Abstract
The effects of the allelochemical benzoxazolin-2-(3H)-one (BOA) were evaluated on growth, lignin content and its monomers p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S) in roots, stems and leaves of soybean. BOA decreased the lengths and fresh weights of roots and stems, and the fresh weights and areas of leaves. Reductions in the growth were accompanied by enhanced lignin content in all tissues. In roots, the allelochemical increased the content of H, G and S monomers as well as the overall amount of lignin (referred to as the sum of H+G+S), but did not alter the S/G ratio. In stems and leaves, BOA increased the H, G, S and H+G+S contents while decreasing the S/G ratio. In brief, BOA-induced inhibition of soybean may be due to excessive production of monomers that increase the degree of polymerization of lignin, limit cell expansion, solidify the cell wall and restrict plant growth.
Keywords: allelochemical, benzoxazolinones, glycine max, lignification, lignin monomers
Abbreviations
- Benzoxazolin-2-(3H)-one
BOA
- 2
4-dihydroxy-1, 4(2H)-benzoxazin-3-one, DIBOA
- guaiacyl
G
- p-hydroxyphenyl
H
- reactive oxygen species
ROS
- syringyl
S
Introduction
Benzoxazinoid derivatives constitute a group of chemical compounds subdivided into hydroxamic acids, lactams, benzoxazolinones, and methyl derivatives of hydroxamic acids. Benzoxazolinones are the most common allelochemicals found in different species of Poaceae (Gramineae) among monocot plants, including the crops Secale cereale, Triticum aestivum and Zea mays. The well-known allelochemical benzoxazolin-2-(3H)-one (BOA), a more stable product of hydrolysis of 2,4-dihydroxy-1,4(2H)-benzoxazin-3-one (DIBOA), has been investigated for phytotoxic effects and herbicidal activity.1
Many plant species are sensitive to BOA, including monocot and dicot weeds and crops. BOA is readily taken up by plants2 and affects many physiological processes, particularly seed germination and seedling growth. Different mechanisms have been suggested for the inhibition of plant growth by BOA.3 These authors proposed multiple modes of action for BOA toxicity, which may in general be due to oxidative damage, auxin inhibition or interference caused by both effects. The former effects of BOA seem to be due to an inhibition of antioxidant systems followed by an accumulation of reactive oxygen species (ROS) that alters the cell redox status and, concomitantly, inhibits auxin. An alteration in redox status can cause protein denaturation and lipid peroxidation, with increases in membrane permeability and ion leakage, inhibition of ATPase activity and changes in membrane structure. Interestingly, supposed activation of the phenylpropanoid pathway by BOA can increase lignification. This process reduces cell wall extensibility, which can also be due to auxin inhibition, followed by a reduction in cell turgor and altered water status. Taken together, these cascading effects have drastic consequences for plant growth.
Despite the relevance of the cell membrane as a possible site of BOA action, the cell wall is also one of the first tissues affected by stress signals, which are then transmitted to the cell interior and influence metabolic processes.4 Cell wall lignification is one of the most striking events in the development of higher plants.5,6 It is a dynamic, flexible process important for many specialized functions, such as regulation of water flow, hydrophobicity, rigidity and protection against biotic and abiotic stresses. Lignin is a heterogeneous polymeric end product of the phenylpropanoid pathway generated by combinatorial radical coupling of p-hydroxycinnamyl alcohols (called monolignols). The first rate-limiting enzyme in this pathway is phenylalanine ammonia-lyase, which in association with enzymes such as cinnamate 4-hydroxylase, 4-coumarate:CoA ligase, 4-hydroxycinnamoyl-CoA:shikimate/quinate 4-hydroxycinnamoyltransferase, p-coumarate 3-hydroxylase and cinnamoyl-CoA reductase, among others, leads to the synthesis of p-coumaral-, coniferal-, and sinapaldehydes. By the action of cinnamyl alcohol dehydrogenase, these metabolites are converted into 3 corresponding monolignols, i.e., p-coumaryl, coniferyl and sinapyl alcohols. Finally, oxidative polymerization of these 3 monolignols, by the action of cell wall-bound peroxidases, generates the p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S) monomers of lignin.7
Whereas much is known about the effects of BOA on physiological events in plants, there is little information on its effects on lignification. A survey of the literature reveals a single report related to this subject.8 After exposure of Avena sativa to DIMBOA (2,4-dihydroxy-7-methoxy-(2H)-1,4-benzoxazin-3(4H)-one), the authors noted that its inhibitory effect on the growth of coleoptile segments was associated with an increase in cell wall peroxidase activities and premature lignification of cell walls. Hitherto, however, we found no report related to the effects of BOA on soybean. For these reasons and due to the relevant role of lignification in plant growth, the question in the current research was whether BOA affects growth, lignin production and its monomeric composition in roots, stems and leaves of soybean, an important crop plant species.
