Abstract
Background
Trichoscopic findings of hair loss have been well described for the differential diagnosis of alopecia; however, critical findings were not thoroughly investigated or compared among all ethnic groups, including Asians.
Objective
We aimed to find any characteristic trichoscopic findings in Korean alopecia patients and to verify whether those findings are closely related to previously reported observations.
Methods
Three hundred and twenty-seven patients with hair loss of various causes and 160 normal scalps were analyzed. Trichoscopic examination was performed with a polarized-light handheld dermoscope.
Results
A total of 35 patterns of trichoscopic features were represented, and certain features were significantly common or observed exclusively in a particular type of alopecia as follows: yellow dots, exclamation mark hairs, and proximal tapering hairs (alopecia areata), trichoptilosis and pointed hairs (trichotillomania), corkscrew hairs, septate hyphae hairs, and comma hairs (tinea capitis), diffuse white area, fibrotic white dots, and tufting hairs (primary cicatricial alopecia), hair diameter diversity and peripilar sign (androgenetic alopecia), and short nonvellus hairs (telogen effluvium).
Conclusion
The characteristic trichoscopic features for the differential diagnosis of alopecia in Koreans, shown as follicular, perifollicular, and hair shaft patterns, are similar to those of Caucasians; however, the frequencies of the pigment patterns are different between Koreans and Caucasians because of the contrast effect of the skin and hair color. Therefore, racial difference should be considered in the trichoscopic evaluation for differential diagnosis.
Keywords: Alopecia, Asians, Dermoscopy, Koreans
INTRODUCTION
Trichoscopy by using a videodermoscope or handheld dermoscope can enhance the clinical diagnosis of various hair and scalp diseases1,2,3. In recent years, the handheld-type dermoscope has been considered a reliable, easy-to-use device as shown by its comparable results to those of videodermoscopes4,5,6,7,8,9. Trichoscopic findings of hair loss have been well described for the differential diagnosis of alopecia; however, most previous studies were primarily performed in Caucasian patients. Therefore, we performed this study to find any distinct pattern in the trichoscopic findings of normal persons and patients with alopecia due to various causes in Korean, and analyzed the trichoscopic patterns to verify any relation with known predisposing findings reported earlier in Caucasian alopecia patients.
MATERIALS AND METHODS
Patients
We examined 327 patients with alopecia (149 males, 178 females) and 160 healthy individuals (80 men, 80 women) designated as controls. The age range of the patient group was 2~81 years (mean age, 34.0±18.2 years), compared with 1~84 years (mean age, 40.5±22.7 years) in normal subjects (Table 1). All alopecia patients and normal subjects were Koreans with Fitzpatrick skin type III or IV, and black hair. The patients were first divided into two groups based on the alopecia pattern: localized and diffuse. The localized types included alopecia areata (AA, patchy and ophiasis type), trichotillomania (TM), tinea capitis (TC), traumatic alopecia (TA), and primary cicatricial alopecia (PCA). The diffuse types included AA (diffuse and incognita type), androgenetic alopecia (AGA), and telogen effluvium (TE) cases. The differential diagnosis of alopecia was clinically established according to distinctive clinical features, trichogram, microscopic hair examination, fungus culture, psychological counseling, and further confirmed with scalp biopsy in ambiguous cases or the cicatricial type (PCA). Patients having more than two different types of alopecia were excluded to avoid a confounding factor. The study was approved by an institutional review board of Chonbuk National University Hospital (IRB No. CUH 2012-06-019), and informed consent was obtained from all participants before study participation.
Table 1. Main characteristics of the patients and control subjects.
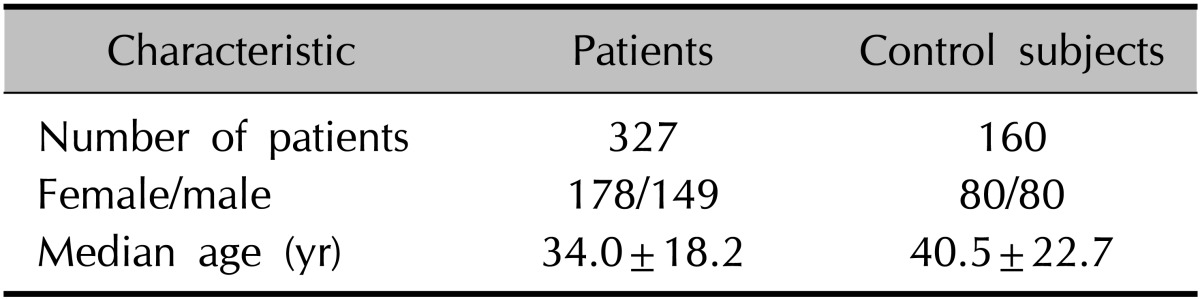
Values are presented as number only or mean±standard deviation.
Trichoscopic examination
Trichoscopic examination was performed with a polarized- light handheld dermoscope (Dermlite DL3; 3Gen LLC, San Juan Capistrano, CA, USA) without the use of liquid medium. All examined lesions were photographed with a digital camera (Canon DSRL; EOS50D, Tokyo, Japan), of a 3- or 4-fold optical zoom, connected to the dermoscope. From the central to the peripheral parts of the alopecia, the lesions were examined routinely in patients with localized alopecia, while the natural separation lines of the hair part at two sites (vertex and occiput) were examined in the diffuse types of alopecia and in healthy controls. The trichoscopic images were evaluated later by two independent dermatologists who have sufficient knowledge and experience in dermoscopic examination. On the basis of previous studies and our personal experience1,3,4,5,9, the checklists were predetermined, including 35 distinct trichoscopic features as possible discriminant variables, and they were grouped into the following four categories: follicular, perifollicular, interfollicular, and hair shaft patterns.
Statistical analysis
By using χ2 tests, we performed intergroup comparisons between patients and normal subjects. The intragroup classifications of alopecia were compared and analyzed in the same manner. A p-value of <0.05 was considered to be statistically significant. Statistical analysis was performed by using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA).
RESULTS
The diagnosis and demographic profiles of the patients in this study are shown in Table 2.
Table 2. Demographic profiles of patients with alopecia.
