Abstract
In order to treat Toll like receptor 4 (TLR4)-mediated diseases, we generated a potent antagonistic antibody directed against human TLR4, Hu 15C1. This antibody's potency can be modulated by engaging not only TLR4 but also Fcγ receptors (FcγR), a mechanism that is driven by avidity and not cell signaling. Here, using various formats of the antibody, we further dissect the relative contributions of the Fv and Fc portions of Hu 15C1, discovering that the relationship to potency of the different antibody arms is not linear. First, as could be anticipated, we observed that Hu 15C1 co-engages up to 3 receptors on the same plasma membrane, i.e., 2 TLR4 molecules (via its variable regions) and either FcγRI or FcγRIIA (via the Fc). The Kd of these interactions are in the nM range (3 nM of the Fv for TLR4 and 47 nM of the Fc for FcγRI). However, unexpectedly, neutralization experiments revealed that, due to the low level of cell surface TLR4 expression, the avidity afforded by engagement through 2 Fv arms was significantly limited. In contrast, the antibody's neutralization capacity increases by 3 logs when able to exploit Fc-FcγR interactions. Taken together, these results demonstrate an unforeseen level of contribution by FcγRs to an antibody's effectiveness when targeting a cell surface protein of relatively low abundance. These findings highlight an exploitable mechanism by which FcγR-bearing cells may be more powerfully targeted, envisioned to be broadly applicable to other reagents aimed at neutralizing cell surface targets on cells co-expressing FcγRs.
Keywords: TLR4, antibody, Fc gamma receptors, affinity maturation, avidity
Abbreviations
- DAMP
damage-associated molecular pattern
- Fc
fragment crystallizable
- FcγR
Fc gamma receptor
- Fv
fragment variable
- Ig
immunoglobulin
- IL
interleukin
- IVIg
intravenous immunoglobulin
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- PAMP
pathogen-associated molecular pattern
- TLR
Toll-like receptor
Introduction
Toll-like receptor 4 (TLR4) is a cell surface protein that homo-dimerizes upon ligand interaction,1,2 causing cells involved in inflammation to release various cytokines and chemokines such as tumor necrosis factor, interleukin (IL)-6 and CCL5.3 TLR4 detects lipopolysaccharide (LPS) from Gram-negative bacteria, playing a fundamental role in pathogen recognition. However, TLR4 is also activated by a range of other pathogen-derived molecules also called pathogen-associated molecular patterns (PAMPs), as well as endogenously-sourced damage-associated molecular pattern molecules (DAMPs), which are proteins produced as a result of cell damage and inflammatory processes.4 Uncontrolled activation of TLR4 induces a systemic release of proinflammatory cytokines that, in an acute setting, results in sepsis5,6 and more chronically appears to influence long-term diseases such as type 1 or 2 diabetes and rheumatoid arthritis.7,8 Thus, interfering with TLR4 activation represents a plausible intervention for a plethora of diseases in which PAMPs and DAMPs underlie disease pathogenesis.
Monoclonal antibodies (mAbs) constitute a huge proportion of medicines that effectively treat patients with inflammatory based diseases. When creating a therapeutic antibody that inhibits TLR4 signaling, we identified an antibody that exploits a novel FcγR-binding mechanism.9 The humanized antibody, Hu 15C1, was engineered to engage both FcγRI and FcγRII, but not FcγRIII.10 The FcγR engagement affords increased inhibitory potency and a prolonged duration of effect on inflammatory cells when blocking TLR4-mediated cell activation. Simultaneous binding of Hu 15C1 to TLR4 and FcγRs is dependent on receptor clustering within lipid rafts, which FcγRI does constitutively and TLR4 and FcγRII do in inflammatory conditions.10 The increased efficacy of the antibody to inhibit TLR4 responses occurs independently of FcγR intracellular signaling.10
To further understand the Fc-involved mechanism of action, we investigated the relative contributions of both the Fv and Fc regions toward the potency of Hu 15C1. Due to its structure, an IgG can potentially bind 2 targets via its variable regions and one FcγR through its Fc portion. To better understand how Hu 15C1 interacts with different receptors at the surface of cells, multiple antibody formats, including Fab, F(ab)’2, monovalent and whole IgG, were generated. In addition, to further dissect the mechanism of action of the mAb, the affinity of the variable region for TLR4 and the affinity of its Fc portion for FcγRs were modulated by affinity maturation and by the use of FcγR blocking reagents. We demonstrate that Hu 15C1 simultaneously interacts with both TLR4 and FcγR at the cell surface and that the avidity mediated by this co-engagement dramatically increases the inhibitory potency of this mAb. In addition, we show that an Fv affinity increase for TLR4 can partially compensate this avidity effect when FcγRI is not available. However, we also found that upon FcγRI engagement, Hu 15C1 reaches a saturable level of blocking potency that cannot be further improved by Fv affinity increase.
Results
A hierarchical influence of Fc-FcγR interactions revealed when blocking TLR4 signaling with an IgG antibody
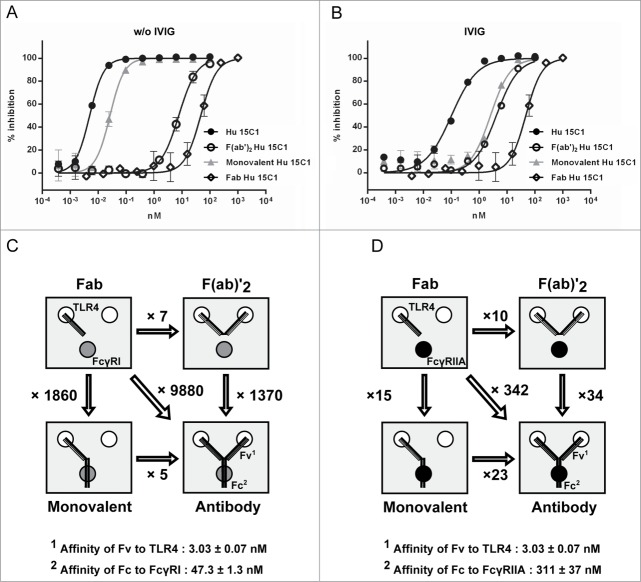
Using Hu 15C1, experiments were designed to evaluate whether a hierarchy of receptor interaction exists for delivering potency when engaging the various receptor combinations, i.e., TLR4, FcγRI, FcγRIIA and FcγRIIB. For this, baseline values of the TLR4 binding arms, with or without Fc interaction, were established using full-length IgG or F(ab)’2 fragments of Hu 15C1, respectively (Fig. 1A). THP1 human monocytic cells were used to perform dose-response experiments, as they have a response that is similar to what is observed in human whole blood when Hu 15C1 blocks the LPS-induced activation of TLR4.9 The results revealed a 3-log gain in potency of the full IgG (IC50 = 0.01 nM) versus the F(ab)’2 fragment (IC50 = 6.5 nM). Furthermore, the inclusion of intravenous immunoglobulins (IVIg), which preferentially blocks the FcγRI, in combination with a blocking anti-FcγRII antibody (IV.3 antibody) shifted the inhibition profile (IC50 = 3 nM) to a level equivalent to that of the F(ab)'2 fragments, confirming the role of these receptors in the mechanism. Addressing hierarchy, when Hu 15C1 was incubated with IV.3, i.e., blocking FcγRIIA and FcγRIIB while allowing FcγRI co-engagement to occur, little shift in potency (IC50 = 0.015 nM) was observed compared to the whole mAb alone, revealing that FcγRI alone is sufficient to promote the highest gain of potency. In contrast, when FcγRI was blocked with IVIg, allowing co-engagement with FcγRIIA or B, the shift was significant (IC50 = 0.25 nM). However, despite decreased potency, the antibody is still better than the F(ab)’2 fragment (IC50 = 6.5 nM). This result indicated that the FcγRII also plays a significant role, but only when FcγRI is not available. This IC50 value was not influenced by the addition of another reagent, 2B6, (IC50 = 0.25 nM) that specifically blocked the interaction of FcγRIIB only, demonstrating that this receptor does not play a role in this system. Taken together, these results reveal that FcγRs have a hierarchy at potentiating the inhibitory effects of Hu 15C1.
