Abstract
Experimental evidences support that the circadian rhythm regulates the transcription levels of genes encoding the enzymes involved in plant metabolism. However, there is no paper to refer the correlation of the circadian rhythms and the metabolic processes for facilitating pollen tube growth. In this study, we found that many central components of the circadian clock were highly enriched and specifically present in the in vivo grown Arabidopsis pollen tubes. Our analysis also identified the significant differentially expressed genes encoding co-expressed enzymes in the consecutive steps of fatty acid β-oxidation II, pentose phosphate pathway (oxidative branch) and phosphatidic acid biosynthesis pathway in the in vivo grown Arabidopsis pollen tubes during pollination. Thus, it is implicated that the circadian rhythms of pollen tube may be adjusted and have a greater probability of the direct or indirect functional relationship with enhanced intracellular Ca2+ dynamics and ATP production for facilitating pollen tube growth in vivo.
Keywords: Arabidopsis, circadian clock, metabolic, pollen tube, pollination
When compatible pollen grains land on the stigma surface, the pollen grains germinate pollen tubes that grow through the pistil tissue toward the embryo sacs. At the same time, stigmas undergo an accelerated process of senescence in response to pollination.1 Genetic and genomic approaches have advanced our understanding of the regulated network of various cellular processes contributing to the fast growth of pollen tubes in stigma.2,3 However, there is little published literature to gain insight into the role of the circadian clock in the coordination of metabolism with other physiological processes in female and male reproductive tissues in compatible pollination. It is generally known that pollen tubes grow deeply within a solid style, making it extremely difficult to obtain in vivo-grown pollen tubes for microarray analysis. Recently, Qin et al. and Lin et al. respectively use the difference method (semi-in vivo/pollen-specific promoter) to identified the mRNAs specifically enriched in vivo–elongating pollen tubes.4,5 Qin et al. first defined the transcriptome of pollen tubes that have grown through pistil tissues using a semi-in vivo pollen tube growth system for Arabidopsis. Second, they performed comparative microarray analysis with RNA isolated from dry, un-germinated pollen (dry pollen), pollen grown in vitro for 0.5 hours, or for 4 hours and pollen germinated and grown through the stigma and style. Lastly, pairwise comparisons of pearson correlation coefficients were used to explored the distinct set of genes define pollen tube growth in vitro and in a pistil. Notably, semi-in vivo pollen tube shares a set of 871 genes that are not expressed in the 3 other pollen samples analyzed.4 Lin et al. termed three different samples, IP-bud (bud, ST11–12, developing microspore, and mature pollen grains), IP-in vivo (ST14-ST15a, the enrichment in grains undergoing tube elongation and fertilization), and IP-in vitro (in vitro–cultured pollen tubes).5 They use a pollen-specific promoter (ProLAT52) to generate epitope-tagged polysomal-RNA complexes that could be affinity purified, they obtained mRNAs undergoing transcriptome of in vivo–grown pollen tubes from self-pollinated gynoecia of Arabidopsis. The polysomal mRNAs isolated from 3 samples were further analyzed to identify gene transcripts specifically enriched in the in vivo elongating pollen tubes. Lastly, by systematic comparison of data sets, there were 519 mRNAs specifically enriched in vivo–elongating pollen tubes.5 The gene ontology enrichment analysis revealed that many significant differentially expressed genes between in vivo and in vitro cultured samples involved in core circadian clock regulation and cell division, which indicates that circadian rhythms might play important roles in the pollination process. In our previous study, we developed a data-driven computational pipeline using the “guilt by association” principle to analyze the transcriptional co-expression profiles of enzymatic genes in the consecutive steps for metabolic routes in the fast-growing pollen tube and stigma during pollination. Our analysis identified an inferred pattern of pollen tube-stigma ethanol coupling.6 Here, we give an additional inferred correlation of circadian clock and metabolic involved in the fast-growing pollen tube during pollination.
As shown in the Fig. 1, the inferred mechanism analyzed in detail below:
Figure 1.
