmic P-gp positivity was assessed when positive staining was present in ≥40% of the tumor cells according to the median value (median value 40). Membrane P-gp was classified positive when immunostaining was present in >0% of the tumor cells examined (median value 0). SR1 protein expression was localized in the cytoplasm of tumor cells. The tumor tissues were defined as SR1 positive when the percentage of SR1 expression was ≥45% of the tumor cells according to the median value (median value 45). HIF-1α protein expression was localized in the cytoplasm and in the nuclei of tumor cells. Cytoplasmic HIF-1α expression was classified positive when present in ≥70% of the tumor cells examined (median value 70). Nuclear HIF-1α tumors were positive when ≥8% of nuclei were stained according to the median value (median value 8).
Statistical analysis
All the analyses were performed using the SPSS 14.0 statistical software (SPSS Inc., Chicago, IL USA). Fisher's exact test was used to evaluate differences in the correlation between expression of NHERF1, P-gp, SR1, HIF-1α and clinicopathological characteristics. Univariate and multivariate logistic regression models were used to investigate the relationship between the outcomes of interest and these proteins, adjusting for the effect of some confounding factors. The results of univariate analysis were presented as column frequencies. The results of multivariate analysis were presented in terms of Odds Ratio (OR) with corresponding 95% confidence intervals. Statistical significance was defined as a p-value of <0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Rossana Daprile for technical assistance. The authors would also like to thank Caroline Oakley for manuscript revision and Giusi Graziano for statistical analyses.
References
- 1.Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur J Cancer 2014; 50(7):1330-44; PMID:24650579; http://dx.doi.org/ 10.1016/j.ejca.2014.01.029 [DOI] [PubMed] [Google Scholar]
- 2.Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy. Cancer 2000; 88(4):921-32; PMID:10679663; http://dx.doi.org/ 10.1002/(SICI)1097-0142(20000215)88:4<921::AID-CNCR24>3.0.CO;2-S [DOI] [PubMed] [Google Scholar]
- 3.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al.. ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376(9742):687-97; PMID:20728210; http://dx.doi.org/ 10.1016/S0140-6736(10)61121-X; Erratum in: Lancet. 2010 Oct 16;376(9749):1302 [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2010; 362(9):858-9; PMID:20200397; http://dx.doi.org/ 10.1056/NEJMc0911925 [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR, et al.. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008; 358(1):36-46; PMID:18172173; http://dx.doi.org/ 10.1056/NEJMoa073149 [DOI] [PubMed] [Google Scholar]
- 6.Huang S, Chen M, Shen Y, Shen W, Guo H, Gao Q, Zou X. Inhibition of activated Stat3 reverses drug resistance to chemotherapeutic agents in gastric cancer cells. Cancer Lett 2012; 315(2):198-205; PMID:22104727; http://dx.doi.org/ 10.1016/j.canlet.2011.10.011 [DOI] [PubMed] [Google Scholar]
- 7.Wang B1, Zhang W, Hong X, Guo Y, Li J. Phase I dose-escalating study of 24-h continuous infusion of 5-fluorouracil in combination with weekly docetaxel and cisplatin in patients with advanced gastric cancer. Cancer Chemotherapy Pharmacol 2009; 63(2):213-8; PMID:18343924; http://dx.doi.org/ 10.1007/s00280-008-0728-4 [DOI] [PubMed] [Google Scholar]
- 8.Xie J1, Li DW, Chen XW, Wang F, Dong P. Expression and significance of hypoxia-inducible factor-1α and MDR1/P-glycoprotein in laryngeal carcinoma tissue and hypoxic Hep-2 cells. Oncol Lett 2013; 6(1):232-238; PMID:23946810; http://dx.doi.org/ 10.3892/ol.2013.1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovalev AA, Tsvetaeva DA, Grudinskaja TV. Role of ABC-cassette transporters (MDR1, MRP1, BCRP) in the development of primary and acquired multiple drug resistance in patients with early and metastatic breast cancer. Exp Oncol 2013; 35(4):287-90; PMID:24382439 [PubMed] [Google Scholar]
