Abstract
Background
Topical corticosteroids (TCS) are the first-line agents for the treatment of atopic dermatitis (AD). However, a vague phobia concerning the use of TCS exists among parents of children with AD because of misinformation, and studies on the subject are insufficient.
Objective
To assess the current state of steroid phobia among parents of atopic children in Korea and to investigate the importance of education in its reduction.
Methods
By using a structured questionnaire, 126 parents of children with AD (22.2% fathers, 77.8% mothers) were interviewed. After the questionnaire administration, all participants were educated about TCS use.
Results
Overall, 67.5% of the parents showed steroid phobia. A statistical correlation was found between steroid phobia and knowledge of potential adverse events, experience of TCS use, and adherence to treatment (p<0.05). Adherence to treatment tended to be lower among parents with steroid phobia. The most recognized adverse effects of TCS were skin atrophy and thinning (71.9%). The most prevalent information source leading to steroid phobia was the Internet (49.2%). The risk factors for steroid phobia were AD severity (odds ratio [OR]=5.332 [moderate], 9.040 [severe] vs. mild; p=0.001) and the knowledge of potential adverse events (OR=2.658; p=0.021).
Conclusion
We found a high prevalence of steroid phobia among parents of patients with AD, and here show the impact of this phobia on treatment adherence. We emphasize the important role of dermatologists as providers of accurate information and appropriate education about the use of TCS.
Keywords: Atopic dermatitis, Steroid phobia, Topical corticosteroid
INTRODUCTION
Atopic dermatitis (AD) is a chronic, inflammatory, eczematous skin condition that mainly occurs in childhood1,2. The adherence to treatment for AD is critically important for the improvement of the condition. Poor control of AD may result in mental or social problems, as well as considerable socioeconomic deficits3,4. Topical corticosteroids (TCS) are the first-line therapeutic agents for AD and are particularly effective during disease exacerbation5. However, some parents of atopic children are reluctant to use TCS because of a vague steroid phobia, which could result in AD exacerbation.
When TCS were first introduced in Korea, there were no basic prescription criteria based on bioavailability. This led to TCS abuse, causing several adverse effects and possibly originating the currently prevalent steroid phobia6. After the growth of mass media in the 1990s, information related to adverse effects associated with TCS overuse became readily available, further fuelling the unwarranted fear of TCS. The wide use of traditional Korean medicines and alternative medicines that include herbs, acupuncture, moxibustion, and homeopathy may also have an influence on this phenomenon7. Steroid phobia may severely affect the parents of atopic children, particularly because the age at which AD is most prevalent is also the age at which the parent-child attachment is at its peak.
The negative connotations associated with TCS have been described as fear, concern, anxiety, scare, and phobia8,9,10,11,12,13. Studies concerning steroid phobia have been undertaken in the United Kingdom, France, Hong Kong, Australia, and Japan, but not in Korea9,10,11,12,13. Therefore, in this study, we aimed to assess the current state of steroid phobia in Korea and to investigate the importance of education in its reduction, to improve AD management.
MATERIALS AND METHODS
Study subjects
Parents of children with AD diagnosed according to the Hanifin and Rajka criteria, and treated in the department of dermatology at Kangdong Sacred Heart Hospital and Kangwon National University Hospital (Seoul, Korea), were consecutively recruited for this prospective study between March and August 201314. Only parents of <16-year-old children with AD were eligible. Parents of children taking TCS for other inflammatory dermatoses were excluded. Of the 143 parents (mothers or fathers) who participated, 17 were excluded because of incomplete or flawed responses, and 126 were finally enrolled. This study was approved from the institutional review board of the Kangdong Sacred Heart Hospital (IRB No. 2013-2-07) and Kangwon National University Hospital (IRB No. 2013-04-001-005).
Questionnaire and education
The questionnaire was divided into 3 parts. The first part assessed the clinical background, including demographic data, degree of AD severity, and level of AD control on the basis of the participants' judgment, by using the patient-oriented eczema measure (POEM). Generally, POEM scores are categorized as follows: 0~2=clear/almost clear, 3~7=mild, 8~16=moderate, 17~24=severe, and 25~28=very severe. However, the present study abbreviated the severity bands into 3 groups as: <8=mild, 8~16=moderate, and >16=severe15,16. When possible, a dermatologist determined the SCORing Atopic Dermatitis (SCORAD) index score according to the Korean Atopic Dermatitis Association criteria (<15=mild, 15~40=moderate, and >40=severe). The second part dealt with attitudes toward TCS use. In this part, the Osnabrück scale was used to determine the presence of steroid phobia (Table 1)17. Knowledge of potential adverse events associated with TCS use and the source of information leading to steroid phobia were also investigated. The third part included questions relating to TCS use behavior, constructed according to participants' TCS use, occurrence of adverse effects, and use of alternative agents.
Table 1. The Osnabrück scale.