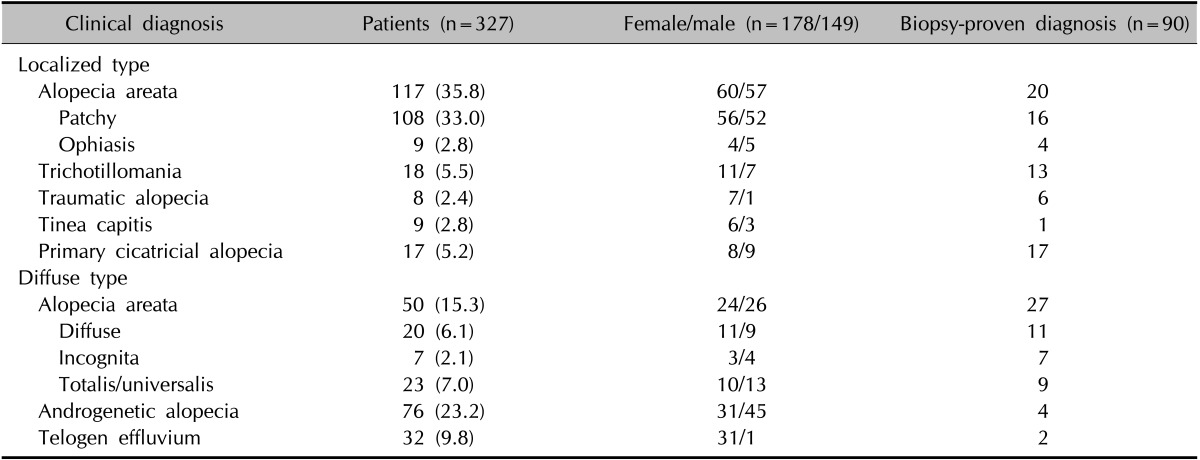
Values are presented as number (%) or number only.
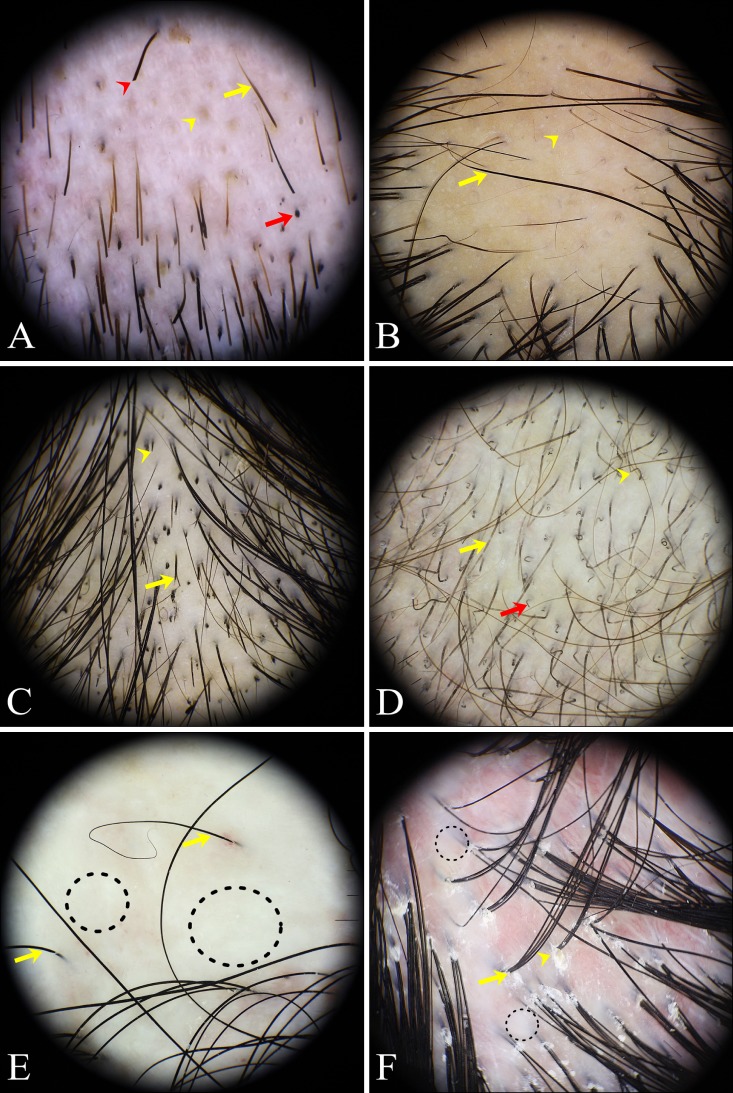
Trichoscopic findings of normal subjects (Fig. 1)
Fig. 1. Trichoscopic findings of normal subjects. (A) simple red loops (yellow arrow), (B) arborizing vessels (yellow arrow), (C) pinpoint white dots (yellow arrow), (D) dirty dots (yellow arrow), and honeycomb pigmentation (dotted circle).
The frequency of trichoscopic findings in normal subjects and alopecia patient groups are listed in Table 3. The most common trichoscopic findings in normal subjects without alopecia were simple red loops (26.9%), followed by arborizing vessels (19.7%), pinpoint white dots (13.1%), perifollicular scales (12.8%), honeycomb pigment networks (9.4%), and dirty dots (8.8%). For a total of 35 trichoscopic features, only simple red loops (pinpoint red dots) were more common in normal subjects than in alopecia patients. Pinpoint white dots, perifollicular scales, dirty dots, and uniform thinning showed no significant difference between the normal and alopecia groups. Among the remaining 30 features less common in the normal group, follicular hyperkeratosis, comma hairs, corkscrew hairs, exclamation mark hairs, pointed hairs, septate hyphae (segmented or Morse-code like) hairs, proximal tapering hairs, tufted hairs, crusts, and trichorrhexis nodosa were never seen in normal subjects.
Table 3. Trichoscopic features of alopecia patients and normal controls.