Figure 1.
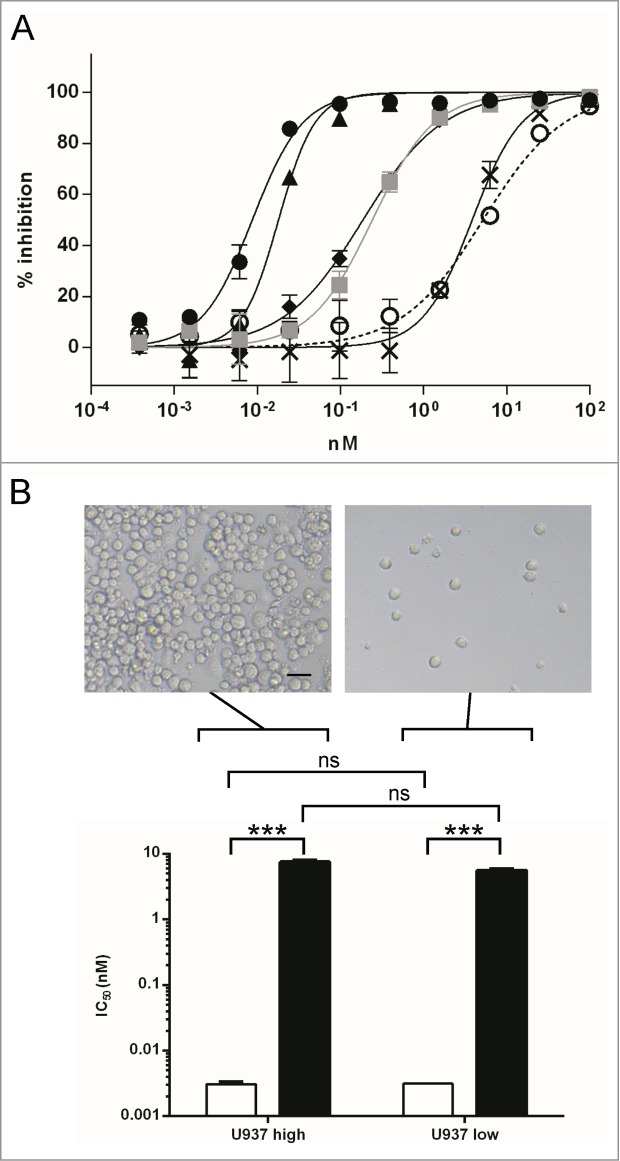
Potency of Hu 15C1 at blocking TLR4 is influenced by Fc-FcγR cis interaction (A) Dose response inhibition of LPS-induced TLR4 activation in THP1-Xblue cells. The potency of Hu 15C1 alone (black circles), after adding IVIg (gray squares), after blocking FcγRII (black triangles), after adding IVIg and blocking FcγRII (black crosses), after adding IVIg and blocking FcγRIIB (black diamonds), were compared to the potency of the F(ab)’2 Hu 15C1 (open circles). Results are expressed as mean ± SD of duplicates. An F test was used to compare the fitted curves of different groups. The p values are presented in the supplementary Table S1. (B) The IC50 of F(ab)’2 Hu 15C1 (black bars) and Hu 15C1 (white bars) at blocking LPS-induced TLR4 activation in U937 cells plated at high (left panel; U937 high) or low confluence (right panel; U937 low). Results are expressed as mean ± SD of 2 independent experiments. An F test was used to compare the different groups. ***P < 0.001, ns: not significant. Bar on photomicrograph represents 20 microns.
The co-engagement of Hu 15C1 with TLR4 and FcγRs occurs on the same cell surface
To determine if Hu 15C1 co-engages TLR4 and FcγRs at the surface of the same cell (i.e., a cis-interaction) or on 2 different cells (i.e., a trans-interaction), the potency of Hu 15C1 vs. F(ab)’2 was assessed by monitoring the antibody inhibition of IL-6 secretion induced by TLR4 activation, using differentiated U937 cells seeded at different densities so that they either remain separate or are in close contact (Figure 1B). In this assay, an adherent cell line is required to control cell confluence to assess cis- or trans-interaction. Therefore, we used the U937 cell line, a human monocytic cell line which can be easily differentiated into adherent macrophages. As observed with human THP1 monocytes (Figure 1A), the activity of whole IgG at blocking LPS-induced TLR4 activation of U937 cells is also dependent on Fc-FcγR interaction as its potency was higher than that of the F(ab)’2 fragments (Figure 1B). Despite different levels of IL-6 secretion between the two experimental conditions, we determined that the IC50 values were similar whether plated at high or low densities for F(ab)’2 (5.5 nM and 7.5 nM, respectively) versus whole IgG (0.003 nM and 0.003 nM, respectively).Therefore, the gain of potency mediated by the Fc-FcγR interaction was independent of the proximity of adjacent cells suggesting that the gain of potency induced by Fc-FcR could happen in cis. Trans-interactions could also occur when cells were seeded at the highest density; however, they do not provide further gain of potency as compared to conditions where only cis-interactions were measured (i.e. when cell were seeded at low density). These results demonstrate that the potency of Hu 15C1 can be increased by the co-engagement in cis of TLR4 and FcγRI or FcγRIIA.