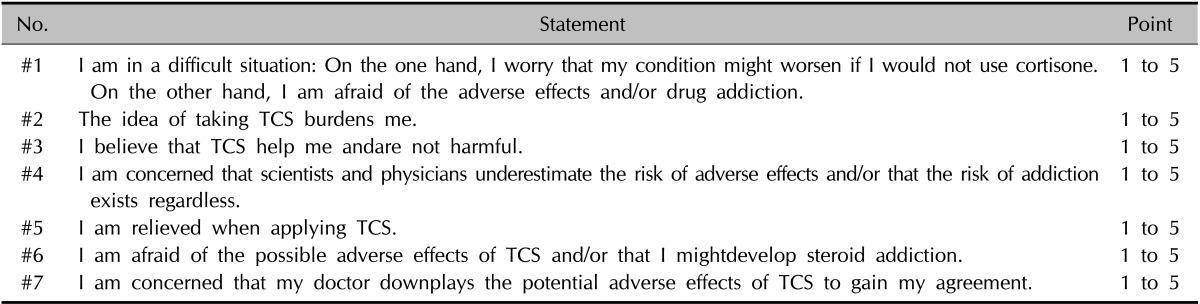
The Osnabrück scale comprises 7 statements referring to attitudes toward the use of topical corticosteroids (TCS). Each statement is rated on a 5-point scale, from 1="I do not agree at all." to 5="I fully agree." If the cutoff point is defined as scale point 3, any person with an average >3.0 across the 7 items is defined as experiencing steroid phobia. The ratings of the positive items #3 and #5 are numerically processed by inverting the rating scale (inverted rating=6-rating).
Once the questionnaire was completed, participants were educated by a dermatologist about aspects of TCS, including application methods and predictable adverse effects. We provided the participants with written instructions on the use of TCS, and measured the index of steroid phobia scores to assess their satisfaction. Pre- and post-education index scores totaled to a 100-point scale and were divided into 5 categories for analysis: concern about resistance, concern about ineffectiveness, concern about temporariness, concern about adverse effects, and distrust of doctors. The scores for all categories were added, and an improvement of >30% was considered to indicate improvement of the phobia.
Statistical analysis
To investigate the correlations of attitude and TCS-use behavior with steroid phobia, nominal data were analyzed by using the χ2 test or Fisher's exact test. Univariable logistic analysis was used to investigate risk factors for steroid phobia. Statistical analysis was performed with PASW Statistics ver. 18.0 for Windows (IBM Co., Armonk, NY, USA), and p-values <0.05 were considered significant.
RESULTS
Patient characteristics
A total of 126 parents (28 [22.2%] fathers and 98 [77.8%] mothers) completed the questionnaire. Their mean age was 36.8±6.4 years. The mean age of the children with AD (58 [46.0%] boys and 68 [54.0%] girls) was 7.5±4.8 years. AD severity, as assessed by using POEM, was reported as mild by 72 (57.1%) parents, moderate by 42 (33.3%) parents, and severe by 12 (9.5%) parents. SCORAD index scores, available for 115 patients, indicated mild AD in 67 (58.3%) patients, moderate AD in 40 (34.8%) patients, and