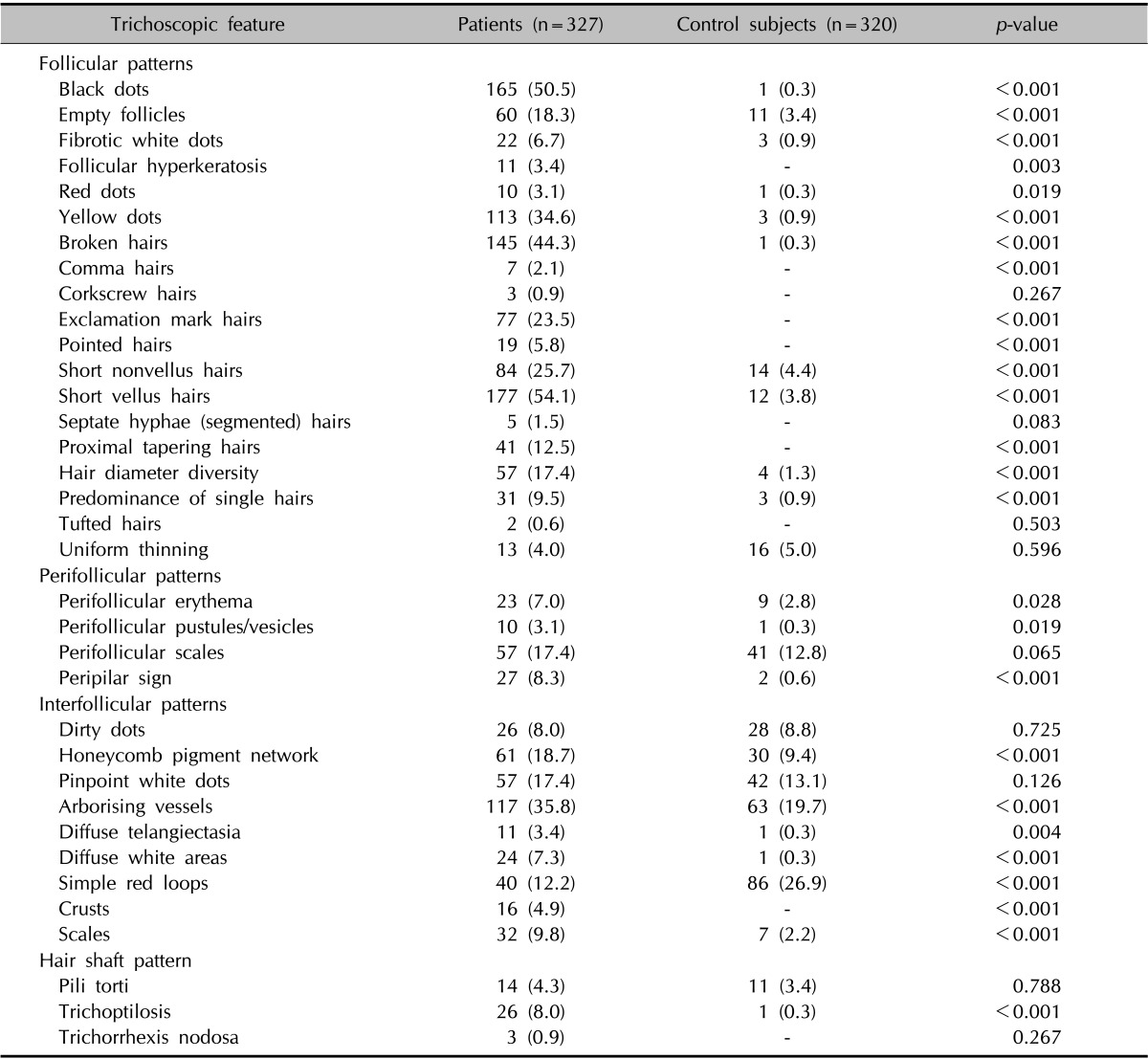
Values are presented as number (%).
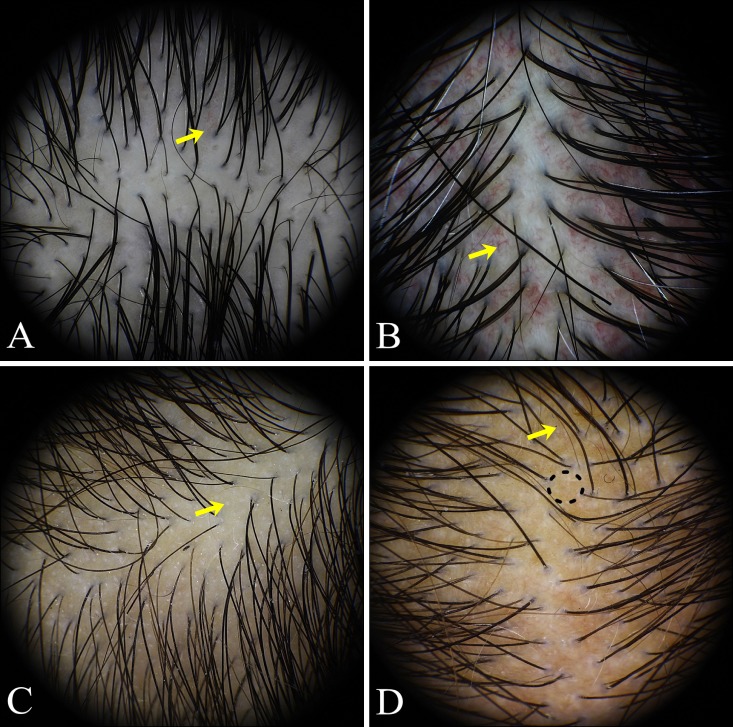
Trichoscopic findings of localized alopecia: AA, TM, TA, TC, and PCA (Fig. 2)
Fig. 2. Trichoscopic findings of localized hair loss. (A) Patchy alopecia areata: exclamation mark hairs (yellow arrow), yellow dots (yellow arrowhead), black dots (red arrow), broken hairs (red arrowhead). (B) Patchy alopecia areata: proximal tapering hairs (yellow arrow), short vellus hairs (yellow arrowhead). (C) Trichotillomania: trichoptilosis (yellow arrow), pointed hairs (yellow arrowhead), black dots, and broken hairs with different lengths. (D) Tinea capitis: septate hyphae hairs (yellow arrow), corkscrew hairs (yellow arrowhead), comma hairs (red arrow). (E) Primary cicatricial alopecia: diffuse white area (dotted circles), predominance of single hair (yellow arrows). (F) Primary cicatricial alopecia: fibrotic white dots (dotted circles), tufted hairs (yellow arrow), perifollicular scales (yellow arrowhead), diffuse erythema.
The frequencies of trichoscopic findings in each localized alopecia group are listed in Table 4.
Table 4. Trichoscopic features of patients with localized alopecia.