- 10.Li W, Song M. Expression of multidrug resistance proteins in invasive ductal carcinoma of the breast. Oncol Let 2014; 8(5):2103-2109; PMID:25295098; http://dx.doi.org/ 10.3892/ol.2014.2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi JH, Lim HY, Joo HJ, Kim HS, Yi JW, Kim HC, Cho YK, Kim MW, Lee KB. Expression of multidrug resistance-associated protein1, P-glycoprotein, and thymidylate synthase in gastric cancer patients treated with 5-fluorouracil and doxorubicin-based adjuvant chemotherapy after curative resection. Br J Cancer 2002; 86(10):1578-85; PMID:12085207; http://dx.doi.org/ 10.1038/sj.bjc.6600305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu HW, Xu L, Hao JH, Qin CY, Liu H. Expression of P-glycoprotein and multidrug resistance-associated protein is associated with multidrug resistance in gastric cancer. J Int Med Res 2010; 38(1):34-42; PMID:20233511; http://dx.doi.org/ 10.1177/147323001003800104 [DOI] [PubMed] [Google Scholar]
- 13.He Q, Zhang G, Hou D, Leng A, Xu M, Peng J, Liu T. Overexpression of sorcin results in multidrug resistance in gastric cancer cells with up-regulation of P-gp. Oncol Rep 2011; 25(1):237-43; PMID:21109982; http://dx.doi.org/ 10.3892/or_00001066 [DOI] [PubMed] [Google Scholar]
- 14.Yamagishi N, Nakao R, Kondo R, Nishitsuji M, Saito Y, Kuga T, Hatayama T, Nakayama Y. Increased expression of sorcin is associated with multidrug resistance in leukemia cells via up-regulation of MDR1 expression through cAMP response element-binding protein. Biochem Biophys Res Commun 2014; 448(4):430-6; PMID:24796664; http://dx.doi.org/ 10.1016/j.bbrc.2014.04.125 [DOI] [PubMed] [Google Scholar]
- 15.Liu B, Qiu Q, Zhao T, Jiao L, Hou J, Li Y, Qian H, Huang W. Discovery of novel P-glycoprotein-mediated multidrug resistance inhibitors bearing triazole core via click chemistry. Chemo Biol Drug Des 2014; 84(2):182-91; PMID:24750961; http://dx.doi.org/ 10.1111/cbdd.12301 [DOI] [PubMed] [Google Scholar]
- 16.Deng L, Su T, Leng A, Zhang X, Xu M, Yan L, Gu H, Zhang G. Upregulation of soluble resistance-related calcium-binding protein (sorcin) in gastric cancer. Med Oncol 2010; 27(4):1102-8; PMID:19885748; http://dx.doi.org/ 10.1007/s12032-009-9342-5 [DOI] [PubMed] [Google Scholar]
- 17.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 2002; 62(12):3387-94; PMID:12067980 [PubMed] [Google Scholar]
- 18.Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo C, Han S, Liu J, Sun S, Han Z, et al.. Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced chemoresistance in gastric cancer. Cancer Sci 2008; 99(1):121-8; PMID:17953712; http://dx.doi.org/ 10.1111/j.1349-7006.2007.00643.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li DW, Dong P, Wang F, Chen XW, Xu CZ, Zhou L. Hypoxia induced multidrug resistance of laryngeal cancer cells via hypoxia-inducible factor-1α. Asian Pac J Cancer Prev 2013; 14(8):4853-8; PMID:24083758; http://dx.doi.org/ 10.7314/APJCP.2013.14.8.4853 [DOI] [PubMed] [Google Scholar]
- 20.Semenza GL. Involvement of hypoxia-inducible factor 1 in human cancer. Intern Med 2002; 41(2):79-83; PMID:11868612; http://dx.doi.org/ 10.2169/internalmedicine.41.79 [DOI] [PubMed] [Google Scholar]
- 21.Lin SC, Liao WL, Lee JC, Tsai SJ. Hypoxia-regulated gene network in drug resistance and cancer progression. Exp Biol Med (Maywood) 2014; 239(7):779-792; PMID:24812122; http://dx.doi.org/ 10.1177/1535370214532755 [DOI] [PubMed] [Google Scholar]