severe AD in 8 (7.0%) patients. The reported degree of AD severity and the SCORAD index score did not match in 12 patients. The mean Osnabrück scale score was 3.22±0.7 (median, 1.73). On the basis of a mean value of ≥3, 85 (67.5%) parents were classified as having steroid phobia and 41 (32.5%) parents were classified as not having the phobia. Other demographic characteristics are shown in Table 2.
Table 2. Demographic and clinical characteristics.
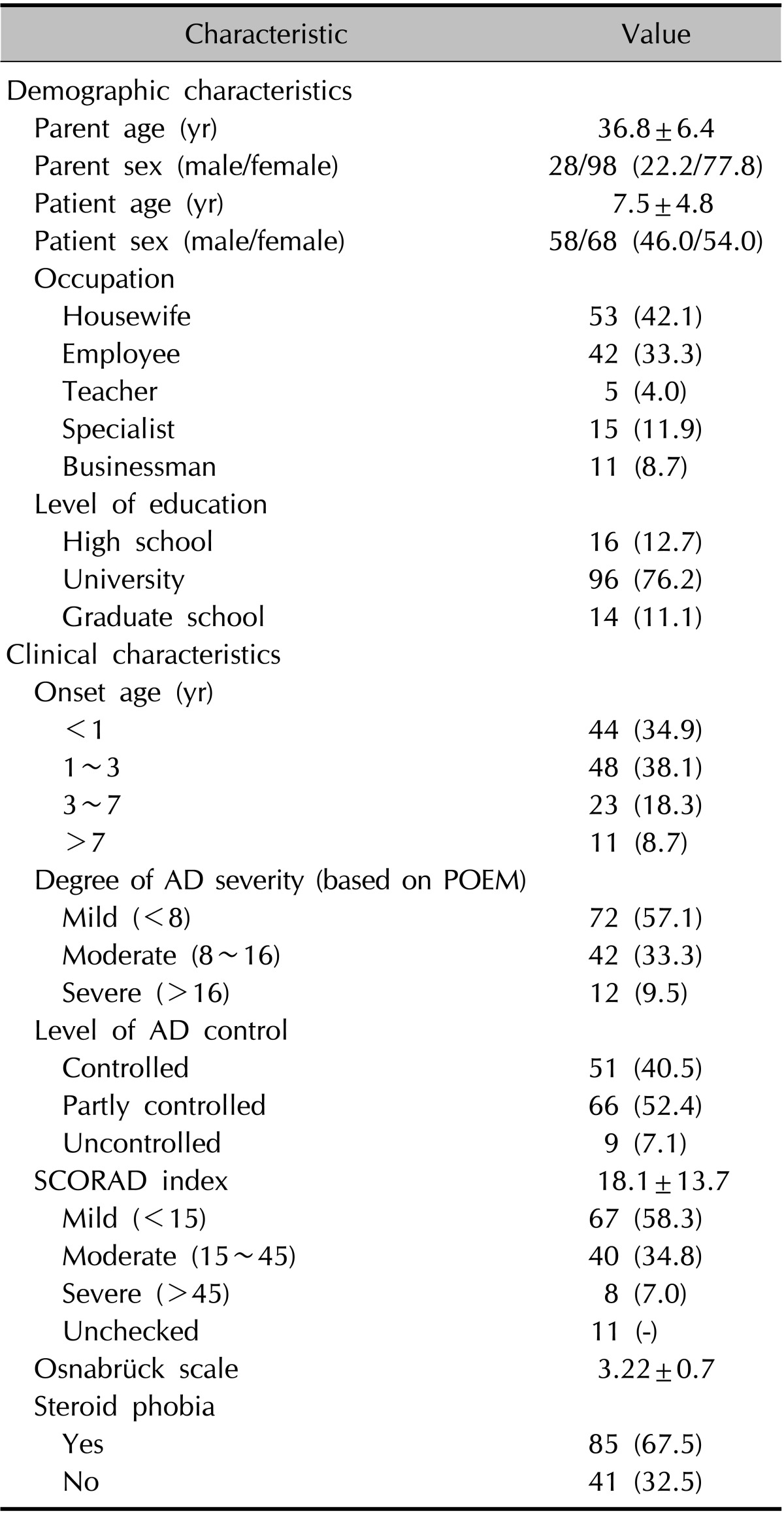
Values are presented as mean±standard deviation or number (%). AD: atopic dermatitis, POEM: patient-oriented eczema measure, SCORAD: SCORing Atopic Dermatitis.
Analysis of correlations with steroid phobia
Of the 5 questionnaire questions evaluating the knowledge of TCS, 3 were strongly correlated with steroid phobia. These were, "When I use the TCS, I am always concerned about the phrase 'only a small amount'" (p=0.004), "TCS will affect my child's immune system in the future" (p=0.019), and "I will stop TCS application as soon as possible" (p=0.034). Knowledge of potential adverse events (p=0.008), experience of TCS use (p=0.008), and treatment adherence (p=0.035) were also strongly related to steroid phobia. The result showed that if the parents had greater knowledge (regardless of its authenticity) of the adverse effects, they had a more severe steroid phobia. However, the occurrence of adverse effect (p=0.148) and use of alternative topical agents such as calcineurin inhibitors, herbal medications, or homeopathic treatments (p=0.072) did not seem to correlate with phobia. After receiving TCS education, the rate of participant satisfaction (p=0.023) and improvement in the perception of TCS use (p=0.013) were correlated with steroid phobia (Table 3). Finally, the phobia index score for TCS was 67.2 before and 38.2 after education was provided, representing an improvement of 43.2%. After excluding the 19 parents children who had never used TCS, the frequency and duration of TCS use did not differ significantly according to the presence of steroid phobia among the remaining 107 parents (Table 4).
Table 3. Questionnaire about steroid phobia (n=126).