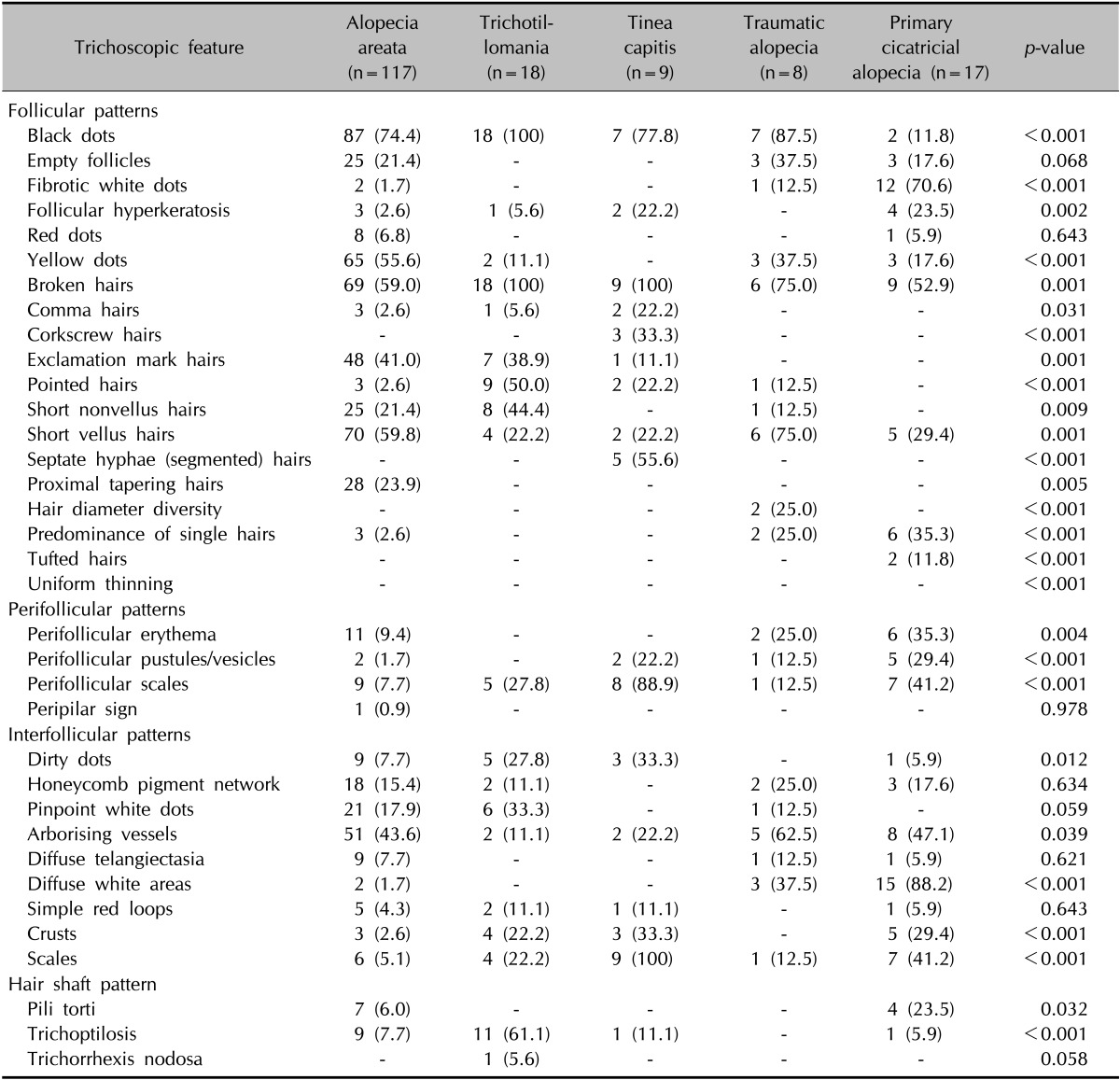
Values are presented as number (%).
1) Localized AA
The most common trichoscopic findings in AA were black dots (74.4%), followed by short vellus hairs (59.8%), broken hairs (59.0%), yellow dots (55.6%), arborizing vessels (43.6%), and exclamation mark hairs (41.0%). Yellow dots, exclamation mark hairs, and proximal tapering hairs (23.9%) were more common in AA compared with other forms of localized alopecia. In particular, proximal tapering hairs were noted solely in AA.
2) TM
Black dots and broken hairs (100%) were seen in all patients with TM. Trichoptilosis (distal longitudinal splitting) (61.1%), pointed hairs (50.0%), and short nonvellus hairs (44.4%) were significantly more common in TM, which were rarely seen in other localized types of alopecia. Exclamation mark hairs (38.9%), short vellus hairs (22.2%), and yellow dots (11.1%), previously thought to be characteristic features of AA, were also observed to a similar or lesser degree in TM compared with that in AA.
3) TC
The most sensitive findings in TC were broken hairs (100.0%) and interfollicular scales (100.0%), followed by perifollicular scales (88.9%), black dots (77.8%), septate hyphae hairs (55.6%), and diffuse erythema (44.4%). Septate hyphae hairs and corkscrew hairs (33.3%) were observed only in the TC group. Comma hairs (22.2%) were also rarely seen in other types of alopecia.
4) TA
In TA, black dots (87.5%), broken hairs (75.0%), short vellus hairs (75.0%), and arborizing vessels (62.5%) were commonly observed, and the prevalence of short vellus hairs and arborizing vessels was higher than in other types of localized alopecia. Diffuse white areas (37.5%), which represent follicular scarring, were less frequently observed in TA.
5) PCA
Diffuse white areas (88.2%) and fibrotic white dots (70.6%) were the two most common trichoscopic findings in PCA. Other common findings of PCA were broken hairs (52.9%), arborizing vessels (47.1%), and scales (41.2%), but they were also encountered in localized noncicatricial alopecia with no statistical significance. On the other hand, a predominance of single hairs (35.3%), perifollicular erythema (35.3%), perifollicular pustules (29.4%), follicular hyperkeratosis (23.5%), pili torti (23.5%), and tufted hairs (11.8%) were significantly more common in PCA than in localized noncicatricial alopecia.
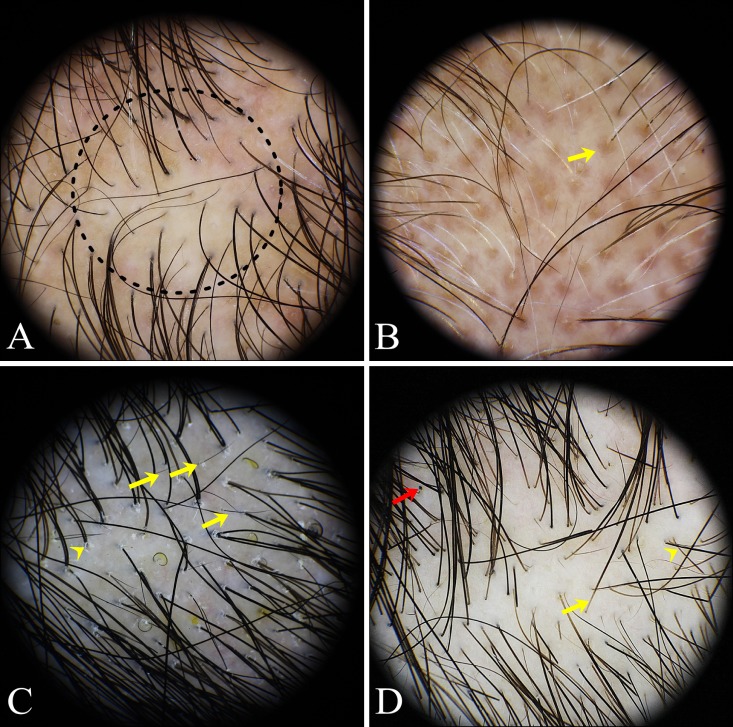
Trichoscopic findings of diffuse alopecia: diffuse AA, AGA, and TE (Fig. 3)
Fig. 3. Trichoscopic findings of diffuse hair loss. (A) Androgenetic alopecia: hair diameter diversity (dotted circle). (B) Androgenetic alopecia: peripilar sign (yellow arrow). (C) Telogen effluvium: short nonvellus hairs (yellow arrows), perifollicular scales (yellow arrowhead). (D) Alopecia areata incognita: proximal tapering hairs (yellow arrow), exclamation mark hairs (yellow arrowhead), black dots (red arrow).