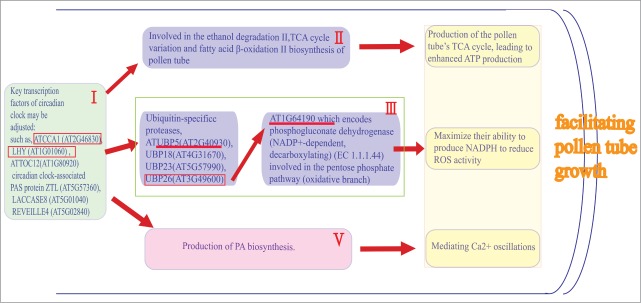
Schematic representation of the inferred correlation of circadian rhythms and metabolism involved in the fast-growing pollen tube in pistil. (I) Key transcription factors of circadian clock may be adjusted and highly enriched and specifically present in the in vivo–grown pollen tubes, such as: ATCCA1 (AT2G46830), LHY (AT1G01060), ATTOC12 (AT1G80920), circadian clock-associated PAS protein ZTL (AT5G57360), LACCASE8 (AT5G01040) REVEILLE4 (AT5G02840). (II) Circadian rhythms triggers a rapid activation of the cooperating enzymes and consecutive steps for the ethanol degradation II, TCA cycle variation and fatty acid β-oxidation II of pollen tube, production of the pollen tube's TCA cycle, leading to enhanced ATP production. (III) Four ubiquitin-specific proteases, UBP18 (AT4G31670), UBP23 (AT5G57990), ATUBP5 (AT2G40930) , UBP26 (AT3G49600) had relations with the central components of the circadian clock ATCCA1 (AT2G46830) and LHY (AT1G01060). UBP26 (AT3G49600) had significant relations with the AT1G64190 which encodes phosphogluconate dehydrogenase (NADP+-dependent, decarboxylating) (EC 1.1.1.44) involved in the pentose phosphate pathway (oxidative branch), which maximize their ability to produce NADPH to reduce ROS level. (IV) Circadian rhythms may have a greater probability of the direct or indirect functional relationship with the cooperation of consecutive steps for production of PA biosynthesis. Intracellular Ca2+ dynamics were induced by the 2 messengers, Ins(1,4,5)P3 and DAG.
(I). A variety of approaches have been taken to describe the spectrum of Arabidopsis proteins involved in circadian regulation. The key transcription factors of the higher plant clock consist of TOC1 (TIMING OF CAB EXPRESSION 1), LHY (LATE ELONGATED HYPOCOTYL), CCA1 (CIRCADIAN CLOCK ASSOCIATED 1) and so on.7,8 Based on the analysis of Qin et al. and Lin et al. comparative microarray data,4,5 we identified the central components of the circadian clock ATCCA1 (AT2G46830), LHY (AT1G01060), ATTOC12 (AT1G80920), circadian clock-associated PAS protein ZTL (AT5G57360), LACCASE 8 (AT5G01040), REVEILLE 4 (AT5G02840) were highly enriched and defined twofold changes in the in vivo–grown pollen tubes. Interestingly, we also identified 4 ubiquitin-specific proteases ATUBP5 (AT2G40930), UBP18 (AT4G31670), UBP23 (AT5G57990), UBP26 (AT3G49600) were specifically presented in the in vivo grown pollen tubes, which regulates the stability of key components of the circadian clock feedback loops.9 It is implicated that these key transcription factors of circadian clock function synergistically in regulating circadian rhythms of Arabidopsis pollen tube for facilitating pollen tube growth.
(II). Metabolism is the complete set of enzyme-catalyzed reactions that allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Much evidence supports that the circadian clock regulates the transcript levels of numerous gene encoding enzymes involved in plant metabolism.10 Using 2-dimensional gel electrophoresis followed by mass spectrometry, Hwang et al. found the rhythmic proteins were functionally classified into photosynthesis, central metabolism, protein synthesis, nitrogen metabolism, stress resistance, signal transduction and unknown.11
It is clear that due to their rapid growth, pollen tubes need to transfer much more energy for the ion fluxes, osmoregulation, synthesis of pectin and proteins, vesicle trafficking, actin dynamics and cyclosis in order to maintain the membrane potential and turgor given the ever-increasing volume. All these processes require ATP hydrolysis either directly or indirectly through GTP. Our study supports the viewpoint that the energy generating pathways may play the critical role in vivo grown Arabidopsis pollen tubes. High-energy nutrients (alcohol/ethanol) are metabolized to acetyl-CoA by ethanol degradation II and pyruvate dehydrogenase bypass in the pollen tubes. Acetyl-CoA is incorporated into the pollen tube's tricarboxylic acid cycle variation, leading to enhanced ATP production for facilitating pollen tube growth.12 In our previous study, we identified the significant differentially expressed genes of Arabidopsis pollen tube encoding co-expressed enzymes in the consecutive steps of ethanol degradation II and TCA cycle variation in response to pollination.6 Now, we found an additional metabolic pathways that catalyze the consecutive steps of fatty acid β-oxidation II involved in the fast-growing pollen tube during pollination (Fig. 2) .