Co-engagement of the Fc and 2 Fv arms of Hu 15C1 with 3 receptors at the cell surface provides maximal inhibitory capacity
To understand the relative contributions of the Fv and Fc regions to the potency of Hu 15C1, we investigated how the antibody co-engages TLR4 and FcγR at the cell surface. For this purpose, different mono- and multivalent antibody formats were generated by engineering or enzymatic cleavage of the IgG. Co-engagement capacity was tested using the following formats: full length IgG (150 kDa), composed of 2 heavy chains (50 kDa) and 2 light chains (25 kDa), for evaluating the co-engagement of 2 TLR4 molecules and one FcγR; F(ab)’2 fragments (100 kDa), consisting of 2 light chains (25 kDa) and 2 VH-CH1 regions (25 kDa), for evaluating the co-engagement of 2 TLR4 molecules; a monovalent format (100 kDa), composed of one light chain (25 kDa), one heavy chain (50 kDa) and one half Fc region (25 kDa), for evaluating the co-engagement of one TLR4 molecule and one FcγR; Fab fragments (50 kDa), composed of one light chain (25 kDa) and one VH-CH1 region (25 kDa), that bind only one TLR4 molecule. The purity and chain composition of each reagent was assessed using an Agilent 2100 bioanalyzer (Fig. 2A). The capacity of the Fv in the 4 antibody formats to bind TLR4 was shown to be similar (Fig. 2B) and their potency at blocking TLR4 activation was then tested on THP1 cells both in the presence or absence of IVIg (Fig. 3A and B).
Figure 2.
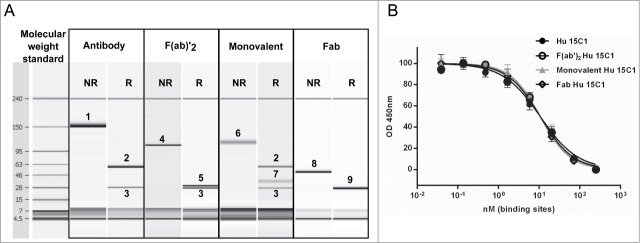
Analyses of the various antibody formats (A) To assess the purity and confirm the size of each reagent, the various antibody formats were analyzed by Agilent 2100 bioanalyzer. Proteins were assessed under non-reduced (NR) and reduced conditions (R). 1, IgG; 2, heavy chain; 3, light chain; 4, F(ab)’2; 5, VH-CH1-hinge; 6, monovalent antibody; 7, hinge-Fc; 8, Fab; 9, light chain and VH-CH1. (B) The binding of Hu 15C1 (black circles), monovalent Hu 15C1 (gray triangles), F(ab)’2 Hu 15C1 (open circles) and Fab Hu 15C1 (open diamonds) to TLR4 was analyzed by competitive ELISA. To compare the different antibody formats, the same number of binding site was used, i.e., the molar concentration of monovalent and Fab is twice the molar concentration of IgG and F(ab)’2. Results are normalized and expressed as mean ± SD of duplicates. An F test was used to compare the fitted curves of different groups. ns: not significant.
Figure 3.
Hu 15C1 co-engages 3 receptors at the cell surface to provide maximal inhibition of TLR4 responses (A) Dose response inhibition of LPS-induced TLR4 activation in THP1-Xblue cells by Hu 15C1 (black circles), monovalent Hu 15C1 (gray triangles), F(ab)’2 Hu 15C1 (open circles) and Fab Hu 15C1 (open diamonds) when FcγRs are available or (B) after adding IVIg. Results are expressed as mean ± SD of duplicates. An F test was used to compare the fitted curves of different groups. The p values are presented in Tables S2 and S3, respectively. (C) and (D) Schematic representation of the different antibody formats used in (A) and (B), respectively, and their relative capacity at binding TLR4 and FcγRs. The number indicated with each arrow linking 2 antibody formats corresponds to the ratio of the IC50 of the first molecule divided by the IC50 of the second molecule.
Either with or without IVIg, the blocking activity of Hu 15C1 F(ab)’2 (Hu 15C1 F(ab)’2 IC50 = 5.2 and 7.5 nM, respectively) was superior to that of Hu 15C1 Fab (Hu 15C1 Fab IC50 ∼ 51 nM in both conditions). In addition, the monovalent Fv format was less potent than the full-length IgG. These results suggest that Hu 15C1 must co-engage 2 TLR4 molecules and one FcγR to reach its maximal potency either in the presence or absence of IVIg (Fig. 3A and B). Moreover, these data also indicate that the blocking activities of Hu 15C1 Fab and F(ab)’2 are not influenced by the binding of a mix of irrelevant human IgG (IVIg) to FcR. The potency of the different formats correlate well with their valency, i.e., the Fab has the lowest potency, the F(ab)’2 and monovalent Fv formats have an intermediate potency and the full length IgG is the most potent. Taken together, these results suggest that the potency of the antagonistic activity of Hu 15C1 depends on the extent to which an antibody molecule interacts via one, 2 or 3 of its arms and which receptor(s) at the cell surface, the most potent being 2 TLR4 molecules via its variable regions and one FcγR through its Fc region. In addition, as observed in Fig. 1, the potencies of the full-length IgG and monovalent Fv antibody formats bearing an Fc portion, are higher when they bind to the higher affinity FcγRI (Fig. 3A and C) compared to their potency when they engage the lower affinity FcγRIIA (Fig. 3B and D). Finally, these findings also indicate that separate engagement of TLR4 and FcγR on the cell surface (i.e., using Fab or F(ab)'2 in combination with IVIg) do not provide any gain of TLR4 blocking activity compared to when the Hu 15C1 monovalent or full-length IgG co-engages TLR4 and FcγR.