Results and Discussion
Exposure to BOA affects the growth and metabolism of many plant species, and this phenomenon prompted us to test its effects on the growth and lignification of soybean. The results obtained revealed that exogenously applied BOA affected roots, stems and leaves as compared to control (Fig. 1). Soybean root length (Fig. 1A) was significantly reduced from 35% to 52% (0.1 to 0.3 mM BOA), while root weight decreased by 43% and 53%, after 0.2 and 0.3 mM BOA treatments. The length and weight of stems (Fig. 1B) were reduced by 18% and 22%, respectively, after 0.2 and 0.3 mM BOA exposure. Whereas 0.3 mM BOA reduced leaf area by 35%, the leaf weight was reduced from 21% to 27% after 0.2 and 0.3 mM BOA treatments (Fig. 1C). Reduced growth is a feature common to the effects of BOA in several crop plant species, such as Avena sativa,9 Cucumis melo, Cucurbita pepo and Zea mays,10 Cucumis sativus,11 Phaseolus aureus,12 Triticum aestivum and Allium cepa,1 Lactuca sativa3,13 and Pisum sativum, Raphanus sativus and Brassica oleracea.14 These effects were also observed in the current research, in which soybean exhibited reductions in roots, stems and leaves with increasing BOA concentrations, indicating that it is a highly BOA-sensitive crop.
Figure 1.
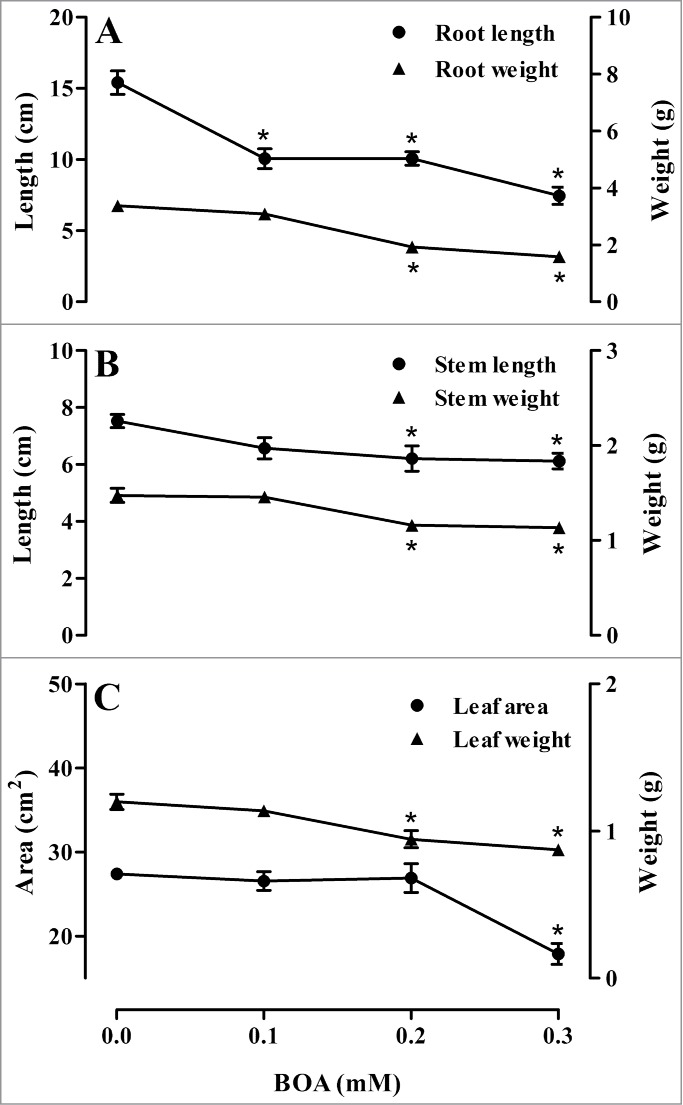
Length and fresh weight of roots, stems and leaf area of soybean plants treated or not with 0.1 to 0.3 mM BOA. Means (N = 10 for roots and stems, and N = 5 for leaves ± standard error of the mean) than the control according to Dunnett's test, significant at P ≤ 0.05 level are marked with *.