The frequencies of the trichoscopic findings in each group of diffuse alopecia are presented in Table 5.
Table 5. Trichoscopic features of patients with diffuse alopecia.
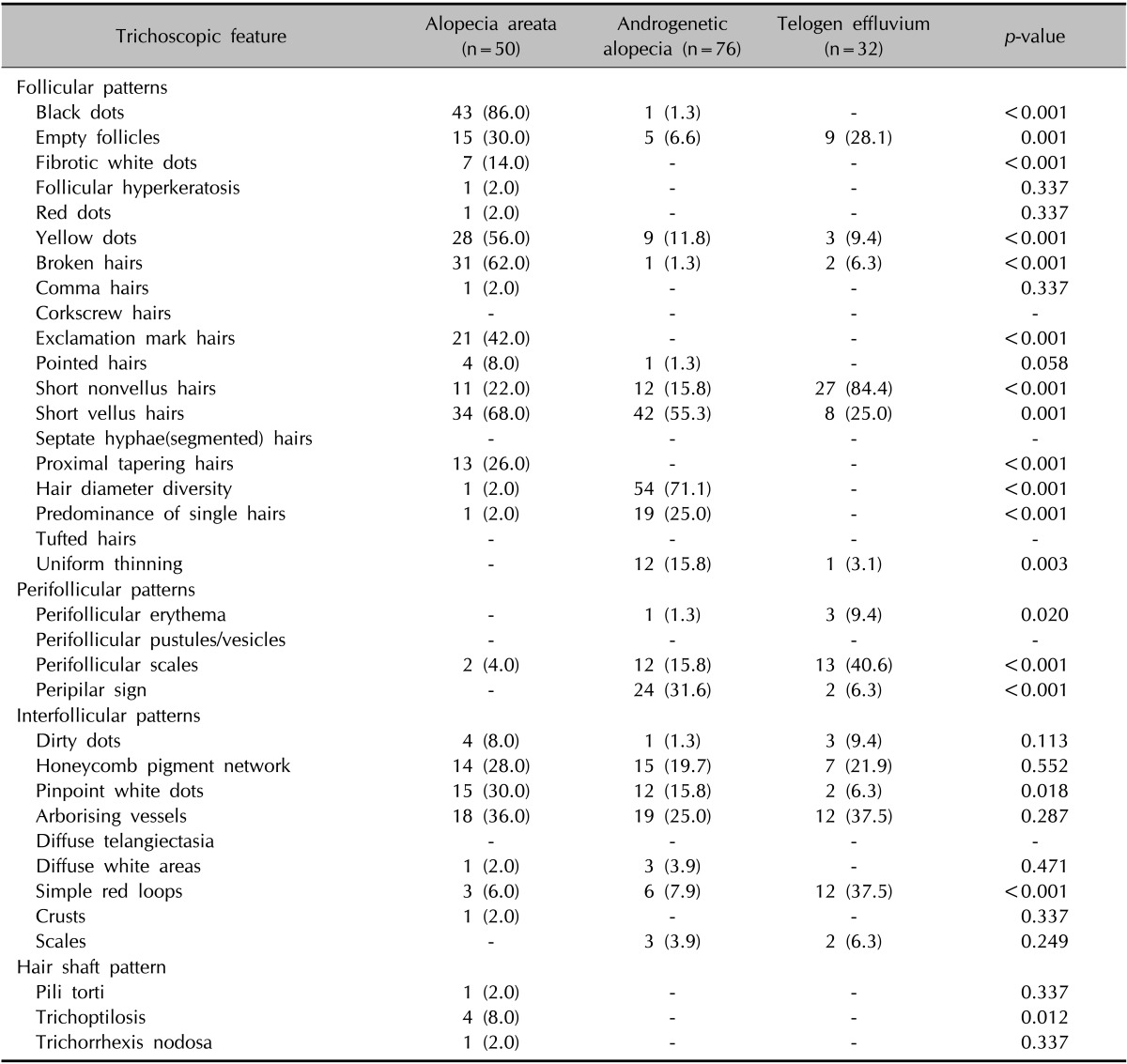
Values are presented as number (%).
1) Diffuse AA
Black dots (86.0%), short vellus hairs (68.0%), broken hairs (62.0%), and yellow dots (56.0%) were common findings in diffuse AA. Black dots, broken hairs, yellow dots, exclamation mark hairs (42.0%), pinpoint white dots (30.0%), proximal tapering hairs (26.0%), and fibrotic white dots (14.0%) showed a higher prevalence rate in AA compared with other forms of diffuse alopecia. In particular, exclamation mark hairs, proximal tapering hairs, and fibrotic white dots were confined to diffuse AA compared with other diffuse types of alopecia.
2) AGA
Hair diameter diversity (71.1%) and short vellus hairs (55.3%) were the most common findings in AGA. Compared with other diffuse types of alopecia, hair diameter diversity, peripilar sign (31.6%), a predominance of single hairs (25.0%), and uniform thinning (15.8%) were more frequently observed in AGA with statistical significance. On the other hand, a honeycomb pigment network (19.7%), previously felt to be a characteristic feature of advanced AGA, was observed to a similar or lesser degree in other diffuse types of alopecia.
3) TE
Short nonvellus hairs (84.4%) were the most sensitive trichoscopic finding in TE. Perifollicular scales (40.6%), arborizing vessels (37.5%), simple red loops (37.5%), empty follicles (28.1%), and short vellus hairs (25.0%) were seen in the stated order of frequency. Among these features, short nonvellus hairs, perifollicular scales, and simple red loops had a higher prevalence rate in TE compared with other types of diffuse alopecia.
