Abstract
Plant's transition from vegetative to reproductive phase is balanced by intricate cascade of genes regulated by both endogenous and environmental inputs. Stress causes suppression of vegetative growth and acceleration of flowering as an emergency response for preservation of the species. Recently, we determined that expression levels of a transcription factor with 2 B-Box motifs, BBX19, is notably reduced in response to accumulation of high levels of Methylerythritol cyclodiphosphate (MEcPP), a plastidial produced isoprenoids intermediate that also functions as a stress-specific retrograde signaling metabolite. We now have identified BBX19 as a repressor of Flower locus T (FT) expression and the corresponding downstream genes, SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1), Leafy (LFY) and Fruitful (FUL), through competition with CONSTANS (CO). Collectively our finding identifies BBX19 as a link between the stress-specific retrograde signal MEcPP and regulation of flowering time by depleting the active CO pool required for transcription of FT.
Keywords: BBX19, flowering time, methylerythritol cyclodiphosphate (MEcPP), retrograde signaling, stress
Plants, as sessile organisms, have evolved complex and sophisticated regulatory networks to survive environmental perturbations. Among the most notable control mechanisms is the bidirectional coordination of signaling cascades between nucleus and organelles. The control of information flow from nucleus to organelles (anterograde signaling) is well studied, but the reciprocal signaling communication from organelles back to nucleus (retrograde signaling) has remained elusive.
Plastids as the metabolic hub of the cell also sense environmental and developmental cues and relay this information back to the nucleus. We have recently identified the methylerythritol phosphate (MEP) pathway produced metabolite methylerythritol cyclodiphosphate (MEcPP), as a stress-specific retrograde signal responsible for communication of environmental perturbations from plastids back to the nucleus.1,2 Furthermore, we have shown that accumulation of MEcPP in a mutant plant designated as ceh1 (constitutively expressing HPL) results in the robust alteration of transcript levels of selected stress responsive nuclear genes. Among the genes with notably reduced expression levels is BBX19, encoding a member of the BBX family of proteins with 2 B-Box (Box1 and 2) motifs.3 Overexpression of BBX19 in ceh1 reverts the early flowering of the mutant plant to a late flowering phenotype (Fig. 1). Similarly, RNAi assisted reduction of expression of BBX19 accelerates flowering, and conversely its constitutive expression delays flowering in the wild type plants.3 Additional studies confirmed that regulatory function of BBX19 in flowering time is restricted to the long day (LD) inductive photoperiods. The mode of BBX19 action is through physical interaction with another BBX family member namely CONSTANS (CO) /AtBBX1,4,5 and thereby depletion of the active CO pool required for transcription of FLOWERING LOCUS T (FT).3 This physical interaction is dependent on the integrity of Box1 motif, since overexpression of BBX19 with a mutation in the Box1 [conserved cysteine 25 (C) to serine (S)] no longer delays the flowering time of wild type plants under LD.3 These results are further confirmed by co-infiltratation assays in tobacco leaves using PFT:LUC together with the 35S:CO and 35S:BBX19 constructs in 1:1:1 or 1:10:1 ratios. These analyses demonstrated LUC bioluminescence suppression in the presence of equal ratios of BBX19 and CO, and full reversion of this suppression in the presence of CO at 10-fold higher levels than that of BBX19. In addition, the coinciding spatial expression patterns of CO and BBX19 in vasculature together with the nuclear co-localization of these 2 BBX family members lend further support for their in vivo physical interaction.6,7 Collectively, these findings strongly support the notion of competition between the 2 BBX transcription factors as well as their opposing function in determining the flowering time.
Figure 1.