Several physiological and biochemical responses have been described to explain the inhibitory effect of BOA on plant growth. BOA typically suppresses seed germination, causing disorders of root growth and inhibiting plant growth. Generation of ROS and oxidative stress have been considered relevant events that alter multiple physiological and biochemical processes such as cellular ultrastructure,11 cell cycle,15 activities of plasma membrane H-ATPase, proteases, α- and β-amylases and antioxidant enzymes,9,12,14,16 energy metabolism,17 lipid metabolism and protein synthesis,3,11,12 and leaf water relationships and photosynthesis.13,18,19 In addition, the induction of premature senescence has been considered as a primary phytotoxic action of BOA.20
It is noteworthy that, in the current research, a decrease in soybean growth (Fig. 1) was associated with an increase in lignin content (Table 1). The data revealed that total lignin in roots, stems and leaves increased about 18%, 32% and 15%, respectively, regardless of the BOA concentration, when compared to their controls. It is also worth noting that increases in lignin content were accompanied by increases in the H, G and S monomers and amount of lignin, defined as the sum of H+G+S. In roots, BOA increased the H monomer content by 26% and 83% (at 0.1 and 0.2 mM); the G monomer by 49% and 38% (at 0.1 to 0.3 mM) and the S monomer by 29%, 40% and 44% (from 0.1 to 0.3 mM), as compared to their respective controls. The allelochemical increased the H+G+S content by 53% and 39% at 0.2 and 0.3 mM, respectively. At 0.2 and 0.3 mM, BOA increased the H monomer content of the stems by 131% and 35%, respectively. The G content increased by 101% and 137%, while the S content increased by 40% and 60%, respectively. Similarly, the allelochemical increased the H+G+S content by 77% and 104%, respectively. Finally, in leaves, and after 0.2 to 0.3 mM allelochemical treatments, the H content increased by 210% and 182% in comparison to control, whereas the G content increased by 73% and 139%. The S content increased by 33% for both concentrations. BOA increased the H+G+S contents by 96% and 110%, respectively.
Table 1.