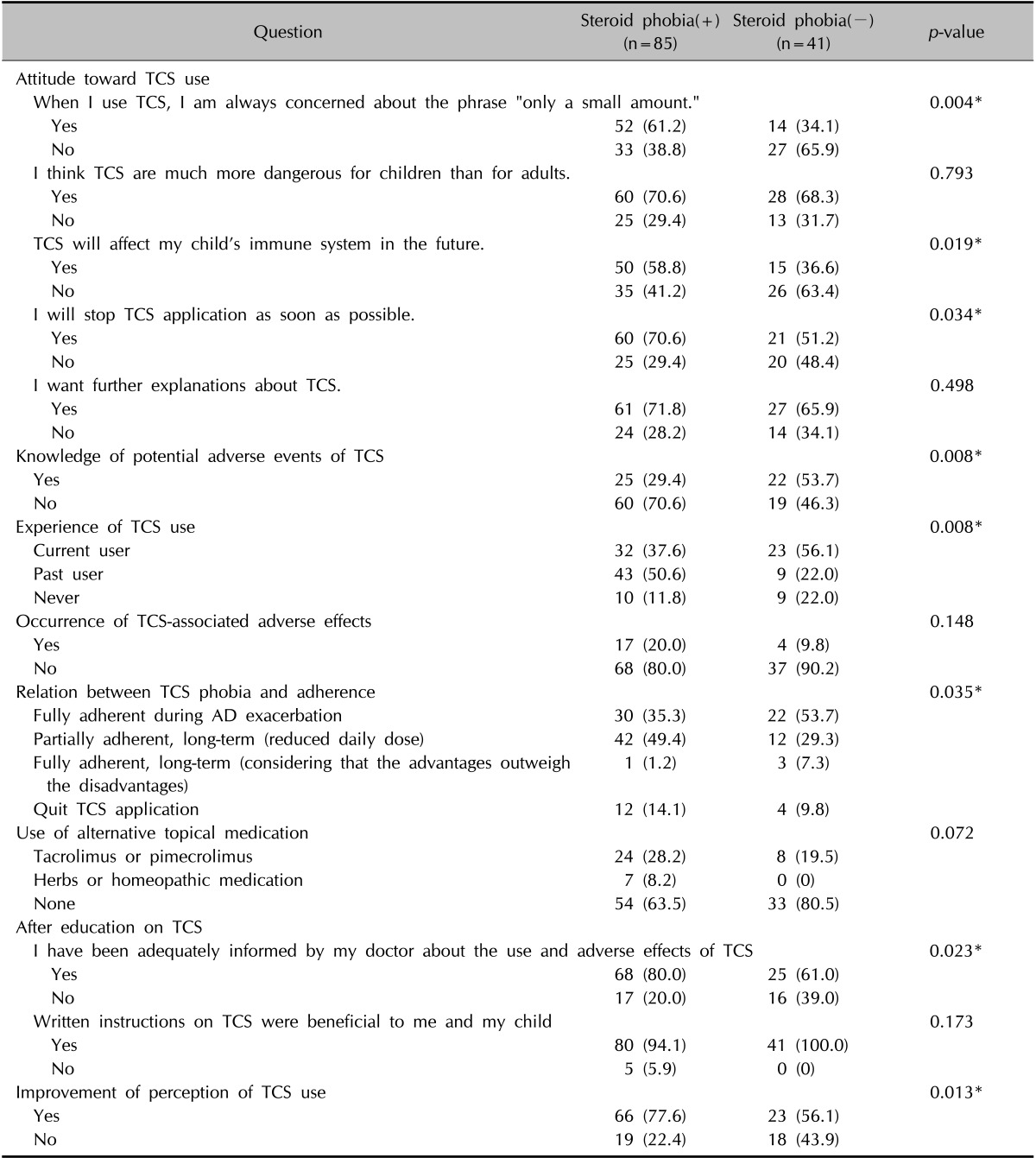
Values are presented as number (%). TCS: topical corticosteroid. *p<0.05.
Table 4. Topical corticosteroid (TCS) use.
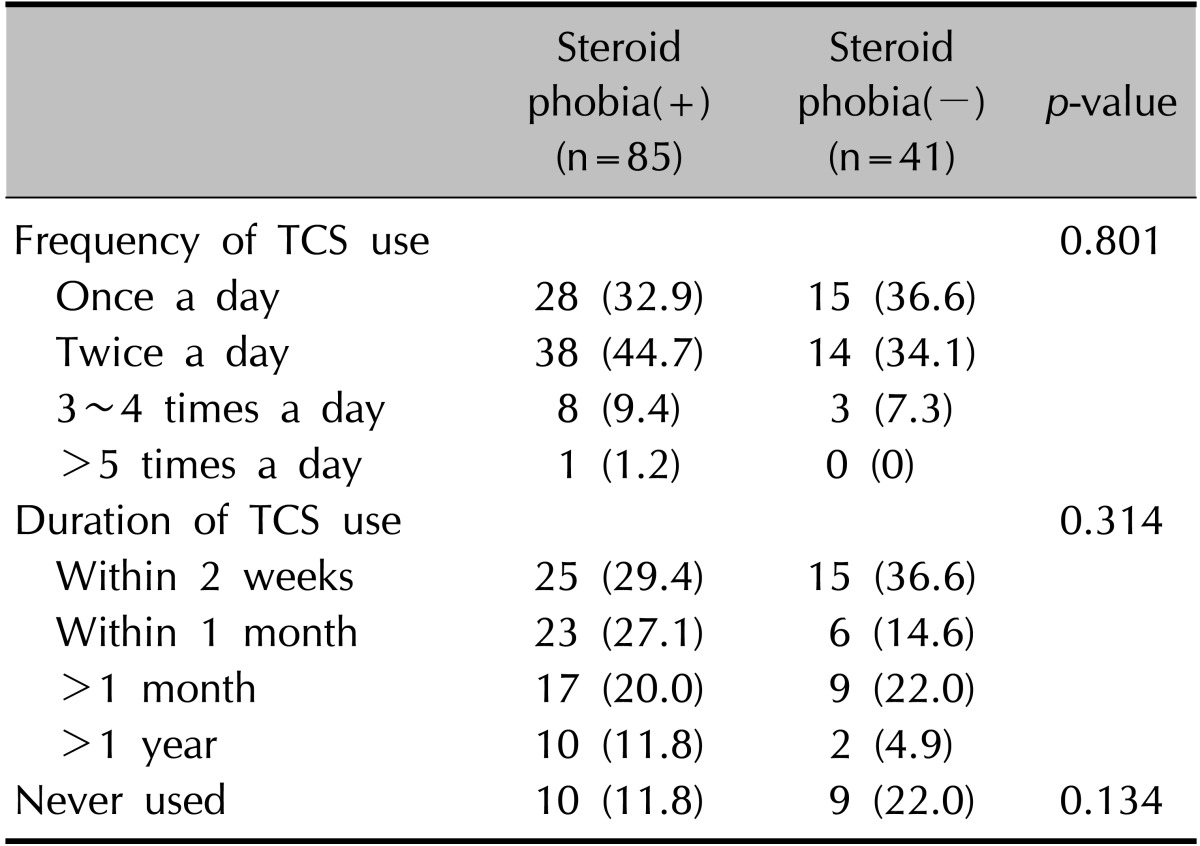
Values are presented as number (%).