The Role of TLR4 and FcγR Density
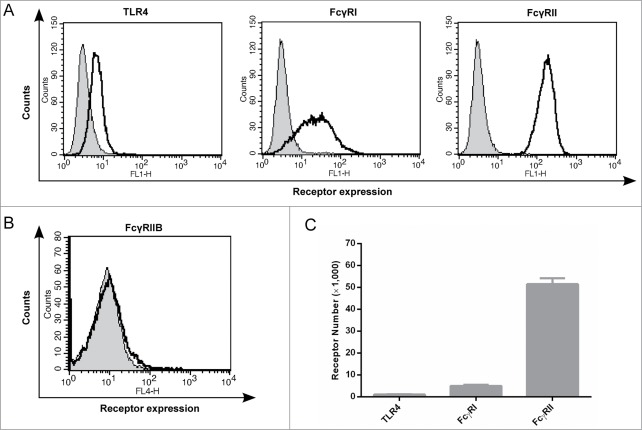
Interestingly, the Fv and Fc contribution to the antibody's potency could not be predicted by the affinity of their respective interactions. As shown in Fig. 3C, we observed that when the Fc region of monovalent or full length Hu 15C1 binds to FcγRI, this interaction induces a gain in potency that is far superior (more than 1000 fold-increase) than the gain of activity mediated by co-engagement of 2 TLR4 molecules by 2 Fv arms (an approximate 6 fold-increase). This observation was unexpected as the affinity of the Fv for TLR4 and Fc for FcγRI are in the nanomolar range (Kd: 3 nM and 47 nM, respectively). Similar results were obtained following addition of IVIg after which the potency of the antibody is mainly influenced by the low affinity Fc-FcγRIIA interaction (Fig. 3D). Although the affinity of the variable region to TLR4 is more than 100-fold higher than the affinity of the Fc to FcγRIIA (Kd: 3 nM and 311 nM, respectively), the gain in potency mediated by the Fc-FcγRIIA interaction is similar to the gain of potency obtained by TLR4 co-engagement by 2 Fv portions. These results demonstrate that the increase in potency does not correlate with the affinities of the Fv and Fc for TLR4 and FcγRs, respectively. This observation prompted us to determine the number of TLR4 and FcγRs molecules on the cell surface because this parameter could influence antibody potency. The expression of TLR4 and FcγR at the surface of THP1 cells was therefore monitored by flow cytometry. Using anti-FcγRI and anti-FcγRII antibodies (10.1 and IV.3 respectively), FACS profiles indicate that the expression of TLR4 is low compared to FcγRI and FcγRII (Fig. 4A). We also controlled FcγRIIb expression (Fig. 4B) and did not detect any signal confirming that this receptor is not expressed by monocytic cell lines as previously described in the literature.11 We further quantified receptor density, using indirect immunofluorescence assay monitored by flow cytometry, in THP1 cell line at early and late stage cell culture passages (5 and 15 cell culture passages, respectively) to control for receptor expression variations. We determined that approximately 1,000 TLR4, 5,000 FcγRI and 51,000 FcγRII molecules are present on THP1 cells and that the relative expression of these different receptors is stable during cell culture passages (Fig. 4C). We also monitored receptor density on U937 cell line and determined similar ratio of TLR4/FcγRI and TLR4/FcγRII compared to THP1 (Fig. S1). Therefore, these data indicated that FcR molecules are far more abundant than TLR4 molecules. These results suggest that the potency of Hu 15C1 is not only dependent on the strength of the Fc-FcγR interaction but also on the number of receptors that are simultaneously engaged.
Figure 4.
Levels of TLR4 and FcγRs expressed at the cell surface of THP1 cells (A) The expression levels of TLR4, FcγRI, and FcγRII at the surface of THP1-Xblue cells as measured by flow cytometry; isotype control (grey); for TLR4, FcγRI and FcγRII; Hu 15C1, 10.1 and IV.3 antibodies were used, respectively (black). (B) The expression level of FcγRIIb at the surface of THP1-Xblue cells as measured by flow cytometry; isotype control (grey); Hu 15C1 (black). (C) Receptor number on THP1-Xblue cells as determined using calibration beads and flow cytometry. The receptors densities were measured in duplicate with cells at passage 5 and 15. The variations between measurements are indicated by the error bars.
Affinity Maturation of the Hu 15C1 Fv
In order to evaluate whether the influence of the Fc-FcγR interaction could be compensated by a stronger interaction of the antibody with TLR4, we generated variant of Hu 15C1 by affinity maturation. The variable regions of Hu 15C1 were diversified and variants with improved affinity were selected by phage display against TLR4. This process led to the identification of the mAb C2E3, which was first characterized in a competition enzyme-linked immunosorbent assay (ELISA) against the parental antibody, Hu 15C1. This assay was used to confirm that C2E3 binds to the same epitope on TLR4 as Hu 15C1 and to determine an increase in binding affinity (Fig. 5A). C2E3 has a 39-fold increase in TLR4 binding compared to Hu 15C1. The C2E3-TLR4 interaction was further characterized by surface plasmon resonance (SPR) analysis (Fig. 5B and C; Table 1). The affinity of C2E3 for human TLR4 was determined to be 80 pM (Kd = 0.082 ± 0.003 nM), which is 37-fold higher than the affinity of Hu 15C1 (Kd = 3.03 ± 0.07 nM), confirming the results obtained by competition ELISA. This increase in affinity is mainly due to a decrease in the Koff dissociation constant of C2E3 compared to Hu 15C1 (Table 1).
Figure 5.
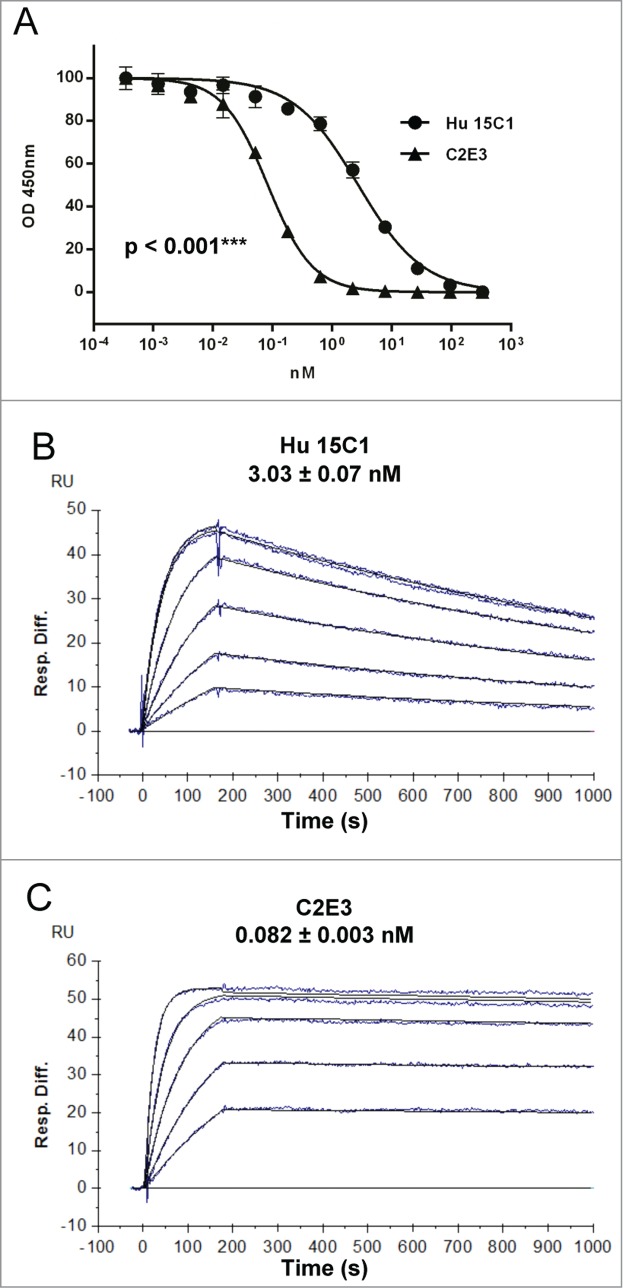
Binding analysis of Hu 15C1 and C2E3 to TLR4 (A) The binding of Hu 15C1 (circles) and C2E3 (triangles) to TLR4 was compared by competitive ELISA. Results are normalized and expressed as mean ± SD of duplicates. An F test was used to compare the different groups. ***p < 0.001. SPR analysis of Hu 15C1 (B) and C2E3 (C) binding to TLR4. Sensorgrams with affinity constants (KD = mean ± SD) shown.