Changes in the lignin content and monomer composition of root, stem and leaf of soybean untreated (0 mM) or treated with BOA
| BOA (mM) | ||||
|---|---|---|---|---|
| 0 | 0.1 | 0.2 | 0.3 | |
| Root | ||||
| Lignin | 126.9 ± 8.7 | 147.4 ± 3.9* | 146.7 ± 1.0* | 156.6 ± 4.2* |
| H | 0.23 ± 0.01 | 0.29 ± 0.02* | 0.65 ± 0.05* | 0.28 ± 0.03 |
| G | 3.19 ± 0.18 | 3.59 ± 0.19 | 4.74 ± 0.37* | 4.41 ± 0.26* |
| S | 1.12 ± 0.08 | 1.44 ± 0.10* | 1.57 ± 0.13* | 1.61 ± 0.13* |
| H + G + S | 4.54 ± 0.23 | 5.32 ± 0.31 | 6.96 ± 1.04* | 6.30 ± 0.40* |
| H : G : S ratio | 5 : 70 : 25 | 5 : 68 : 27 | 9 : 68 : 23 | 4 : 70 : 26 |
| S/G ratio | 0.35 ± 0.03 | 0.40 ± 0.01 | 0.33 ± 0.02 | 0.37 ± 0.01 |
| Stem | ||||
| Lignin | 134.0 ± 5.0 | 175.4 ± 1.7* | 176.5 ± 3.8* | 180.3 ± 0.4* |
| H | 0.26 ± 0.01 | 0.31 ± 0.01 | 0.60 ± 0.05* | 0.35 ± 0.03* |
| G | 7.16 ± 0.36 | 8.25 ± 0.20 | 14.4 ± 1.00* | 17.0 ± 1.09* |
| S | 4.75 ± 0.29 | 4.56 ± 0.38 | 6.64 ± 0.50* | 7.61 ± 0.32* |
| H + G + S | 12.2 ± 0.49 | 13.1 ± 0.56 | 21.6 ± 1.48* | 24.9 ± 1.42* |
| H : G : S ratio | 2 : 59 : 39 | 2 : 63 : 35 | 3 : 66 : 31 | 2 : 68 : 30 |
| S/G ratio | 0.67 ± 0.05 | 0.55 ± 0.03* | 0.46 ± 0.02* | 0.45 ± 0.01* |
| Leaf | ||||
| Lignin | 87.9 ± 2.89 | 100.1 ± 1.7* | 100.9 ± 1.9* | 102.2 ± 0.9* |
| H | 0.29 ± 0.02 | 0.25 ± 0.01 | 0.90 ± 0.02* | 0.82 ± 0.09* |
| G | 0.33 ± 0.02 | 0.39 ± 0.01 | 0.57 ± 0.02* | 0.79 ± 0.04* |
| S | 0.40 ± 0.03 | 0.39 ± 0.01 | 0.53 ± 0.03* | 0.53 ± 0.03* |
| H + G + S | 1.02 ± 0.04 | 1.03 ± 0.02 | 2.00 ± 0.06* | 2.14 ± 0.19* |
| H : G : S ratio | 29 : 32 : 39 | 24 : 38 : 38 | 45: 29 : 26 | 38 : 37 : 25 |
| S/G ratio | 1.24 ± 0.14 | 1.00 ± 0.01 | 0.93 ± 0.06* | 0.67 ± 0.01* |
H, p-hydroxyphenyl; G, guaiacyl; and S, syringyl monomers. Results are expressed as mg monomer g−1 cell wall. Values (N = 4 ± SE) significantly different from the control (P ≤ 0.05, Dunnett's multiple comparison test) are marked with asterisk (*).
In general, the results showed that the BOA concentrations used herein affected soybean growth (Fig. 1), lignin content and also its monomeric composition (Table 1). The findings in this study are of particular interest because perturbations in lignin biosynthesis often affect plant growth. As reported earlier, oxidative stress caused by BOA can alter some metabolic events related to plant growth, and lignification appears to be one of them.11 It is known that stress-induced lignification can limit cell expansion, the capacity for nutrient uptake and the ability to sustain plant growth. Indeed, previous results revealed that the reduced growth of soybean roots caused by hydroxycinnamic derivative allelochemicals was associated with an effective performance of the phenylpropanoid pathway and premature cell wall lignification.21-23 Increases in the content of H, G and S monomers and H+G+S, which is a reasonable estimate of the degree of condensation of the lignin polymer,7 suggest that structural properties of the cell wall can be adversely affected by BOA. It is clear that such effects can significantly limit cell expansion and compromise plant growth.