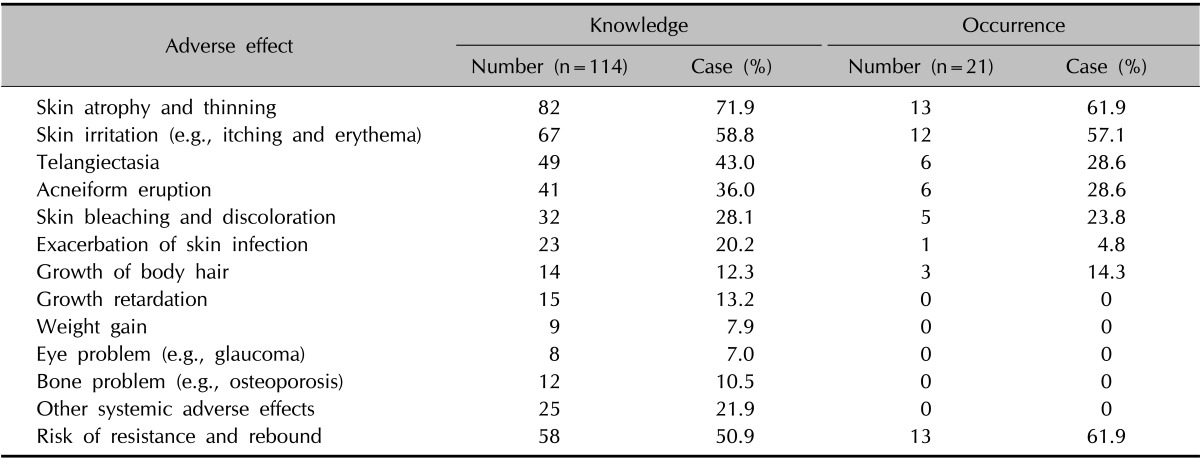
Knowledge and occurrence of adverse effects when using TCS
According to the multiple-choice responses from 114 parents who were aware of TCS-associated adverse effects, skin atrophy and thinning (71.9%) were the most frequently recognized adverse effects, followed by skin irritation (58.8%) and risk of resistance and rebound (50.9%). The children of 21 parents had reportedly experienced adverse effects, and the most commonly occurring adverse effects were skin atrophy and thinning (61.9%) and resistance and rebound (61.9%). No systemic adverse effects were reported (Table 5).
Table 5. Knowledge and occurrence of adverse effects when using topical corticosteroids.
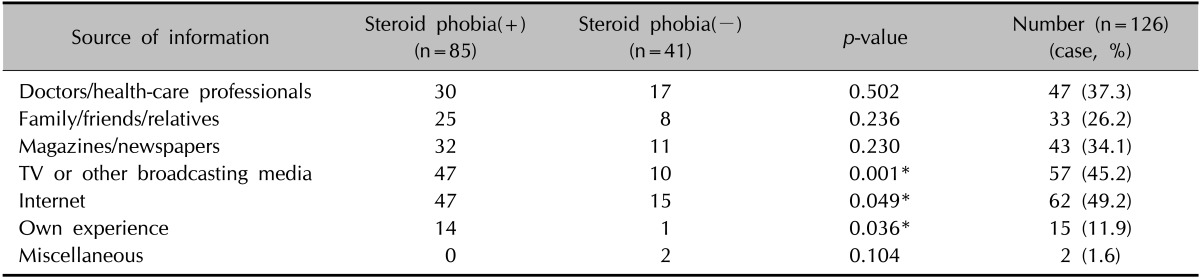
Source of information leading to steroid phobia
Sources of information that could be associated with steroid phobia included the Internet (49.2%), television or other broadcasting media (45.2%), doctors/health-care professionals (37.3%), and magazines/newspapers (34.1%). In particular, parents who cited television or other broadcasting media, the Internet, and personal experience as their information sources were significantly more likely to show a steroid phobia (Table 6).
Table 6. Source of information that can be associated with steroid phobia.
*p<0.05.