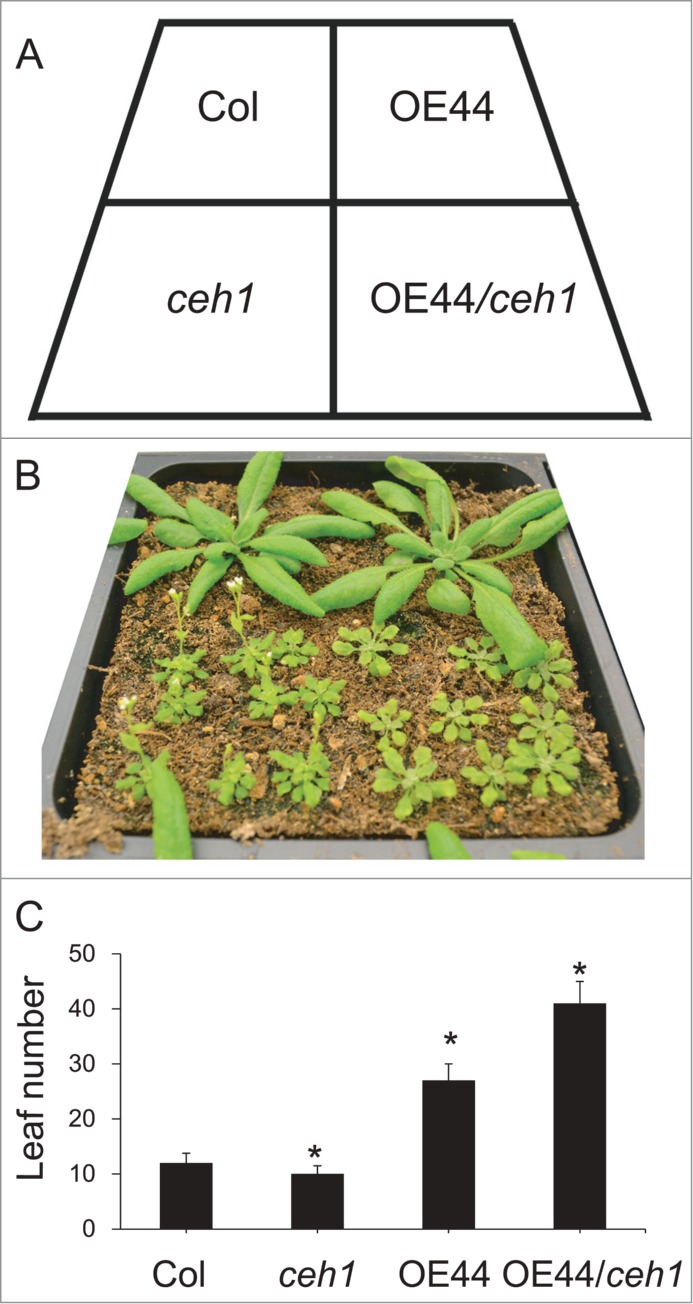
BBX19 is a suppressor flowering time (A) Diagram displaying the arrangement of various genotypes. (B) Phenotypes of 30 day old Col, ceh1, BBX19 overexpression line (OE44) and OE44/ceh1. (C) Leaf number per plants to flowering time of the aforementioned genotypes. Asterisks denote a significant difference in leaf number at flowering time between control (Col) and other genotypes (P < 0.05).
Detailed examination of transcript levels of BBX19, CO and FT, every 6 hours in a 24-hour cycle display the antiphasic circadian rhythm of BBX19 compared to those of FT and CO.3 This antiphasic rhythm is indeed a checkpoint for plants to accurately monitor the day length and precisely time the expression of FT. Specifically during the day BBX19 physically interacts with and depletes the active pool of CO the activator of FT, and thereby prevents CO-activation of FT expression.
It is well established that stress accelerates flowering time in plants as an emergency response for preservation of the species.8,9 As depicted in the schematic model (Fig. 2), we propose that stress-mediated acceleration of flowering is in part through reduction of the BBX19 expression levels in response to accumulation of the stress-specific retrograde signal MEcPP. That is, stress results in elevated levels of MEcPP, the retrograde signal that directly or indirectly suppresses expression of BBX19, that in turn enhances the active pool of CO available for binding to and activating FT expression and ultimately leading to early flowering. However, in the absence of stress, MEcPP functions solely as an intermediate of isoprenoids pathway and as such can no longer accumulate to levels capable of suppressing BBX19 expression. This leads to accumulation of BBX19 at sufficient levels to interact with and deplete the active CO pool required for transcription of FT, which consequently delays flowering.
Figure 2.