Table 1.
Affinity of Hu 15C1 and C2E3 for TLR4
| Antibody | kon (105M−1s−1) ± SD | koff (10−4s−1) ±SD | KD (10−9M) ± SD | KD (fold gain)1 | Relative affinity2 |
|---|---|---|---|---|---|
| Hu 15C1 | 2.26 ± 0.07 | 6.85 ± 0.06 | 3.03 ± 0.07 | 1 | 1 |
| C2E3 | 4.76 ± 0.06 | 0.39 ± 0.01 | 0.082 ± 0.003 | 37 | 39 |
1Affinity improvement are reported as fold gain over Hu 15C1.
2See Material and Methods.
We then tested whether the increase in affinity for TLR4 could compensate for the gain of potency mediated by the Fc-FcγR interaction. For that purpose, we compared the activity of Hu 15C1 and C2E3 at blocking the TLR4 activation of THP1 cells using 2 formats, full length IgG and F(ab)’2 fragments. The activity and purity of the C2E3 F(ab)’2 fragment were assessed as presented in Fig. 2 (data not shown). We first compared the blocking activity of C2E3 F(ab)’2 and Hu 15C1 F(ab)’2 (Fig. 6A). The C2E3 F(ab)’2 (IC50 = 0.2 nM) has a 35-fold increase in potency compared to the Hu 15C1 F(ab)’2 (IC50 = 7 nM), which correlates with the increase in affinity and further validates the measurements obtained by competition ELISA and SPR.
Figure 6.
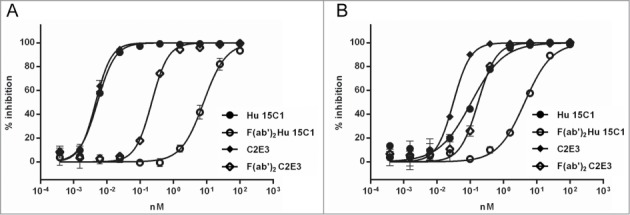
Fc-FcγR interaction creates an avidity effect (A) Dose response inhibition of LPS-induced TLR4 activation in THP1-Xblue cells by Hu 15C1 (circles), C2E3 (diamonds), F(ab)’2 Hu 15C1 (open circles) and F(ab)’2 C2E3 (open diamond) when all FcγR are available (B) or after adding IVIg. Results are expressed as mean ± SD of duplicates. An F test was used to compare the fitted curves of different groups. The p values are presented in Tables S4 and S5, respectively.
Next, the mAbs were tested in an IgG format on THP1 cells where FcγRI was available (i.e., without IVIg). As expected, the Fc-FcγRI interaction enhanced the potency of both C2E3 and Hu 15C1 (Fig. 6A). However, despite C2E3 being approximately 40-fold better at binding TLR4, the blocking activity of C2E3 and Hu 15C1 were equivalent when co-engagement of FcγRI occurred (IC50 = 0.005 nM). These results suggest that with co-engagement of FcγRI, anti-TLR4 antibodies reach a saturable level of blocking activity and that a further increase in TLR4 affinity beyond that of Hu 15C1 will not lead to a further increase in potency.
These experiments were repeated with IVIg to saturate FcγRI in order to eliminate the contribution of the Fc-FcγRI interaction in the potency of the antibodies. As determined previously, the blocking activity of C2E3 F(ab)’2 was found to be better than that of Hu 15C1 F(ab)’2 (Fig. 6B). In addition, we determined that, in the presence of IVIg, full-length C2E3 (IC50 = 0.03 nM) remains better than Hu 15C1 at blocking TLR4 (Fig. 6B). In contrast, we observed that the potency of full length Hu 15C1 (IC50 = 0.2 nM) is similar to C2E3 F(ab)’2 blocking activity when Fc-FcγRIIA mainly influences antibody activity. Thus, the gain of activity mediated by the Fc-FcγRIIA interaction can be compensated by increasing the affinity of the antibody to TLR4. Taken together, these results confirm that the co-engagement of TLR4 and FcγRs creates an avidity effect that is dependent on the nature of recruited FcγRs (i.e., FcγRI or FcγRIIA) and also confirm the hierarchy of FcγRs in the gain of potency.
Discussion
In this study, we demonstrate that the co-engagement of a mAb through the Fc arm and an Fv arm can afford a dramatic increase in potency on monocytic cell lines (i.e., 1,000 fold increase in the case of Fc-FcγRI interaction). This gain of activity mediated by the Fc portion is due, when available, to binding to the higher affinity FcγRI. However, after saturation of the high affinity receptor using IVIg, we observed that the lower affinity Fc-FcγRIIA interaction can also contribute to the potency of the antibody. These results suggest that the potency of Hu 15C1 depends on the affinity of the Fc-FcγR interaction. Moreover, we demonstrated that in case the gain of activity is not mediated by Fc-FcγRI interaction, it can be compensated by increasing the affinity of the Fv part of the antibody to TLR4.