Univariable logistic analysis of steroid phobia
Univariable logistic analysis identified the degree of AD severity (odds ratio [OR]=5.332 [moderate], 9.040 [severe] vs. mild; p=0.001) and knowledge of potential adverse events of TCS (OR=2.658; p=0.021) as potential risk factors significantly associated with steroid phobia (Table 7).
Table 7. Univariable logistic analysis of steroid phobia.
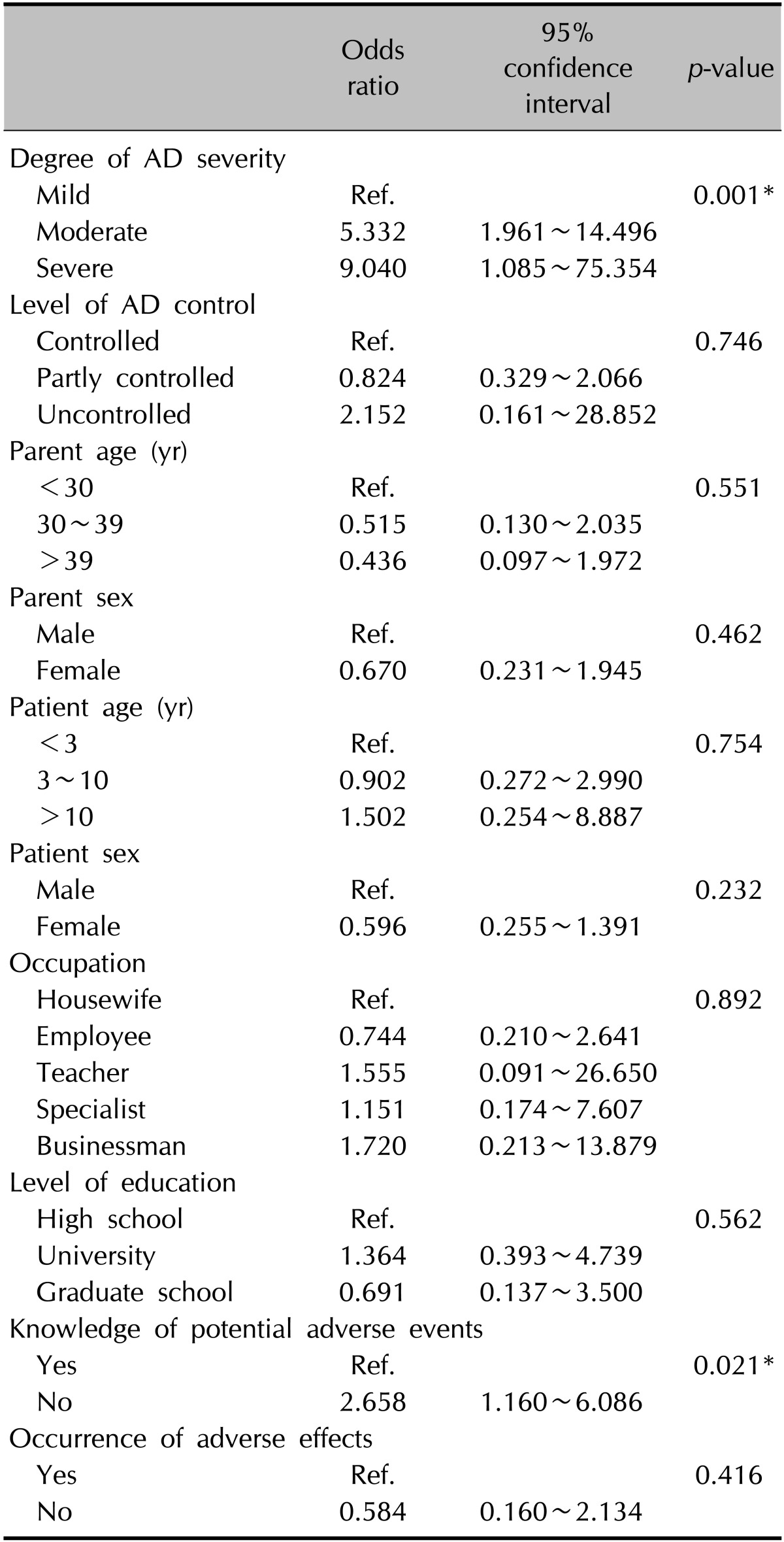
AD: atopic dermatitis, Ref.: reference. *p<0.05.
DISCUSSION
The prevalence of AD became higher owing to the development of industrialization, and this burden of AD on children has become one of the most serious problems in Korea. Frequent skin lesion exacerbation and uncontrolled pruritus critically affect atopic children, as these may lead to learning disability, insomnia, depression, and even social phobia2,18. Applying TCS when needed is important for improving the ability of atopic children to maintain a high quality of life and to continue their normal daily functions. Unfortunately, some parents of children with AD discontinue TCS treatment because of steroid phobia19. Although the systemic adverse effects of TCS are very rarely reported and their long-term toxicity is extremely low, the adverse effect of TCS is largely exaggerated to the public. This results in the efficacy and safety of TCS becoming biased and ignored by the public, which brings about steroid phobia.