DISCUSSION
As shown in this observation, some distinct trichoscopic patterns are predominant in certain types of alopecia, and this aids in predicting the correct diagnosis. However, the interpretation of trichoscopic findings should be done carefully because a pigmented pattern of the scalp can be influenced by several factors, including the light type of dermoscope, hair-washing habit, use of hair dye or camouflage products (dark makeup powder or foundation, cosmetic hair fibers), as well as the skin type and hair color. Koreans, or the northern Mongoloids, have distinct skin and hair characteristics such as yellow or light brown skin, and straight and black hair that is coarse with a thicker diameter and lower density compared with Caucasoid hair. Thus, there are some differences in trichoscopic findings of the scalp and hair between Koreans and Caucasians. We examined the frequency of various trichoscopic findings of normal persons and alopecia patients from the Korean population, because most of the previous studies were primarily performed in Caucasian patients, with a few others in Japanese patients7,9,10. Although a previous trichoscopic study in Koreans has been reported, the observation was rather confined to localized hairless patches in a cross-sectional study with a restrictive statistical analysis of only 14 trichoscopic features as discriminative variables, and without a normal control group11. In this study, we extended the distinctive trichoscopic features to 35, consisting of the 31 previously known trichoscopic features and 4 new trichoscopic features that could be characteristic or helpful in the diagnosis of both localized and diffuse alopecia, and we compared them with normal control subjects through an appropriate statistical analysis.
In the present study, there were no specific trichoscopic findings confined to the normal scalp, although simple red loops were more common. In addition, the frequency of pinpoint white dots, perifollicular scales, dirty dots, and uniform thinning did not show a significant difference between the normal and alopecia groups. Therefore, these five features could be considered nonspecific or of no diagnostic significance when they are shown independently. Some trichoscopic findings showed a unique distribution and prevalence rate depending on age. Unlike in an earlier study by Fu et al.12, dirty dots, nonmicrobial environmental particles appearing as brown and black particulate dots or loose fibers, revealed a bimodal peak distribution and were predominantly seen in children (<10 years old) and older patients (>60 years old). We considered that the decreased sebaceous activity resulting from the immature and regressed sebaceous glands in these age groups may affect inability to eliminate the environmental particles. Uniform thinning, empty follicles, and short vellus hairs were predominantly seen in the older group, a pattern that seems to reflect a normal physiologic aging process.
Several reports supporting the value of trichoscopy in the differential diagnosis of localized hair loss have been published. Yellow dots, black dots, broken hairs, exclamation mark hairs, and short vellus hairs in AA; broken hairs with different lengths in TM; comma hairs and corkscrew hairs in TC; and diffuse white areas in PCA were shown to be hallmark features in a previous study10,13,14,15,16,17. However, in our observation, black dots, broken hairs, and short vellus hairs were variably encountered in all types of localized noncicatricial alopecia; thus, seem to have less diagnostic value especially in the differential diagnosis of localized noncicatricial alopecia. On the other hand, the following trichoscopic features were significantly more common in particular types of localized noncicatricial alopecia, which are thought to be valuable characteristic features: yellow dots, exclamation mark hairs, proximal tapering hairs in AA, trichoptilosis and pointed hairs in TM, and corkscrew hairs, septate hyphae, and comma hairs in TC. Although exclamation mark hairs and yellow dots were known as hallmark features of AA in an earlier study, those features also appeared with a similar frequency in the TM and TA groups, respectively. Interestingly, proximal tapering hairs were observed only in AA; therefore, this could be considered as a pathognomonic feature of AA. Proximal tapering hairs, long elongated hair shafts that are narrowed down to the follicles without breakage, are different from short broken exclamation mark hairs in that their distal ends are not visible within the field of the dermoscopic view. A tapering hair alone may often be confused with a normal short regrowing hair with a distal tip; therefore, the term "proximal tapering hair" indicates its overall shape rather than tapering. Trichoptilosis and the pointed hairs of TM were also valuable characteristic features, but were not absolutely diagnostic. Pointed hairs, named by us in this study, are a kind of short broken hairs (<5 mm) that particularly look like a "bayonet" with artificially tapered, ragged distal tip predominantly in lower magnification. We consider that it may be analogous to the distal longitudinal splitting of the hair shaft resulting from mechanical manipulations. In TC, comma hairs, corkscrew hairs, and septate hyphae hairs (hair shafts divided into compartments separated by fungi) seem to be specific markers in this study. Comma hairs are short, bent, comma- shaped hairs with homogeneous thickness and share a diagonal end. Corkscrew hairs, broken hairs showing a spiral shape, differ from comma hairs with the presence of multiple twists and intensively coiled appearance. Septate hyphae hairs, also known as Morse code-like hairs, show multiple transverse white bands across the hair shaft. Although the trichoscopic findings of TC in relation to clinical and mycological features remain unclear, completely broken comma hairs or partially broken corkscrew/ septate hyphae hairs are probably associated with the severity of, or different types of fungal infection (i.e., endothrix or ectothrix). Although arborizing vessels and short vellus hairs were more common in TA than in other alopecia groups, they do not seem to be specific features; therefore, a detailed history can be very important in diagnosing TA. Diffuse white areas and fibrotic white dots, related to scar formation, were prominent features of PCA, which is similar to previous results; however, these features can also be seen in some cases of longstanding TA and AA18. In addition to scarring features, a predominance of single hairs, perifollicular erythema, pustules, follicular hyperkeratosis, pili torti, and tufted hairs, which are observed predominantly on the periphery of active lesions, were more common in the PCA group; therefore, they can be helpful in distinguishing PCA from other forms of localized alopecia. Among them, tufted hairs are thought be a diagnostic feature of PCA.
Trichoscopic findings of diffuse hair loss have not been investigated extensively in comparison with those of localized hair loss. Most of the previous studies were performed with a videodermoscope with a high magnification, and there have been insufficient comparative studies1,2,3. Hair diameter diversity (≥20%) and the peripilar sign are important distinguishing features of AGA19,20,21,22; however, there are no established sensitivities or specific clues for the diagnosis of TE1,22. In nonbald AA incognita, which can be easily confused with AGA or TE, yellow dots and short regrowing hairs are known as the characteristic features23,24. In this study, the overall trichoscopic pattern of diffuse AA was similar to that seen in localized AA, with a higher prevalence rate of black dots, short vellus hairs, broken hairs, yellow dots, and exclamation mark hairs. Proximal tapering hairs and exclamation mark hairs are considered the most specific features of AA for the differential diagnosis of diffuse alopecia. In addition to hair diameter diversity and peripilar sign, a predominance of single hairs and uniform thinning (defined as ≥80% thin hairs in one trichoscopic fieldview) were also distinguishing features of AGA in this study. In TE, a group of short nonvellus hairs, newly emerging just above surface of the scalp, were the most commonly observed finding. Short nonvellus hairs are also short and thin, but differ from short vellus hairs by their firm appearance with intense pigmentation, pointed distal end, and upright position. Perifollicular scales and simple red loops were also more common in the TE group compared with other types of diffuse alopecia, although those findings were observed on the normal scalp. Therefore, trichoscopic features can help distinguish TE from other forms of diffuse hair loss such as AGA.