- 22.Georgescu MM, Morales FC, Molina JR, Hayashi Y. Roles of NHERF1/EBP50 in cancer. CurrMol Med 2008; 8(6):459-68; PMID:18781953; http://dx.doi.org/ 10.2174/156652408785748031 [DOI] [PubMed] [Google Scholar]
- 23.Voltz JW, Weinman EJ, Shenolikar S. Expanding the role of NHERF, a PDZ-domain containing protein adapter, to growth regulation. Oncogene 2001; 20(44):6309-14; PMID:11607833; http://dx.doi.org/ 10.1038/sj.onc.1204774 [DOI] [PubMed] [Google Scholar]
- 24.Hegedüs T, Sessler T, Scott R, Thelin W, Bakos E, Váradi A, Szabó K, Homolya L, Milgram SL, Sarkadi B. C-terminal phosphorylation of MRP2 modulates its interaction with PDZ proteins. Biochem Biophys Res Commun 2003; 302(3):454-61; PMID:12615054; http://dx.doi.org/ 10.1016/S0006-291X(03)00196-7 [DOI] [PubMed] [Google Scholar]
- 25.Hoque MT, Cole SP. Down-regulation of Na+/H+ exchanger regulatory factor 1 increases expression and function of multidrug resistance protein 4. Cancer Res 2008; 68(12):4802-9; PMID:18559527; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-6778 [DOI] [PubMed] [Google Scholar]
- 26.Cardone RA, Bellizzi A, Busco G, Weinman EJ, Dell'Aquila ME, Casavola V, Azzariti A, Mangia A, Paradiso A, Reshkin SJ. The NHERF1 PDZ2 domain regulates PKA-RhoA-p38-mediated NHE1 activation and invasion in breast tumor cells. Mol Biol Cell 2007; 18(5):1768-80: PMID:17332506; http://dx.doi.org/ 10.1091/mbc.E06-07-0617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malfettone A, Silvestris N, Paradiso A, Mattioli E, Simone G, Mangia A. Overexpression of nuclear NHERF1 in advanced colorectal cancer: association with hypoxic microenvironment and tumor invasive phenotype. Exp Mol Pathol 2012; 92(3):296-303; PMID:22440733; http://dx.doi.org/ 10.1016/j.yexmp.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 28.Bellizzi A, Malfettone A, Cardone RA, Mangia A. NHERF1/EBP50 in Breast Cancer: Clinical Perspectives. Breast Care (Basel) 2010; 5(2):86-90; PMID:21048827; http://dx.doi.org/ 10.1159/000298962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karn T, Ruckhäberle E, Hanker L, Müller V, Schmidt M, Solbach C, Gätje R, Gehrmann M, Holtrich U, Kaufmann M, et al.. Gene expression profiling of luminal B breast cancers reveals NHERF1 as a new marker of endocrine resistance. Breast Cancer Res Treat 2011; 130(2):409-20; PMID:21203899; http://dx.doi.org/ 10.1007/s10549-010-1333-x [DOI] [PubMed] [Google Scholar]
- 30.Correale P, Fulfaro F, Marsili S, Cicero G, Bajardi E, Intrivici C, Vuolo G, Carli AF, Caraglia M, Del Prete S, Greco E, Gebbia N, Francini G. Gemcitabine (GEM) plus oxaliplatin, folinic acid, and 5-fluorouracil (FOLFOX-4) in patients with advanced gastric cancer. Cancer Chemother Pharmacol 2005; 56(6):563-8; PMID: 16041610; http://dx.doi.org/ 10.1007/s00280-005-1024-1 [DOI] [PubMed] [Google Scholar]
- 31.Santini D, Graziano F, Catalano V, Di Seri M, Testa E, Baldelli AM, Giordani P, La Cesa A, Spalletta B, Vincenzi B, et al.. Weekly oxaliplatin, 5-fluorouracil and folinic acid (OXALF) as first-line chemotherapy for elderly patients with advanced gastric cancer: results of a phase II trial. BMC Cancer 2006; 10(6):125; PMID: 16686939; http://dx.doi.org/ 10.1186/1471-2407-6-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai JL, Wang L, Sabin AA, Broemeling LD, Schutte M, Pan Y. NHERF-1 (Na+/H+ exchanger regulatory factor) gene mutations in human breast cancer. Oncogene 2004; 23(53):8681-8687; PMID:15467753 [DOI] [PubMed] [Google Scholar]
- 33.Mangia A, Chiriatti A, Bellizzi A, Malfettone A, Stea B, Zito FA, Reshkin SJ, Simone G, Paradiso A. Biological role of NHERF1 protein expression in breast cancer. Histopathology 2009; 55(5):600-8; PMID:19912366; http://dx.doi.org/ 10.1111/j.1365-2559.2009.03424.x [DOI] [PubMed] [Google Scholar]
