Dear Editor:
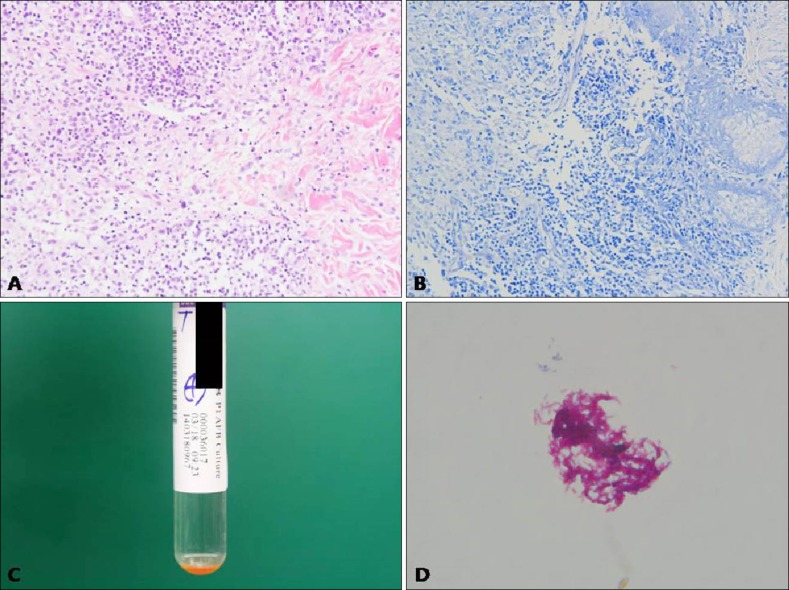
A 56 years old Korean woman presented with asymptomatic multiple rice-sized erythematous papules on left cheek (Fig. 1A). The scaly papules were noted 2 months ago. She had no history of trauma or exposure to fish. She had been learning to swim for 3 months. Culture examination of the smear for bacteria and fungus revealed no growth. The nested polymerase chain reaction (PCR) for Mycobacterium tuberculosis showed negative results. A biopsy specimen taken from the lesion showed granulomatous inflammation with granulation tissue in the deep dermis. The granulomatous infiltration was composed of multiple histiocytes, mononuclear cells, and giant cells (Fig. 2A). Acid-fast bacilli (AFB) staining produced negative findings (Fig. 2B). Hence, the diagnosis was as nonspecific inflammation of the face, and the patient was treated with triamcinolone injection, three times for 6 weeks. After the injection, the lesions improved overall; however, a solitary lesion showed pus formation. Thus, in addition, mycobacterial culture including the pus was performed, which showed growth of yellow pigment-producing Mycobacterium at 32℃ to 33℃ in mycobacterium growth indicator tube (Fig. 2C). Zeihl-Neelsen staining of culture materials showed innumerable AFB (Fig. 2D), and the or ganism was identified as Mycobacterium. Hence, PCR-reverse blot hybridization assay was performed to identify atypical mycobacterial species, which were confirmed as M. marinum or M. ulcerans.
Fig. 1. (A) Multiple, erythematous, various sized, scaly papules on the left cheek. (B) After 4 months, the lesions improved with postinflammatory hyperpigmentation and atrophic scar.
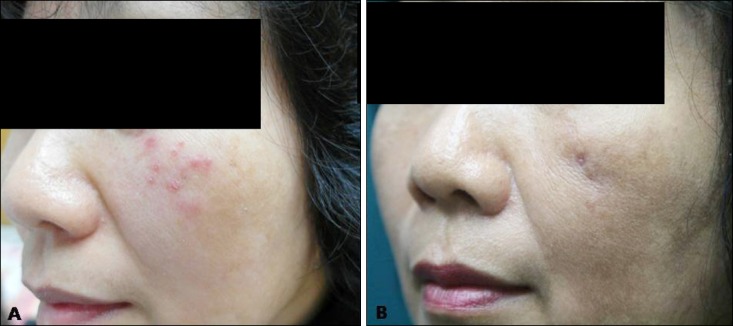
Fig. 2. (A) Granulomatous infiltration in the deep dermis was composed of multiple histiocytes, mononuclear cells, and giant cells (H&E, ×200). (B) Negative findings (acid-fast bacillus, ×100). (C) Growth of yellow pigment producing mycobacterium on 32℃ to 33℃ mycobacterium growth indicator tube after 45 days. (D) Presence of innumerable acid-fast bacilli (Ziehl-Neelsen, ×1,000).
Furthermore, PCR amplification and direct sequencing of 16SrRNA, tuf, rpoB, hsp65 genes were performed for the rapid and accurate identification of the organism. In 16SrRNA and tuf gene analysis, both M. marium and M. ulcerans showed >99% homology; thus, it was not possible to differentiate between the two species. Finally, in the rpoB and hsp65 gene analysis, M. marium showed homology of 100% and 86%, respectively, whereas M. ulcerans showed homology of 99% and 85%, respectively. On the basis of this result, the organism was identified as M. marium and the patient was treated with 200 mg minocycline and 500 mg clarithromycin for 2 months. After the treatment, the lesions improved, showing postinflammatory hyperpigmentation (Fig. 1B).
M. marium is a nontuberculous photochromogenic mycobacterium1. The optimal temperature for its growth is 30℃ to 32℃, and it rarely grows at 37℃. Thus, M. marium infection rarely occurs on the face and most commonly affects the cooler extremities2. Thus far, only seven cases of infection on the face have been reported worldwide3.
The conventional microbiological methods used for M. marium diagnosis are slow and solely rely on phenotypic characteristics. As delayed diagnosis is the main cause of adverse effects, rapid and accurate molecular diagnosis methods are necessary. Rapid detection of mycobacteria by using conventional broad-range PCR has previously been described in the literature4.
Nowadays, M. marinum infection due to the use of pools rarely occurs because of chlorination disinfection of pool water. Thus, we report a rare and interesting case of M. marium infection showing an unusual location in a patient with a history of exposure to pool water. Hence, clinicians should consider the possibility of M. marium infection even if the history and location are atypical.
ACKNOWLEDGMENT
This study was supported by the Laboratory Equipment and Research Fund of Chosun University, in 2015.
References
- 1.Runyon EH. Anonymous mycobacteria in pulmonary disease. Med Clin North Am. 1959;43:273–290. doi: 10.1016/s0025-7125(16)34193-1. [DOI] [PubMed] [Google Scholar]
- 2.Adhikesavan LG, Harrington TM. Local and disseminated infections caused by Mycobacterium marinum: an unusual cause of subcutaneous nodules. J Clin Rheumatol. 2008;14:156–160. doi: 10.1097/RHU.0b013e31817759fe. [DOI] [PubMed] [Google Scholar]
- 3.Ko DY, Song KH. Mycobacterium marinum infection occurring on the face. J Dermatol. 2013;40:773–774. doi: 10.1111/1346-8138.12213. [DOI] [PubMed] [Google Scholar]
- 4.Chia JH, Wu TL, Su LH, Kuo AJ, Lai HC. Direct identification of mycobacteria from smear-positive sputum samples using an improved multiplex polymerase chain reaction assay. Diagn Microbiol Infect Dis. 2012;72:340–349. doi: 10.1016/j.diagmicrobio.2011.12.008. [DOI] [PubMed] [Google Scholar]