Another interesting observation is that alterations in lignin monomeric composition were accompanied by changes in the S/G ratio (Table 1). This parameter indicates the degree and nature of polymeric cross-linking, and has profound effects on lignin structure.24 Whereas S units contribute to a more linear lignin with a low degree of polymerization, G-rich lignin has a branched structure with a high degree of polymerization. Therefore, a lower S/G ratio implies more covalent cross-linking and rigidity in the plant cell wall. As noted, the results gave very similar S/G ratios in roots of seedlings treated with BOA in comparison to the control. However, in stems and leaves, the S/G ratios decreased with increasing BOA concentrations when compared to the respective controls. Based on our experimental findings (Table 1), there is no doubt that the decreases in S/G ratios were due to G units increasing significantly more than S units. At least in stems and leaves, the H:G:S ratios strengthen this statement. An enhanced production of highly branched G-rich lignin contributes to hardening of the cell wall, associated with reduced soybean growth (Fig. 1). Evidence supports these findings. A remarkable increase in G units due to the action of the allelochemical ferulic acid has also associated with reduced soybean growth.25
Overall, our study suggests that all the monomeric compositional shifts caused by BOA can have repercussions on the structure of lignin and, as a consequence, on cell wall properties. For these reasons it seems plausible to assume that BOA-induced inhibition of soybean may be attributed to excessive production of lignin monomers that limit cell expansion, solidify the cell wall and restrict the growth. Carrying out gene expression profiles related to enzymes in the phenylpropanoid pathway, such as ferulate 5-hydroxylase, caffeic acid O-methyltransferase and cinnamyl alcohol dehydrogenase, which are specific for the biosynthesis of monomers G and S, is one approach to strengthening this hypothesis. Ultimately, our study reveals useful insights concerning the effects of BOA on the growth and lignification of soybean, and provides a good opportunity to begin to comprehensively investigate this approach in other plant species to determine how widespread this phenomenon is.
Materials and Methods
General procedures
Soybean (Glycine max L. Merr. cv. BRS-232) seeds were surface-sterilized with 2% sodium hypochlorite for 3 min, rinsed extensively with deionized water and dark-germinated at 25°C on 2 sheets of moistened filter paper. Three-day-old seedlings of uniform size were supported by an adjustable acrylic plate and dipped into an 8 × 15 cm glass container filled with 350 ml of nutrient solution, pH 6.0.26 The container was kept in a growth room at 25°C, with a light/dark photoperiod of 14/10 h and a photon flux density of 400 μmol m−2 s−1. After 10 d, seedlings were treated with 0.1 to 0.3 mM BOA. A control experiment without BOA was made in parallel. The nutrient solutions were renewed every other day. The roots, stems and leaf area were measured before incubation (10th day) and at the end of the experiment (15th day), and the differences were calculated for all of the samples. The leaf area was obtained by measuring the length and width of the terminal leaflet of the first trifolium by the method of leaf dimension. At the end of the experiment, roots, stems and leaves of the first trifolium were excised and the fresh weights were determined immediately. BOA (Aldrich 15,705–8) was purchased from Sigma-Aldrich and all other reagents used were of the purest grade available or of chromatographic grade.
Quantification of lignin and monomeric composition
Lignin was extracted from dry tissues after the incubation period of the soybean seedlings. After removing other compounds by washing with phosphate buffer, Triton® X-100, NaCl and acetone,27 the lignin content was determined from the protein-free cell wall fraction by the acetyl bromide method28 with slight modifications. A sample (20 mg) of the protein-free cell wall fraction obtained earlier was placed into a screw-cap centrifuge tube containing 0.5 ml of 25% acetyl bromide (v/v in glacial acetic acid) and incubated at 70ºC for 30 min. After complete digestion, the samples were quickly cooled on ice and mixed with 0.9 ml of 2 M NaOH, 0.1 ml of 7.5 M hydroxylamine-HCl and 2 ml of glacial acetic acid. After centrifugation (1,000 × g, 5 min), the absorbance of the supernatant was measured at 280 nm. A standard curve with lignin (alkali, 2-hydroxy-propyl ether, Aldrich 37,096–7) was generated, and the absorptivity (ε) value obtained was 16.4 g−1 L cm−1. The results are expressed as mg lignin g−1 cell wall.