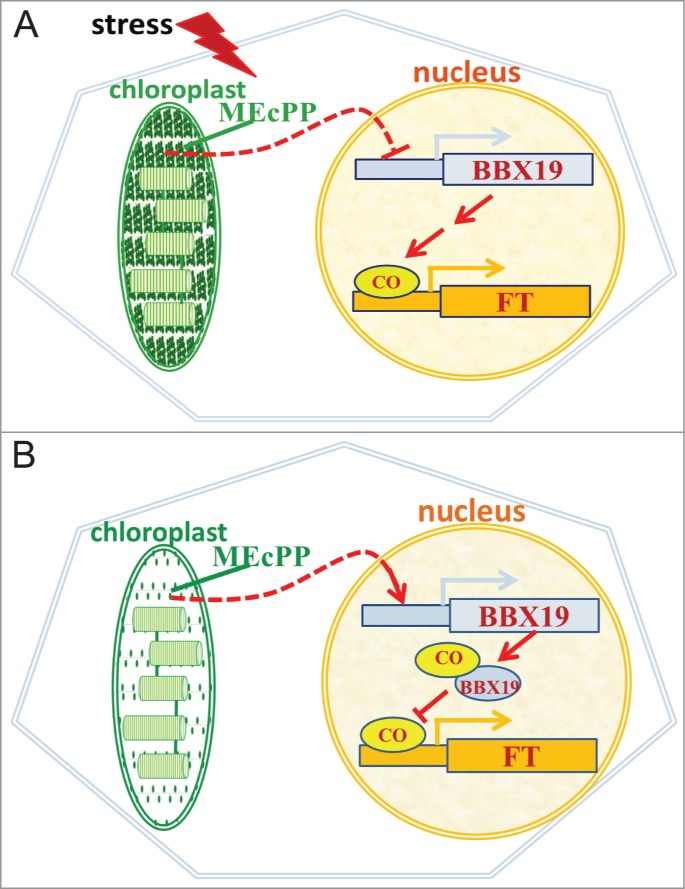
MEcPP levels directly or indirectly regulates flowering time by altering BBX19 transcript levels. (A) MEcPP accumulation in response to stress reduces the BBX19 transcript levels and promotes availability of CO for activation of FT. (B) Under unstressed conditions MEcPP does not accumulate hence allowing expression of BBX19 to sufficiently high levels resulting in its interaction with and depletion of CO pool required for activation of FT.
This finding provides a novel link between a plastidial retrograde signaling molecule MEcPP and BBX19 a flowering time checkpoint.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by National Institute of Health (R01GM107311), and National Science Foundation (IOS-1036491 and IOS-1352478), and Agriculture experiment station (CA-D-PLB-3510-H) grants awarded to KD.
References
- 1.Xiao Y, et al.. Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell 2012; 149(7):1525–35; PMID: 22726439; http://dx.doi.org/ 10.1016/j.cell.2012.04.038 [DOI] [PubMed] [Google Scholar]
- 2.Xiao Y, Wang J, Dehesh K. Review of stress specific organelles-to-nucleus metabolic signal molecules in plants. Plant Sci 2013; 212: 102–7; PMID:24094057; http://dx.doi.org/ 10.1016/j.plantsci.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 3.Wang CQ, et al.. BBX19 interacts with CONSTANS to REPRESS FLOWERING LOCUS T transcription, defining a flowering time checkpoint in Arabidopsis. Plant Cell 2014; 26(9):3589–602; PMID:25228341; http://dx.doi.org/ 10.1105/tpc.114.130252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gangappa SN, Botto JF. The BBX family of plant transcription factors. Trends Plant Sci 2014; 19(7):460–70; PMID:24582145; http://dx.doi.org/ 10.1016/j.tplants.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 5.Khanna R, et al.. The Arabidopsis B-box zinc finger family. Plant Cell 2009; 21(11):3416–20; PMID:19920209; http://dx.doi.org/ 10.1105/tpc.109.069088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An H, et al.. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 2004; 131(15):3615–26; PMID:15229176; http://dx.doi.org/ 10.1242/dev.01231 [DOI] [PubMed] [Google Scholar]
- 7.Kumar SV, et al.. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 2012; 484(7393):242–5; PMID:22437497; http://dx.doi.org/ 10.1038/nature10928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu MY, et al.. Stress-induced early flowering is mediated by miR169 in Arabidopsis thaliana. J Exp Bot 2014; 65(1):89–101; PMID:24336445; http://dx.doi.org/ 10.1093/jxb/ert353 [DOI] [PubMed] [Google Scholar]
- 9.Wada KC, Takeno K. Stress-induced flowering. Plant Signal Behav 2010; 5(8):944–7; PMID:20505356; http://dx.doi.org/ 10.4161/psb.5.8.11826 [DOI] [PMC free article] [PubMed] [Google Scholar]