According to our results, 67.5% of parents showed steroid phobia. Similar to previous reports, a negative attitude toward TCS was correlated with steroid phobia9,10,11,12,13. Surprisingly, the occurrence of adverse effects was not correlated with phobia, but the experience of TCS use showed a correlation. This may be because few persons have experienced TCS-associated adverse effects, suggesting that steroid phobia is not based on practical experience. Parents who initially demonstrated steroid phobia were more likely to report finding education helpful and to show improved perceptions of TCS. Written instructions were also helpful. The detailed methods of our TCS education are as follows. First, a skilled dermatologist explained to the patients how to use the TCS, by using written instructions, for 10~15 min. Thereafter, the dermatologist answered the patients' questions and gave them feedbacks. Written instructions included the lesion on which the TCS should be applied, the amount of the TCS and how often it should be applied, application of a moisturizer, differences in absorbency depending on the body part, the potency of TCS, the adverse effects of TCS with clinical images, and the differences between systemic steroids and TCS. We suggest that a simple educational intervention such as ours is critical, as it enhances patient compliance, especially for a chronic disease like AD. Moreover, this represents an effective method of building better relationships between patients and dermatologists18,20.
Univariable logistic analysis identified the degree of AD severity and knowledge of potential adverse events of TCS as factors significantly associated with steroid phobia. However, previous reports concluded that the severity is not related to steroid phobia9,10,11,12,13. In these previous reports, severity was measured by using an objective indicator such as the SCORAD index. The present study, however, allowed the self-reporting of severity by using POEM, suggesting that parental judgments of disease severity are different from SCORAD index determinations. Parental judgments may be biased because if the parents consider their child's condition to be very serious, they are psychologically burdened about the child's need for prolonged TCS use. Notably, the SCORAD index also allows subjective assessment of patient symptoms, which led to the development of the objective SCORAD; however, its reliability is controversial21. Another factor is the characteristics of the medical environments in Korea. Local dermatology clinics in Korea usually focus on aesthetics, and AD patient care is therefore inconsistent. Many AD patients who transferred to university hospitals had already been disappointed by the poor prolonged treatment of disease at local clinics, and some of them are less likely to adhere to instructions; this is more common in patients with severe AD. Because this study was conducted in Korean university hospitals, this characteristic of the medical environments could affect AD severity being a risk factor for steroid phobia.
TCS are classified from superpotent to mildly potent on the basis of the vasoconstrictor assay, and most dermatologists consider age, anatomical site, vehicle, and severity when prescribing TCS22. Children with AD have a higher surface-to-weight ratio and a more vulnerable epidermal barrier than healthy adults; thus, these patients have a higher possibility of TCS absorption into the body for drugs administered at the same potency and quantity23. Therefore, TCS should be prescribed with caution in children with AD. However, only 1% of TCS are therapeutically active, and some TCS have a relatively low chance of being absorbed into the systemic circulation24. Nevertheless, many parents develop a steroid phobia because of a lack of communication with dermatologists, and because of external factors such as information gathered from the Internet or other broadcasting media22.
Korea has a highly developed information technology infrastructure; therefore, the Internet culture in Korea is very active. According to a 2006 study, a web search for the term "atopy" retrieved 483 websites from NAVER and 418 from YAHOO, the most popular search engines in Korea25. However, information on the Internet may easily be inaccurate because of the prevalence of nonprofessional sources, some of which are aimed at marketing. This flawed or exaggerated information can negatively influence the clinical progress in AD and damage the relationships between dermatologists and patients, which take a long time to build. Unfortunately, appropriate criteria for measuring the validity and accuracy of information available on the Internet have yet to be established. Therefore, legal criteria are needed to regulate the quality of information on the Internet.
This study has some limitations. First, the results may be biased because of the small sample size, and therefore cannot reflect the characteristics of the whole population. Second, because data were based on self-reporting, it was not possible to provide objective data related to practical TCS use, and there is a possibility of social desirability bias. Third, the results could be affected by unmeasured factors such as social status, personality, doctor-patient relationship, conflicting information, and differing views on TCS of individual dermatologists. Finally, the method of providing TCS education by using written instructions and the effect of such education on parents should be studied. Therefore, further study focusing on parental education and its impact on achieving better outcomes is required. Our results confirmed the high rate of steroid phobia among parents of patients with AD and its impact on treatment adherence in Korea. Dermatologists should systematically study the significance of this phobia and help the parents of children with AD to overcome steroid phobia and follow treatment protocols.