An important aspect of our study is that Hu 15C1 can co-engage TLR4 and FcγR at the surface of the same cell using both its Fv and Fc portions (illustrated in Figure 7). In addition, we show that the potency of the anti-TLR4 mAb depends on the extent to which this molecule interacts via 1, 2 or 3 of its arms to increase the avidity of the interaction, which is a basic function of antibody biology. However, a key finding of our study is that, despite important differences in affinity constants between Fv-TLR4, Fc-FcγRI and Fc-FcγRIIa interactions (Kd = 3 nM, 47 nM and 300 nM, respectively),10 the contribution of a second Fv arm to the antibody potency was very limited in comparison to the gain of antibody potency due to Fc-FcR co-engagement. Our data suggest that the relatively low numbers of TLR4 molecules expressed at the cell surface (approximately 1,000 molecules/cell) limits the avidity effect mediated by the Fv arms and thus that co-engagement of two TLR4 molecules by the same antibody is probably a rare event. In the case of Hu 15C1 targeting TLR4, the Fc-arm of the antibody can more frequently interact with the more abundant FcγRI (5,000 molecules/cell) and FcγRII (51,000 molecules/cell) available at the cell surface. Importantly, exploiting FcγRI binding delivers a saturable effect of TLR4 inhibition for which increasing affinity of the Fv portion to the target no longer provided improvement in blocking receptor signaling. This could be explained by the affinity of the Fc portion for FcγRI and the molecular ratio of FcγRI versus TLR4, meaning that the antibody could saturate all available TLR4 proteins at the surface of the cell. Nevertheless, due to its high affinity for human IgG1, IgG3 and IgG4, it is commonly assumed that FcγRI are occupied in vivo, suggesting that the effect observed in this study may be mainly driven by interaction with FcγRIIa under physiological conditions.
Figure 7.
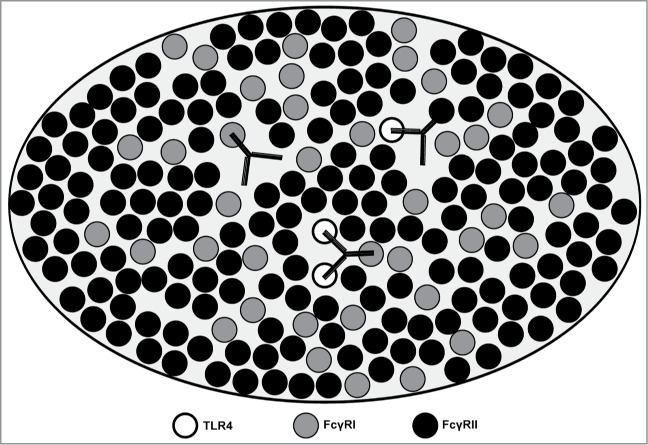
Model to illustrate the potential engagement scenario of Hu 15C1 with TLR4 and FcγRs Schematic representation of TLR4 (open circle) and FcγRs molecules (filled circle) in the membrane environment (light gray). The interactions of Fv vs. Fc portions of Hu 15C1 with the membrane proteins are shown. The presence of ligand induces aggregation of molecules into lipid rafts where the potency of Hu 15C1 depends on the co-engagement of 3 receptors at the cell surface, 2 TLR4 and one FcγR.10 The interaction of the Fc part of Hu 15C1 with the FcγR has a predominant role in the activity of the antibody by increasing the global affinity of the antibody to TLR4.
The comparison between Fab and F(ab)’2 formats indicates that Hu 15C1 is capable of co-engaging 2 TLR4 molecules at the cell surface thus suggesting that 2 TLR4 molecules can be brought into close structural proximity. Alternatively, preformed TLR4 dimers may also exist at the cell surface in an inactive conformation, such as described for other cell membrane receptors including EPOR and GHR,12,13 which may facilitate antibody co-engagement. In any case, as Hu 15C1 is devoid of any agonistic activity, its Fv domains block TLR4 active dimer conformation necessary to induce receptor signaling.2 Ongoing crystallographic studies of the complex of Hu 15C1 with TLR4 will add additional insight into antibody-antigen interaction allowing a full understanding of the inhibition of TLR4 by the Fv portion of Hu 15C1.
In contrast to several studies which report that IgG-FcR interactions occurring between adjacent cells modulate the activity of an antibody,14,15 we highlight an unsuspected role of FcγRs in the increase of the potency of a mAb by enhancing antibody/target interaction on the same cell; however, it can be envisioned that both cis- and trans-interactions could contribute to the increase of the potency of Hu 15C1. Recently, the anti-CD115 antibody, H27K15, was described to have a similar dependence on co-engagement of FcγRs.16 Thus, as our data demonstrate, the relationship between Fv and Fc arms, antibody binding and potency is not necessarily linear. We envision that this imbalance will be exploitable for other cell surface targets for which FcγR co-engagement is plausible.
Materials and Methods
Reagents
The Mu 15C1 mAb (mouse IgG1, κ) has been described previously.9 The anti-human TLR4 mAb Hu 15C1 is a humanized 4-28/A26 version of Mu 15C1 on a human IgG1, κ backbone containing the N325S and L328F mutations in the CH2 domain. These mutations abolish binding of the Fc portion of the mAb to both FcγRIII and C1q.10 Anti-human FcγRI 10.1 (mouse IgG1, κ) was from BioLegend; blocking anti-human FcγRII IV.3 (mouse IgG2b, κ) was from StemCell. Human IgG1, κ, mouse IgG1, κ and mouse IgG2b, κ isotype control were from Novimmune. The C2E3 sequence was selected by phage display. Briefly, libraries were generated introducing diversity into the complementary determining regions (CDR) of Hu 15C1 and selected against human TLR4 expressing cells. A panel of variants was selected and characterized. The detailed methodology allowing their selection will be described elsewhere. THP1-XBlue™-MD2-CD14 cells were purchased from Invivogen. The U937 and Chinese hamster ovary (CHO) cell lines were obtained from the American Type Culture Collection. IVIg, a pool of human intravenous IgG which can be used to preferentially block human FcγRI, are from CSL Behring (Privigen). LPS from Salmonella Minnesota R595 (Re) was purchased from List Biological Laboratories, Inc.
Cloning and expression of the anti-human FcγRIIB antibody 2B6
2B6 is an anti-human FcγRIIB antibody competing for immunoglobulin binding to FcγRIIB, suggesting that it directly recognizes the Fc-binding region of the receptor.22 2B6 variable heavy chain (VH) and variable light chain (VL) nucleotide sequences were synthesized by DNA2.0 (Menlo Park, CA, USA) according to the sequences described in the patent entitled ″Humanized FcγRIIB specific antibodies and methods of uses thereof″ (International Publication number WO 2008/105886 A2). 2B6 VH and VL sequences were sub-cloned into vectors containing the human IgG1 backbone, and human constant Igκ for expression in mammalian cells. 2B6 was expressed in CHO cells and purified using the MabSelect Sure resin (GE Healthcare).