- 34.Shibata T, Chuma M, Kokubu A, Sakamoto M, Hirohashi S. EBP50, a beta-catenin-associating protein, enhances Wnt signaling and is over-expressed in hepatocellular carcinoma. Hepatology 2003; 38(1):178-86; PMID:12830000; http://dx.doi.org/ 10.1053/jhep.2003.50270 [DOI] [PubMed] [Google Scholar]
- 35.Hu WQ, Peng CW, Li Y. The expression and significance of P-glycoprotein, lung resistance protein and multidrug resistance-associated protein in gastric cancer. J Exp Clin Cancer Res 2009; 28:144; PMID:19930704; http://dx.doi.org/ 10.1186/1756-9966-28-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffiths EA, Pritchard SA, Valentine HR, Whitchelo N, Bishop PW, Ebert MP, Price PM, Welch IM, West CM. Hypoxia-inducible factor-1alpha expression in the gastric carcinogenesis sequence and its prognostic role in gastric and gastro-oesophageal adenocarcinomas. Br J Cancer 2007; 96(1):95-103; PMID:17179985; http://dx.doi.org/ 10.1038/sj.bjc.6603524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Oliveira J1, Felipe AV, Neto RA, Oshima CT, de Souza Silva M, Forones NM. Association between ABCB1 immunohistochemical expression and overall survival in gastric cancer patients. Asian Pac J Cancer Prev 2014; 15(16):6935-8; PMID:25169549; http://dx.doi.org/ 10.7314/APJCP.2014.15.16.6935 [DOI] [PubMed] [Google Scholar]
- 38.Ding Z, Yang L, Xie X, Xie F, Pan F, Li J, He J, Liang H. Expression and significance of hypoxia-inducible factor-1 alpha and MDR1/P-glycoprotein in human colon carcinoma tissue and cells. J Cancer Res Clin Oncol 2010; 136(11):1697-707; PMID:20217131; http://dx.doi.org/ 10.1007/s00432-010-0828-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cardone RA, Bagorda A, Bellizzi A, Busco G, Guerra L, Paradiso A, Casavola V, Zaccolo M, Reshkin SJ. Protein kinase A gating of a pseudopodial-located RhoA/ROCK/p38/NHE1 signal module regulates invasion in breast cancer cell lines. Mol Biol Cell 2005; 16(7):3117-27; PMID:15843433; http://dx.doi.org/ 10.1091/mbc.E04-10-0945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayashi Y, Molina JR, Hamilton SR, Georgescu MM. NHERF1/EBP50 is a new marker in colorectal cancer. Neoplasia 2010; 12(12):1013-22; PMID:21170265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paradiso A, Scarpi E, Malfettone A, Addati T, Giotta F, Simone G, Amadori D, Mangia A. Nuclear NHERF1 expression as a prognostic marker in breast cancer. Cell Death Dis 2013; 4:e904; PMID:24201803; http://dx.doi.org/ 10.1038/cddis.2013.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song J, Bai J, Yang W, Gabrielson EW, Chan DW, Zhang Z. Expression and clinicopathological significance of oestrogen-responsive ezrin-radixin-moesin-binding phosphoprotein 50 in breast cancer. Histopathology 2007; 51(1):40-53; PMID:17593079; http://dx.doi.org/ 10.1111/j.1365-2559.2007.02730.x [DOI] [PubMed] [Google Scholar]
- 43.Stemmer-Rachamimov AO, Wiederhold T, Nielsen GP, James M, Pinney-Michalowski D, Roy JE, Cohen WA, Ramesh V, Louis DN. NHE-RF, a merlin-interacting protein, is primarily expressed in luminal epithelia, proliferative endometrium, and estrogen receptor-positive breast carcinomas. Am J Pathol 2001; 158(1):57-62; PMID:11141479; http://dx.doi.org/ 10.1016/S0002-9440(10)63944-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lv XG, Lei XF, Ji MY, Guo XF, Wang J, Dong WG. Clinical significance of EBP50 overexpression assessed by quantum dot analysis in gastric cancer. Oncol Lett 2013; 5(6):1844-1848; PMID:23833653; http://dx.doi.org/ 10.3892/ol.2013.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al.. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45(2):228-47; PMID:19097774; http://dx.doi.org/ 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]