The common or critical trichoscopic findings in this study for the differential diagnosis of alopecia, with regard to the follicular, perifollicular, hair shaft, and vascular patterns, revealed similarities to earlier studies primarily done in Caucasians (Table 6)1,3,8,9,14,19,20. Arborizing vessels and dirty dots in normal scalp (19.7% and 8.8% vs. 25.7%~26.6% and 11.1%); broken hairs in AA, TM, and TC (59.9%, 100.0%, and 100.0% vs. 57.1%~67.0%, 100.0%, and 83.0%); and exclamation mark hairs in AA (41.3% vs. 42.9%~71.0%) were relatively shown to have similar frequencies compared with Caucasians. However, the pigmentation patterns (i.e., peripilar signs, honeycomb pigment network, yellow dots, pinpoint white dots, and fibrotic white dots) and hair shaft patterns such as black dots and short vellus hairs were significantly different between Caucasians and Koreans. The incidence of the peripilar sign and honeycomb pigment network in AGA, and yellow dots in both AA and AGA was 31.6% and 19.7%, 55.7% and 11.8%, respectively, which are lower than those in Caucasians (59.3%~100.0% and 18.7%~37.3%, 66.0%~95.0% and 8.0%~66.0%, respectively). This may be influenced by the concealing light effect of the Asian skin color, with its yellow or brown pigmentation, which makes these features more difficult to detect5,9,19,23,24,25,26. In contrast, light-colored pinpoint white dots in normal scalp (13.1% vs. 0%); short vellus hairs in AA (62.3% vs. 27.1%~53.0%); black dots in AA, TM, and TC (77.8%, 100.0%, 77.8% vs. 53.3%~63.3%, 27.0%, 25.0%); and fibrotic white dots in PCA (70.6% vs. 52.4%) were more frequently observed in Koreans in comparison with Caucasians9. This is also thought to be the result of the light contrast effects between the hypopigmented pattern and darker background color of the skin. Contrary to our expectations, the frequencies of the remaining features, such as simple red loop, perifollicular scales, trichoptilosis, comma hairs, corkscrew hairs, and tufted hairs, also appeared differently from previous studies on Caucasians. This could be influenced by several factors, including the type of light and magnification of the dermoscope, hair-washing habit, and the subtype or severity of each alopecia group, but not by racial difference.
Table 6. Comparison of common or critical trichoscopic features of alopecia and normal scalp between Koreans and Caucasians.
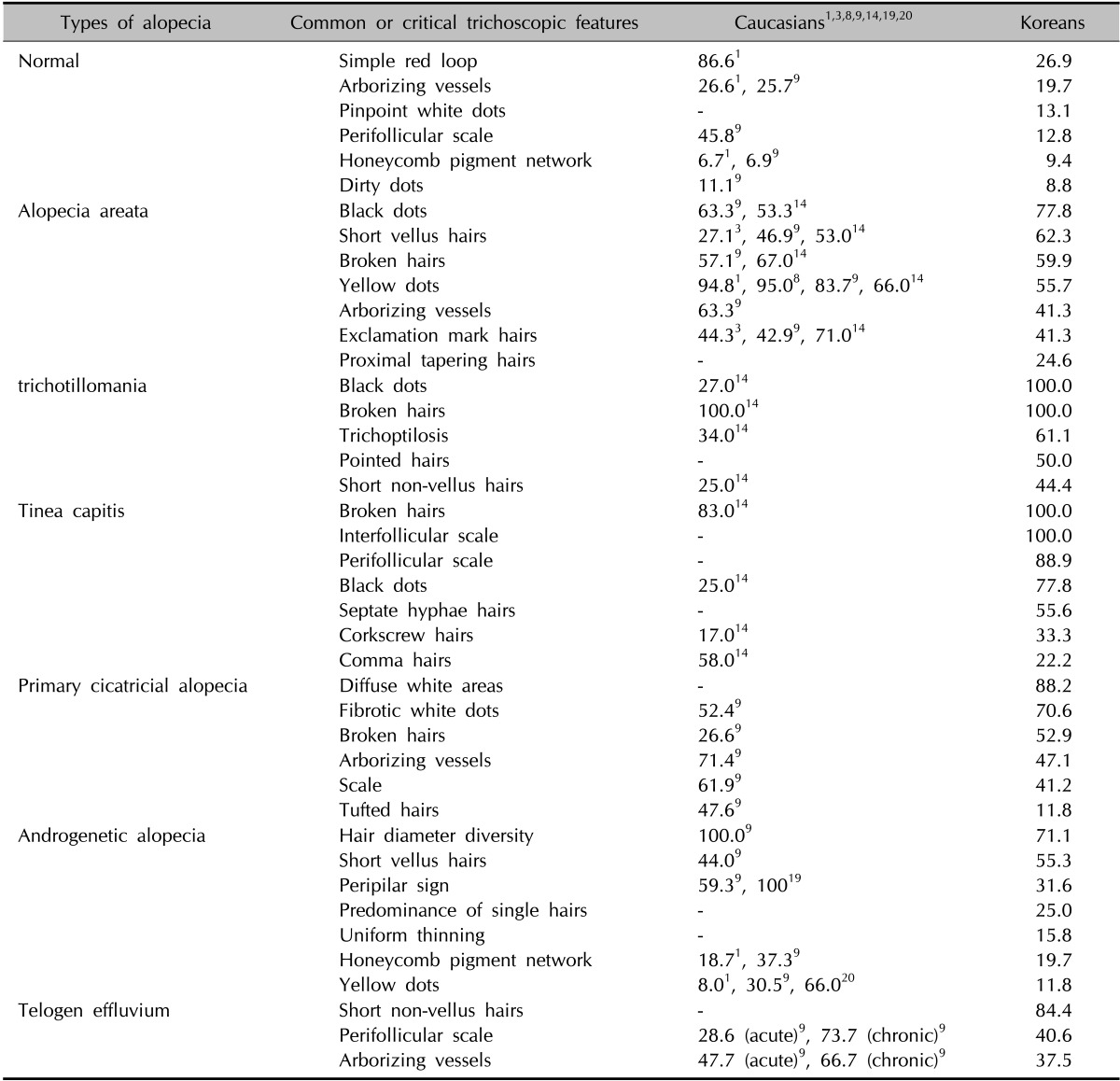
Values are presented as percentage.
Yet, our study also bears several limitations. First, the progressive acute AA to totalis/universalis patients may be categorized into the diffuse type during the early stage of examination. Second, the stage and disease duration of TE patients were somewhat diverse because of causative factors that were not considered thoroughly in this study.
Third, more cases need to be analyzed to obtain higher statistical power. Therefore, additional well-designed prospective studies with more alopecia patients are necessary to draw a conclusion regarding this subject. In conclusion, we identified some distinct trichoscopic patterns of localized and diffuse alopecia patients and normal persons in Korean. Most of the critical trichoscopic features of Korean alopecia patients are similar to those of Caucasian patients; however, racial difference must be considered in some pigmentation patterns that may vary compared with Caucasians.