References
- 1.Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–137. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams HC. Clinical practice. Atopic dermatitis. N Engl J Med. 2005;352:2314–2324. doi: 10.1056/NEJMcp042803. [DOI] [PubMed] [Google Scholar]
- 3.Chamlin SL. The psychosocial burden of childhood atopic dermatitis. Dermatol Ther. 2006;19:104–107. doi: 10.1111/j.1529-8019.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- 4.Yang HJ, Jeon YH, Pyun BY. Evaluation of patients subjective severity using various scoring system in Korean children with atopic dermatitis. Asian Pac J Allergy Immunol. 2010;28:130–135. [PubMed] [Google Scholar]
- 5.Zuberbier T, Orlow SJ, Paller AS, Taïeb A, Allen R, Hernanz-Hermosa JM, et al. Patient perspectives on the management of atopic dermatitis. J Allergy Clin Immunol. 2006;118:226–232. doi: 10.1016/j.jaci.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Kim SY, Lee SD, Kim HO, Park YM. A survey of the awareness, knowledge, and behavior of topical steroid use in dermatologic outpatients of the university hospital. Korean J Dermatol. 2008;46:473–479. [Google Scholar]
- 7.Chin HW, Jang HS, Jang BS, Jo JH, Kim MB, Oh CK, et al. A study on utilization of alternative medicine for patients with atopic dermatitis. Korean J Dermatol. 2005;43:903–911. [Google Scholar]
- 8.David TJ. Steroid scare. Arch Dis Child. 1987;62:876–878. doi: 10.1136/adc.62.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol. 2000;142:931–936. doi: 10.1046/j.1365-2133.2000.03473.x. [DOI] [PubMed] [Google Scholar]
- 10.Hon KL, Kam WY, Leung TF, Lam MC, Wong KY, Lee KC, et al. Steroid fears in children with eczema. Acta Paediatr. 2006;95:1451–1455. doi: 10.1080/08035250600612298. [DOI] [PubMed] [Google Scholar]
- 11.Smith SD, Hong E, Fearns S, Blaszczynski A, Fischer G. Corticosteroid phobia and other confounders in the treatment of childhood atopic dermatitis explored using parent focus groups. Australas J Dermatol. 2010;51:168–174. doi: 10.1111/j.1440-0960.2010.00636.x. [DOI] [PubMed] [Google Scholar]
- 12.Aubert-Wastiaux H, Moret L, Le Rhun A, Fontenoy AM, Nguyen JM, Leux C, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808–814. doi: 10.1111/j.1365-2133.2011.10449.x. [DOI] [PubMed] [Google Scholar]
- 13.Kojima R, Fujiwara T, Matsuda A, Narita M, Matsubara O, Nonoyama S, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013;30:29–35. doi: 10.1111/j.1525-1470.2012.01808.x. [DOI] [PubMed] [Google Scholar]
- 14.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1980;60(Suppl 92):44–47. [Google Scholar]
- 15.Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients perspective. Arch Dermatol. 2004;140:1513–1519. doi: 10.1001/archderm.140.12.1513. [DOI] [PubMed] [Google Scholar]
- 16.Charman CR, Venn AJ, Ravenscroft JC, Williams HC. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br J Dermatol. 2013;169:1326–1332. doi: 10.1111/bjd.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burrichter A, Schlippe AV, Szczepanski R. Kortisonangst bei Asthma bronchiale Eine Elternbefragung [Fear of cortisone in bronchial asthma. A parent survey] Monatsschr Kinderh. 2006;154:979–985. [Google Scholar]
- 18.Ohya Y, Williams H, Steptoe A, Saito H, Iikura Y, Anderson R, et al. Psychosocial factors and adherence to treatment advice in childhood atopic dermatitis. J Invest Dermatol. 2001;117:852–857. doi: 10.1046/j.0022-202x.2001.01475.x. [DOI] [PubMed] [Google Scholar]
- 19.Fukaya M. Why do patients with atopic dermatitis refuse to apply topical corticosteroids? Dermatology. 2000;201:242–245. doi: 10.1159/000018495. [DOI] [PubMed] [Google Scholar]
- 20.Bewley A Dermatology Working Group. Expert consensus: time for a change in the way we advise our patients to use topical corticosteroids. Br J Dermatol. 2008;158:917–920. doi: 10.1111/j.1365-2133.2008.08479.x. [DOI] [PubMed] [Google Scholar]
- 21.Oranje AP, Glazenburg EJ, Wolkerstorfer A, de Waard-van der Spek FB. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007;157:645–648. doi: 10.1111/j.1365-2133.2007.08112.x. [DOI] [PubMed] [Google Scholar]
- 22.Rao VU, Apter AJ. Steroid phobia and adherence--problems, solutions, impact on benefit/risk profile. Immunol Allergy Clin North Am. 2005;25:581–595. doi: 10.1016/j.iac.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54:1–15. quiz 16-18. doi: 10.1016/j.jaad.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Cook LJ, Freinkel RK, Zugerman C, Levin DL, Radtke R. Iatrogenic hyperadrenocorticism during topical steroid therapy: assessment of systemic effects by metabolic criteria. J Am Acad Dermatol. 1982;6:1054–1060. doi: 10.1016/s0190-9622(82)70090-8. [DOI] [PubMed] [Google Scholar]
- 25.Kwon HJ, Kim YJ, Park SB, Yu DS, Kim JW. Study of atopic dermatitis information on the internet in Korea. Korean J Dermatol. 2006;44:137–140. [Google Scholar]