The oxidation of alkaline nitrobenzene was used to determine the monomeric composition of the lignin.23 The protein-free cell wall fraction (50 mg) obtained above was sealed in a Pyrex® ampule containing 1 ml of 2 M NaOH and 0.1 ml of nitrobenzene prior to heating at 170ºC for 90 min with occasional shaking during the reaction. The sample was then cooled to room temperature, washed twice with chloroform, acidified to pH 2.0 with 2 M HCl and extracted twice with chloroform. The organic extracts were combined, dried and resuspended in 1 ml of a mixture of methanol and 4% acetic acid in water (20:80, v/v). All the samples were filtered through a 0.45-μm disposable syringe filter, and 20 μl aliquots were analyzed in an high performance liquid chromatography (HPLC) system equipped with an LC-10AD pump, a Rheodyne® injector, an SPD-10A UV detector, a CBM-101 Communications Bus Module, and a Class-CR10 workstation system. A reversed-phase Shimpack® CLC-ODS column (150 mm × 4.6 mm, 5 μm), protected with an equivalent pre-column, was used at 30°C. The mobile phase consisted of a mixture of methanol and 4% acetic acid in water (20:80, v/v) with a flow rate of 1.2 ml min−1 for an isocratic run of 20 min. Quantification of the monomer aldehyde products (p˗hydroxybenzaldehyde, vanillin and syringaldehyde) released by nitrobenzene oxidation was performed at 290 nm using the corresponding standards. The results are expressed as mg monomer g−1 cell wall.
Statistical design
The experimental design was completely randomized, with each point on the plot representing one glass container with one seedling. The data are expressed as the means of 3 to 6 independent experiments ± standard error of the mean. Significant differences were verified by one-way analysis of variance (ANOVA) using the GraphPad Prism® package (Version 5.0, GraphPad Software Inc…, USA). Differences between parameters were evaluated by Dunnett´s multiple comparison test, and P values ≤0.05 were adopted as the minimum criterion for statistical significance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to Aparecida M. D. Ramos for technical assistance.
Funding
This work was supported by grants from The Brazilian Council for Scientific and Technological Development - CNPq (no. 470705/2011–6). Maria de Lourdes L. Ferrarese and O. Ferrarese-Filho are research fellows of CNPq. Angela V. Parizotto is the recipient of a CAPES fellowship.
References
- 1. Macías FA, Marín D, Oliveros-Bastidas A, Castellano D, Simonet AM, Molinillo JM. Structure-activity relationships (SAR) studies of benzoxazinones, their degradation products and analogues. phytotoxicity on standard target species (STS). J Agric Food Chem 2005; 53:538-48; PMID:15686399; http://dx.doi.org/ 10.1021/jf0484071 [DOI] [PubMed] [Google Scholar]
- 2. Chiapusio G, Pellissier F, Gallet C. Uptake and translocation of phytochemical 2-benzoxazolinone (BOA) in radish seeds and seedlings. J Exp Botany 2004; 55:1587-92; PMID:15181106; http://dx.doi.org/ 10.1093/jxb/erh172 [DOI] [PubMed] [Google Scholar]
- 3. Sánchez-Moreiras AM, Reigosa MJ. Whole plant response of lettuce after root exposure to BOA (2(3H)-benzoxazolinone). J Chem Ecol 2005; 31:2689-703; PMID:16273435; http://dx.doi.org/ 10.1007/s10886-005-7620-z [DOI] [PubMed] [Google Scholar]