Figure 2.
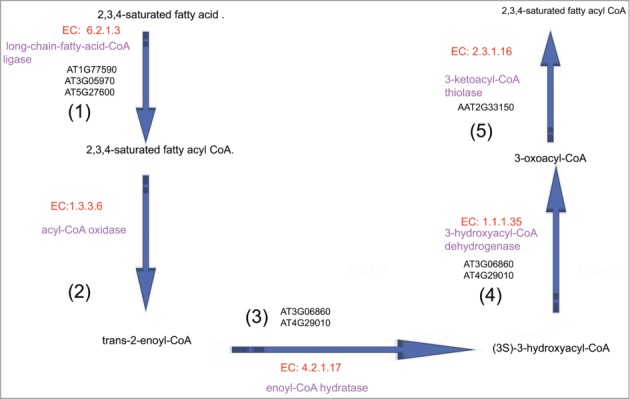
Consecutive steps of fatty acid β-oxidation II in vivo–grown Arabidopsis pollen tube. (1): Three gene loci, AT1G77590, AT3G05970, AT5G27600, encode long-chain-fatty-acid-CoA synthetase (EC 6.2.1.3), which catalyzes the conversion of 2,3,4-saturated fatty acid into 2,3,4-saturated fatty acyl CoA. (2): Our multiomics network analyses showed no differentially expressed genes encoding encode acyl-CoA oxidase (EC 1.3.3.6), which catalyzes the conversion of 2,3,4-saturated fatty acyl CoA into trans-2-enoyl-CoA. However, after re-checking the source datasets of the expression of these genes, we did find that the expression of these genes did not significantly change. (3): Two gene loci, AT3G06860, AT4G29010, encode enoyl-CoA hydratase (EC 4.2.1.17), which catalyzes the conversion of trans-2-enoyl-CoA into (3S)-3-hydroxyacyl-CoA. (4): Two gene loci, AT3G06860, AT4G29010, encode 3-hydroxyacyl-CoA dehydrogenase (EC 1.1.1.35), which catalyzes the conversion of (3S)-3-hydroxyacyl-CoA into 3-oxoacyl-CoA. (5): The gene locus, AT2G33150, encodes 3-ketoacyl-CoA thiolase (EC 2.3.1.16), which catalyzes the conversion of 3-oxoacyl-CoA into 2,3,4-saturated fatty acyl CoA.
β-oxidation is a multi-step process in which fatty acids are broken down to produce energy, which also reflected an increase in lipid oxidation for the production of energy. Thus, it is implicated that the circadian clock may coordinates the expression of enzymes in these pathways in pollen tube, which might be involved in the TCA cycle of pollen tube, leading to enhanced ATP production for facilitating pollen tube growth. However, the extent of circadian regulation of enzyme activities is unknown.
(III). In Arabidopsis, ubiquitin-mediated protein regulates the stability of key components of the circadian clock feedback loops.9 It is generally known that protein–protein interactions are essential for circadian clock function. Protein complexes that regulate both clock gene transcription and protein stability have been reported.8,10,11 In this study, 4 ubiquitin-specific proteases, ATUBP5 (AT2G40930), UBP18 (AT4G31670), UBP23 (AT5G57990), UBP26 (AT3G49600) were identified to be specifically present in the in vivo grown pollen tubes. Based on the analysis of proteome-wide binary protein–protein interaction map of Arabidopsis.13 and plant metabolic pathway database (PMN/PlantCyc) (http://www.plantcyc.org), we found that ATUBP5 had relations with the central components of the circadian clock ATCCA1 (AT2G46830) and LHY (AT1G01060). Interestingly, we also found that ATUBP26 had significant relations with the gene locus AT1G64190 which encodes phosphogluconate dehydrogenase (NADP+-dependent, decarboxylating) (EC 1.1.1.44) involved in the pentose phosphate pathway (oxidative branch). Pentose phosphate pathway contributes to the control of the cell redox status, which is the key metabolic pathway for the generation of reducing equivalents (NADPH) to scavenge reactive oxygen species. Thus, when the pollen grains germinate and pollen tubes grow through the pistil tissue toward the embryo sacs, the glucose is metabolized through the pentose phosphate pathway to generate NADPH. Therefore, it is possible that circadian clock function synergistically in regulating pentose phosphate pathway of Arabidopsis pollen tube to maximize their ability to produce NADPH and eliminate ROS for facilitating pollen tube growth.