ACKNOWLEDGMENT
This paper was supported by the Fund of Biomedical Research Institute, Chonbuk National University Hospital.
References
- 1.Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799–806. doi: 10.1016/j.jaad.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 2.Toncić RJ, Lipozencić J, Pastar Z. Videodermoscopy in the evaluation of hair and scalp disorders. Acta Dermatovenerol Croat. 2007;15:116–118. [PubMed] [Google Scholar]
- 3.Lacarrubba F, Dall'Oglio F, Rita Nasca M, Micali G. Videodermatoscopy enhances diagnostic capability in some forms of hair loss. Am J Clin Dermatol. 2004;5:205–208. doi: 10.2165/00128071-200405030-00009. [DOI] [PubMed] [Google Scholar]
- 4.Rudnicka L, Olszewska M, Rakowska A, Slowinska M. Trichoscopy update 2011. J Dermatol Case Rep. 2011;5:82–88. doi: 10.3315/jdcr.2011.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudnicka L, Rakowska A, Olszewska M. Trichoscopy: how it may help the clinician. Dermatol Clin. 2013;31:29–41. doi: 10.1016/j.det.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol. 2012;67:1040–1048. doi: 10.1016/j.jaad.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Tosti A, Torres F. Dermoscopy in the diagnosis of hair and scalp disorders. Actas Dermosifiliogr. 2009;100(Suppl 1):114–119. doi: 10.1016/s0001-7310(09)73176-x. [DOI] [PubMed] [Google Scholar]
- 8.Tosti A, Duque-Estrada B. Dermoscopy in hair disorders. J Egypt Women Dermatol Soc. 2010;7:1–4. [Google Scholar]
- 9.Karadağ Köse Ö, Güleç AT. Clinical evaluation of alopecias using a handheld dermatoscope. J Am Acad Dermatol. 2012;67:206–214. doi: 10.1016/j.jaad.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Inui S, Nakajima T, Nakagawa K, Itami S. Clinical significance of dermoscopy in alopecia areata: analysis of 300 cases. Int J Dermatol. 2008;47:688–693. doi: 10.1111/j.1365-4632.2008.03692.x. [DOI] [PubMed] [Google Scholar]
- 11.Shim WH, Jwa SW, Song M, Kim HS, Ko HC, Kim BS, et al. Dermoscopic approach to a small round to oval hairless patch on the scalp. Ann Dermatol. 2014;26:214–220. doi: 10.5021/ad.2014.26.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu JM, Starace M, Tosti A. A new dermoscopic finding in healthy children. Arch Dermatol. 2009;145:596–597. doi: 10.1001/archdermatol.2009.58. [DOI] [PubMed] [Google Scholar]
- 13.Abraham LS, Torres FN, Azulay-Abulafia L. Dermoscopic clues to distinguish trichotillomania from patchy alopecia areata. An Bras Dermatol. 2010;85:723–726. doi: 10.1590/s0365-05962010000500022. [DOI] [PubMed] [Google Scholar]
- 14.Rakowska A, Slowinska M, Olszewska M, Rudnicka L. New trichoscopy findings in trichotillomania: flame hairs, V-sign, hook hairs, hair powder, tulip hairs. Acta Derm Venereol. 2014;94:303–306. doi: 10.2340/00015555-1674. [DOI] [PubMed] [Google Scholar]
- 15.Tangjaturonrusamee C, Piraccini BM, Vincenzi C, Starace M, Tosti A. Tinea capitis mimicking folliculitis decalvans. Mycoses. 2011;54:87–88. doi: 10.1111/j.1439-0507.2009.01761.x. [DOI] [PubMed] [Google Scholar]
- 16.Slowinska M, Rudnicka L, Schwartz RA, Kowalska-Oledzka E, Rakowska A, Sicinska J, et al. Comma hairs: a dermatoscopic marker for tinea capitis: a rapid diagnostic method. J Am Acad Dermatol. 2008;59(5 Suppl):S77–S79. doi: 10.1016/j.jaad.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Lacarrubba F, Verzì AE, Micali G. Newly described features resulting from high-magnification dermoscopy of tinea capitis. JAMA Dermatol. 2015;151:308–310. doi: 10.1001/jamadermatol.2014.3313. [DOI] [PubMed] [Google Scholar]
- 18.Kossard S, Zagarella S. Spotted cicatricial alopecia in dark skin. A dermoscopic clue to fibrous tracts. Australas J Dermatol. 1993;34:49–51. doi: 10.1111/j.1440-0960.1993.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 19.Deloche C, de Lacharrière O, Misciali C, Piraccini BM, Vincenzi C, Bastien P, et al. Histological features of peripilar signs associated with androgenetic alopecia. Arch Dermatol Res. 2004;295:422–428. doi: 10.1007/s00403-003-0447-y. [DOI] [PubMed] [Google Scholar]
- 20.Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Rudnicka L. Dermoscopy in female androgenic alopecia: method standardization and diagnostic criteria. Int J Trichology. 2009;1:123–130. doi: 10.4103/0974-7753.58555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inui S, Nakajima T, Itami S. Scalp dermoscopy of androgenetic alopecia in Asian people. J Dermatol. 2009;36:82–85. doi: 10.1111/j.1346-8138.2009.00593.x. [DOI] [PubMed] [Google Scholar]
- 22.de Lacharrière O, Deloche C, Misciali C, Piraccini BM, Vincenzi C, Bastien P, et al. Hair diameter diversity: a clinical sign reflecting the follicle miniaturization. Arch Dermatol. 2001;137:641–646. [PubMed] [Google Scholar]
- 23.Molina L, Donati A, Valente NS, Romiti R. Alopecia areata incognita. Clinics (Sao Paulo) 2011;66:513–515. doi: 10.1590/S1807-59322011000300027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tosti A, Whiting D, Iorizzo M, Pazzaglia M, Misciali C, Vincenzi C, et al. The role of scalp dermoscopy in the diagnosis of alopecia areata incognita. J Am Acad Dermatol. 2008;59:64–67. doi: 10.1016/j.jaad.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 25.Werner B, Mulinari-Brenner F. Clinical and histological challenge in the differential diagnosis of diffuse alopecia: female androgenetic alopecia, telogen effluvium and alopecia areata--part II. An Bras Dermatol. 2012;87:884–890. doi: 10.1590/S0365-05962012000600010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudnicka L, Olszewska M, Rakowska A, Czuwara J. In: Atlas of trichoscopy. 1st ed. Rudnicka L, Olszewska M, Rakowska A, editors. London: Springer-Verlag; 2012. pp. 206–235. [Google Scholar]