- 4. Komatsu S, Kobayashi Y, Nishizawa K, Nanjo Y, Furukawa K. Comparative proteomics analysis of differentially expressed proteins in soybean cell wall during flooding stress. Amino Acids 2010; 39:1435-49; PMID:20458513; http://dx.doi.org/ 10.1007/s00726-010-0608-1 [DOI] [PubMed] [Google Scholar]
- 5. Bhuiyan NH, Selvaraj G, Wei Y, King J. Role of lignification in plant defense. Plant Signaling Behavior 2009; 4:158-9; PMID:19649200; http://dx.doi.org/ 10.4161/psb.4.2.7688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhong R, Ye ZH. Transcriptional regulation of lignin biosynthesis. Plant Signaling Behavior 2009; 4:1028-34; PMID:19838072; http://dx.doi.org/ 10.4161/psb.4.11.9875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Acker R, Vanholme R, Stormel V, Mortimer JC, Dupree P, Boerjan W. Lignin biosynthesis perturbations affect secondary cell wall composition and saccharification yield in arabidopsis thaliana. Biotechnol Biofuels 2013; 6:46; PMID:23622268; http://dx.doi.org/ 10.1186/1754-6834-6-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. González LF, Rojas MC. Role of wall peroxidases in oat growth inhibition by DIMBOA. Phytochemistry 1999; 50:931-7; http://dx.doi.org/ 10.1016/S0031-9422(98)00635-9 [DOI] [Google Scholar]
- 9. Friebe A, Roth U, Kuck P, Schnabl H, Schulz M. Effects of 2,4-dihydroxy-1,4-benzoxazin-3-ones on the activity of plasma membrane H+ ATPase. Phytochemistry 1997; 44:979-83; http://dx.doi.org/ 10.1016/S0031-9422(96)00677-2 [DOI] [Google Scholar]
- 10. Burgos NR, Talbert R. Differential activity of allelochemicals from secale cereale in seedling bioassays. Weed Science 2000; 48:302-10; PMID:15139316; http://dx.doi.org/ 10.1614/0043-1745(2000)048%5b0302:DAOAFS%5d2.0.CO;215139316 [DOI] [Google Scholar]
- 11. Burgos NR, Talbert RE, Kim KS, Kuk YI. Growth inhibition and root ultrastructure of cucumber seedlings exposed to allelochemicals from rye (secale cereale). J Chem Ecol 2004; 30:671-89; PMID:15139316; http://dx.doi.org/ 10.1023/B:JOEC.0000018637.94002.ba [DOI] [PubMed] [Google Scholar]
- 12. Batish DR, Singh HP, Setia N, Kaur S, Kohli RK. Two-Benzoxazolinone (BOA) induced oxidative stress, lipid peroxidation and changes in some antioxidant enzyme activities in mung bean (phaseolus aureus). Plant Physiol Biochem 2006; 44:819-27; PMID:17107811; http://dx.doi.org/ 10.1016/j.plaphy.2006.10.014 [DOI] [PubMed] [Google Scholar]
- 13. Hussain MI, Reigosa MJ. Seedling growth, leaf water status and signature of stable carbon isotopes in C3 perennials exposed to natural phytochemicals. Aust J Botany 2012; 60:676-84; http://dx.doi.org/ 10.1071/BT12072 [DOI] [Google Scholar]
- 14. Chum M, Batish DR, Singh HP, Kohli RK. Phytotoxic effect of 2-benzoxazolinone (BOA) against some vegetable crops. J Environ Biol 2012; 33:21-5; PMID:23033638 [PubMed] [Google Scholar]
- 15. Sánchez-Moreiras AM, de la Pena TC, Reigosa MJ. The natural compound benzoxazolin-2(3H)-one selectively retards cell cycle in lettuce root meristems. Phytochemistry 2008; 69:2172-9; PMID:18597799; http://dx.doi.org/ 10.1016/j.phytochem.2008.05.014 [DOI] [PubMed] [Google Scholar]