Cloning, expression and modification of antibodies
The sequences coding the VH and VL of mAbs were cloned into vectors containing the human IgG1 backbone, and human constant Igκ for expression in mammalian cells. To have the same backbone as Hu 15C1, the mutations N325S and L328F were introduced into the CH2 domain using the QuickChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies). Antibodies were expressed in CHO cells by co-transfecting vectors coding the heavy chain (with the mutations N325S, L328F) and the light chain, and purified using the MabSelect Sure resin (GE Healthcare). The sequence coding the human IgG1 Fc region described in the patent entitled Treatment method (United States patent application US 2011/0262436 A1, SEQ ID NO:13) was cloned into a vector for expression in mammalian cells. A leader sequence of immunoglobulin has been introduced in the N-terminal part of the hinge region. The mutations N325S and L328F were introduced into the CH2 domain as described above. In parallel, the mutation H435R was introduced into the vector coding the heavy chain of the antibody (cloning described above) to abrogate the binding to the protein A (mutation described in the patent entitled: Readily isolated bispecific antibodies with native immunoglobulin format, publication number EP2445936 A1). Monovalent antibodies were expressed in CHO cells by co-transfection of vectors coding the heavy chain (with the mutations N325S, L328F and H435R), the light chain and the Fc region (with the mutations N325S and L328F), and then purified through 2 affinity purification steps (Figure S1). The MabSelect Sure resin (GE Healthcare) was used for the first step and the IgG-CH1 resin (BAC B.V.) was used for the second one. The sequence coding the VH and CH1 region was cloned into a vector for expression in mammalian cells. Fabs were expressed in CHO cells by co-transfecting the last vector and the one coding for the light chain and purified using the IgG-CH1 resin. F(ab)’2 were obtained cleaving mAb using the FragIT™ Microspin Kit (Genovis). The quality of the proteins was evaluated using the Agilent protein 230 Kit, and then analyzed using the Agilent 2100 Bioanalyser.
THP1 Assay
THP1-XBlue cells were grown in RPMI 1640 (Sigma) with 10% heat-inactivated fetal bovine serum (FBS, Sigma), 200 μg/ml Zeocin™ (Invitrogen) and 250 μg/ml of G418 (Life technologies). THP1-XBlue cells were plated in 96-well plate at 1.105 cells/well in 30 μl of medium. IVIg, the IV.3 antibody and the 2B6 antibody were diluted in medium at a final concentration of 1 mg/ml, 10 μg/ml and 10 μg/ml, respectively, and 30 μl of the dilution were added to the cells for 30 min at 37°C. Antibodies or antibody fragments were serial diluted in medium and 30 μl were added to the cells for 30 min at 37°C. LPS was diluted in medium to have a final concentration of 10 ng/ml, and 30 μl/well were added for 24 h at 37°C. Supernatant were collected and 20 μl were added to 180 μl of QUANTI-Blue™ (Invivogen) and incubated for 3 h at 37°C. Finally the absorbance at 650 nm was measured using a spectrophotometer.
U937 assay
U937 cells were grown in RPMI 1640 (Sigma) with 10% heat-inactivated FBS and 2 mM glutamine (Sigma). U937 cells were differentiated into macrophages with 25 nM of phorbol myristate acetate (Sigma) during 72 h in order to increase their responsiveness to LPS and establish adherent cell conditions. To determine cell density influence on antibody potency, differentiated cell were plated in a flat bottom 96-well plate at 1.105 cells/well or 2.104 cells/well in 30 μl of medium. Hu 15C1 and Hu 15C1 F(ab)’2 were serial diluted in medium and 30 μl were added to the cells for 30 min at 37°C. Human IgG1 isotype control was used at 100 nM final. Then, LPS was diluted in medium to a final concentration of 1 ng/ml, and 30 μl/well were added for 24 h at 37°C. IL-6 secretion in the culture supernatant was monitored by ELISA (Human IL-6 ELISA, BD biosciences). The percentage (%) of inhibition of IL-6 was calculated using the following formula: % IL-6 inhibition = 100-(IL-6 of sample×100/IL-6 of isotype control).
Competitive enzyme-linked immunosorbent assay
F96 Maxisorp plates (Nunc) were coated with 8 μg/ml of Hu 15C1 and incubated at 4°C overnight. Plates were washed with phosphate-buffered saline (PBS)-Tween 0.05% and blocked with PBS-bovine serum albumin (BSA) 3% 1 h at 37°C. Samples were serial diluted in PBS-BSA 1%, and soluble His-tagged human TLR4 (R&D Systems) was diluted at 0.025 μg/ml. Hu 15C1 was used as the reference sample. Blocked plates were washed, 50 μl of diluted sample and 50 μl of TLR4 were successively added to the plates for 1.5 h at 37°C. After washing, plates were incubated with 100 μl of Penta-His HRP conjugate (1:2000, Qiagen) for 1 h at 37°C. Plates were washed and incubated with 50 μl of TMB (Sigma) for 30 min at room temperature. Immediately after stopping the reaction by the addition of 50 μl of H2SO4 (1 M), the absorbance at 450 nm was measured using a precision microplate reader (Molecular Devices). The relative binding affinity of the antibodies to TLR4 was calculated applying the following formula: Relative binding affinity = IC50 reference/IC50 sample.
Receptor quantification
The 15C1, 10.1 and IV.3 antibodies were used for the labeling of TLR4, FcγRI and FcγRII, respectively. Briefly, THP1-XBlue cells were incubated in 1X BD CellFIX™ (BD biosciences) for 15 min at 4°C. For this assay, antibodies were deglycosylated using the deGlycIT™ Microspin columns (Genovis) to avoid unwanted binding to FcγRs through their Fc region.18 Antibodies were diluted in PBS-BSA 2% at 100 μg/ml. Cells were washed with PBS-BSA 2%, and incubated with the diluted antibody for 15 min at 4°C. After washing, cells, setup and calibration beads (QIFIKIT™, Dako) were incubated with 10 μg/ml of Biotin-SP-AffiniPure F(ab’)2 Fragment Goat Anti-Mouse IgG (Jackson ImmunoResearch) for 15 min at 4°C. Then, cells and beads were washed and stained with Streptavidin-FITC (1:100 dilution, PharMingen) for 15 min at 4°C. After washing, cells and beads were analyzed using a FACSCalibur flow cytometer (BD biosciences) in the FL-1 channel. The bead fluorescence intensity was then used as a calibration curve to determine the number of receptors on the cells.