- 16. Kato-Noguchi H, Macías FA, Molinillo JMG. Structure–activity relationship of benzoxazinones and related compounds with respect to the growth inhibition and α-amylase activity in cress seedlings. J Plant Physiol 2010; 167:1221-5; PMID:20605653; http://dx.doi.org/ 10.1016/j.jplph.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 17. Niemeyer HM, Calcaterra NB, Roveri OA. Inhibition of energy metabolism by benzoxazoli-2-one. Compar Biochem Physiol 1987; 87:35-9; PMID:2956049; http://dx.doi.org/ 10.1016/0300-9629(87)90421-X [DOI] [PubMed] [Google Scholar]
- 18. Sánchez-Moreiras AM, Oliveiros-Bastidas A, Reigosa MJ. Reduced photosynthetic activity is directly correlated with 2-(3H)-benzoxazolinone accumulation in lettuce leaves. J Chem Ecol 2010; 36:205-9; PMID:20143137; http://dx.doi.org/ 10.1007/s10886-010-9750-1 [DOI] [PubMed] [Google Scholar]
- 19. Hussain MI, González L, Chiapusio G, Reigosa MJ. Benzoxazolin-2(3H)-one (BOA) induced changes in leaf water relations, photosynthesis and carbon isotope discrimination in lactuca sativa. Plant Physiol Biochem 2011; 49:825-34; PMID:21665486; http://dx.doi.org/ 10.1016/j.plaphy.2011.05.003 [DOI] [PubMed] [Google Scholar]
- 20. Sánchez-Moreiras AM, Martinez-Penalver A, Reigosa MJ. Early senescence induced by 2-3H-benzoxazolinone (BOA) in arabidopsis thaliana. J Plant Physiol 2011; 168:863-70; PMID:21237530; http://dx.doi.org/ 10.1016/j.jplph.2010.11.011 [DOI] [PubMed] [Google Scholar]
- 21. Santos WDd, Ferrarese MdLL, Finger A, Teixeira ACN, Ferrarese-Filho O. Lignification and related enzymes in glycine max root growth-inhibition by ferulic acid. J Chem Ecol 2004; 30:1199-208; PMID:15303323 [DOI] [PubMed] [Google Scholar]
- 22. Zanardo DIL, Lima RB, Ferrarese MdLL, Bubna GA, Ferrarese-Filho O. Soybean root growth inhibition and lignification induced by p-coumaric acid. Environ Exp Bot 2009; 66:25-30; PMID:21489652; http://dx.doi.org/ 10.1016/j.envexpbot.2008.12.01421489652 [DOI] [Google Scholar]
- 23. Bubna GA, Lima RB, Zanardo DIL, Santos WDd, Ferrarese MdLL, Ferrarese-Filho O. Exogenous caffeic acid inhibits the growth and enhances the lignification of the roots of soybean (glycine max). J Plant Physiol 2011; 168:1627-33; PMID:21489652; http://dx.doi.org/ 10.1016/j.jplph.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 24. Ferrer JL, Austin MB, Stewart JC, Noel JP. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol Biochem 2008; 46:356-70; PMID:18272377; http://dx.doi.org/ 10.1016/j.plaphy.2007.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Santos WDd, Ferrarese MdLL, Ferrarese-Filho O. Ferulic acid: an allelochemical troublemaker. Functional Plant Sci Biotechnol 2008; 2:47-55 [Google Scholar]
- 26. Dong J, Wu FB, Zhang GP. Influence of cadmium on antioxidant capacity and four microelement concentrations in tomato seedlings (lycopersicon esculentum). Chemosphere 2006; 64:1659-66; PMID:16497361; http://dx.doi.org/ 10.1016/j.chemosphere.2006.01.030 [DOI] [PubMed] [Google Scholar]
- 27. Ferrarese MdLL, Rodrigues JD, Ferrarese-Filho O. Phenylalanine ammonia-lyase activity in soybean roots extract measured by reverse-phase high performance liquid chromatography. Plant Biol 2000; 2:152-3; http://dx.doi.org/ 10.1055/s-2000-9162 [DOI] [Google Scholar]
- 28. Morrison IM. A semi-micro method for the determination of lignin and its use in predicting the digestibility of forage crops. J Sci Food Agric 1972; 23:455; PMID:5029974; http://dx.doi.org/ 10.1002/jsfa.2740230405 [DOI] [PubMed] [Google Scholar]