Surface plasmon resonance
SPR experiments were carried out at 25°C using a BIAcore 2000 (GE Healthcare). A CM5 chip was coated with a Penta-His antibody (Qiagen). The CM5 surface was activated for 7 min at 10 μL/min with a 1:1 mix containing EDC and NHS solutions. The Penta-His antibody was diluted at 1 μg/mL in 10 mM acetate pH 4.5 buffer (GE Healthcare) and the immobilization step was performed at the same flow rate for 7 min. Unused activated chip surface was blocked injecting 1 M ethanolamine for 7 min. This process resulted in the immobilization of 1700 RU of Penta-His antibody. A reference surface was activated and blocked without antibody coating for further referencing. The BIAcore was primed with HBS-EP running buffer (GE Healthcare). Kinetics were performed at a flow rate of 20 μL/min. Human TLR4-MD2 (R&D Systems) was captured for 3 min at 5 μg/mL. After a 5 min stabilization time, Fabs were injected for 3 min at concentrations ranging from 100 nM to 6.25 nM. The highest concentration was injected twice and the dissociation phase was monitored for 15 min. A solution of 10 mM glycine pH 2.0 was injected for 1 min for regeneration of the Penta-His surface. To avoid nonspecific binding artifacts and running buffer effects, data were processed by subtracting signals obtained from the control surface and from a blank injection using the BIAevaluation v.4.1 software. Kinetic constants were determined according to the Langmuir 1:1 binding model.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Emmanuel Monnet, Dr. Giovanni D’Ario and Dr. Gérard Didelot for their help in the preparation of the manuscript.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Botos I, Segal DM, Davies DR. The structural biology of Toll-like receptors. Structure 2011; 19:447-59; PMID:21481769; http://dx.doi.org/ 10.1016/j.str.2011.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009; 458:1191-5; PMID:19252480; http://dx.doi.org/ 10.1038/nature07830 [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010; 11:373-84; PMID:20404851; http://dx.doi.org/ 10.1038/ni.1863 [DOI] [PubMed] [Google Scholar]
- 4.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol 2010; 87:989-99; PMID:20179153; http://dx.doi.org/ 10.1189/jlb.1209775 [DOI] [PubMed] [Google Scholar]
- 5.Daubeuf B, Mathison J, Spiller S, Hugues S, Herren S, Ferlin W, Kosco-Vilbois M, Wagner H, Kirschning CJ, Ulevitch R, et al. . TLR4MD-2 monoclonal antibody therapy affords protection in experimental models of septic shock. J Immunol 2007; 179:6107-14; PMID:17947685; http://dx.doi.org/ 10.4049/jimmunol.179.9.6107 [DOI] [PubMed] [Google Scholar]
- 6.Wiersinga WJ. Current insights in sepsis: from pathogenesis to new treatment targets. Curr Opin Crit Care 2011; 17:480-6; PMID:21900767; http://dx.doi.org/ 10.1097/MCC.0b013e32834a4aeb [DOI] [PubMed] [Google Scholar]
- 7.Kim JK. Fat uses a TOLL-road to connect inflammation and diabetes. Cell Metab 2006; 4:417-9; PMID:17141623; http://dx.doi.org/ 10.1016/j.cmet.2006.11.008 [DOI] [PubMed] [Google Scholar]
- 8.McCormack WJ, Parker AE, O’Neill LA. Toll-like receptors and NOD-like receptors in rheumatic diseases. Arthritis Res Ther 2009; 11:243; PMID:19835640; http://dx.doi.org/ 10.1186/ar2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn-Siegrist I, Leger O, Daubeuf B, Poitevin Y, Depis F, Herren S, Kosco-Vilbois M, Dean Y, Pugin J, Elson G. Pivotal involvement of Fcgamma receptor IIA in the neutralization of lipopolysaccharide signaling via a potent novel anti-TLR4 monoclonal antibody 15C1. J Biol Chem 2007; 282:34817-27; PMID:17921137; http://dx.doi.org/ 10.1074/jbc.M706440200 [DOI] [PubMed] [Google Scholar]
- 10.Shang L, Daubeuf B, Triantafilou M, Olden R, Depis F, Raby AC, Herren S, Dos SA, Malinge P, Dunn-Siegrist I, et al. . Selective antibody intervention of Toll like Receptor 4 activation through Fc gamma receptor tethering. J Biol Chem 2014; 289:15309-18; PMID:24737331; http://dx.doi.org/ 10.1074/jbc.M113.537936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogarth PM, Pietersz GA. Fc receptor-targeted therapies for the treatment of inflammation, cancer and beyond. Nat Rev Drug Discov 2012; 11:311-31; PMID:22460124; http://dx.doi.org/ 10.1038/nrd2909 [DOI] [PubMed] [Google Scholar]
- 12.Gent J, van KP, Roza M, Bu G, Strous GJ. Ligand-independent growth hormone receptor dimerization occurs in the endoplasmic reticulum and is required for ubiquitin system-dependent endocytosis. Proc Natl Acad Sci U S A 2002; 99:9858-63; PMID:12105275; http://dx.doi.org/ 10.1073/pnas.152294299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livnah O, Stura EA, Middleton SA, Johnson DL, Jolliffe LK, Wilson IA. Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science 1999; 283:987-90; PMID:9974392; http://dx.doi.org/ 10.1126/science.283.5404.987 [DOI] [PubMed] [Google Scholar]
- 14.White AL, Chan HT, Roghanian A, French RR, Mockridge CI, Tutt AL, Dixon SV, Ajona D, Verbeek JS, Al-Shamkhani A, et al. . Interaction with FcgammaRIIB is critical for the agonistic activity of anti-CD40 monoclonal antibody. J Immunol 2011; 187:1754-63; PMID:21742972; http://dx.doi.org/ 10.4049/jimmunol.1101135 [DOI] [PubMed] [Google Scholar]
- 15.Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D, Li Y, Pitti R, Totpal K, Yee S, et al. . An Fcgamma receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell 2011; 19:101-13; PMID:21251615; http://dx.doi.org/ 10.1016/j.ccr.2010.11.012 [DOI] [PubMed] [Google Scholar]
- 16.Haegel H, Thioudellet C, Hallet R, Geist M, Menguy T, Le PF, Marchand JB, Toh ML, Duong V, Calcei A, et al. . A unique anti-CD115 monoclonal antibody which inhibits osteolysis and skews human monocyte differentiation from M2-polarized macrophages toward dendritic cells. MAbs 2013; 5:736-47; PMID:23924795; http://dx.doi.org/ 10.4161/mabs.25743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veri MC, Gorlatov S, Li H, Burke S, Johnson S, Stavenhagen J, Stein KE, Bonvini E, Koenig S. Monoclonal antibodies capable of discriminating the human inhibitory Fcgamma-receptor IIB (CD32B) from the activating Fcgamma-receptor IIA (CD32A): biochemical, biological and functional characterization. Immunology 2007; 121:392-404; PMID:17386079; http://dx.doi.org/ 10.1111/j.1365-2567.2007.02588.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nose M, Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci U S A 1983; 80:6632-6; PMID:6579549; http://dx.doi.org/ 10.1073/pnas.80.21.6632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.