(IV). In plants, phosphatidic acid is a lipid-signaling mediator involved in various cellular processes, such as in the regulation of the tip growth of the pollen tube and the root hair.14 Numerical detailed studies have shown that PtdIns(4, 5)P2 leads to increased [Ca2+] cyt, whereas Ins(1, 4, 5)P3 causes a transient [Ca2+] cyt increase of similar magnitude15,16. Depending on the Ca2+ concentration, PLC is able to hydrolyze different substrates. At low Ca2+ concentrations, PtdIns(4, 5)P2 is hydrolyzed, whereas at higher Ca2+ concentrations, PLC preferentially uses phosphatidylinositol phosphate (PIP) as the substrate.17 Monteiro et al. showed that PtdIns(4, 5)P2 and Ins(1, 4, 5)P3, together with PA, play a vital role in the regulation of cytoplasmic [Ca2+] levels, endo/exocytosis, and vesicular trafficking in the apex of pollen tubes.18 In addition, Monteiro et al also demonstrated that PtdIns(4, 5)P2 and Ins(1, 4, 5)P3 signaling consists of a feedback loop from the cytosol to the plasma membrane through a multiple pathway system that involves the regulation of [Ca2+] cyt levels, endo/exocytosis, actin cytoskeleton dynamics, and vesicular trafficking.18
Our previous study established that Arabidopsis phosphatidylinositol 3-kinase (AtVPS15) functions in the development and germination of pollen by catalyzing the biosynthesis of phosphatidylinositol 3-phosphate (PI3P).19 Germination ratio of pollen from the atvps15/+ genotype is about half when compared to that of the wild type. When supplied with PI3P, in vitro pollen germination of the atvps15/+ plant is greatly improved. Another study has revealed the cooperating enzymes and consecutive steps for the production of phosphatidic acid in Arabidopsis and maize stigmas.6 In this study, when the significantly differentially expressed genes between probe pair sets in vitro, semi-in vivo and in vivo grown pollen tube were mapped to the Arabidopsis genome-scale enzyme correlation network model, we also observed that encoded co-expressed enzymes and consecutive steps in the phosphatidic acid biosynthesis pathway in the in vivo–grown Arabidopsis pollen tubes during pollination (Fig. 3).
Figure 3.
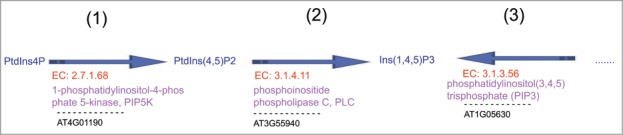
Consecutive steps in the phosphatidic acid biosynthesis pathway in Arabidopsis pollen tube during pollination. (1). The gene locus, AT4G01190 encodes 1-phosphatidylinositol-4-phosphate 5-kinase, PIP5K (EC: 2.7.1.68) catalyzing the conversion of PtdIns4P to PtdIns(4,5)P2. (2). The gene locus, AT3G55940 encodes phosphoinositide phospholipase C, PLC (EC: 3.1.4.11) catalyzing the conversion of PtdIns(4,5)P2 to Ins(1,4,5)P3. (3). The gene locus, AT1G05630 encodes an inositol polyphosphate 5-phosphatase (EC: 3.1.3.56) with phosphatase activity toward only Ins(1,4,5)P3.
Recent study indicates that metabolites might play a role in regulating the clock in plants.3 Nitrate, glutamate and glutamine treatments of Arabidopsis seedlings grown in low nitrate media are able to shift the phase of oscillations of circadian clock.20 The mechanism which the circadian clock regulate Ca2+ oscillations or Ca2+ oscillations synchronise circadian rhythms is unknown. Our study implicated that the circadian rhythms may have a direct or indirect functional relationship with the cooperation of phosphatidic acid mediating Ca2+ oscillations for facilitating pollen tube growth.
Metabolic networks display very complex regulation at multiple levels involving transcriptional, posttranslational and allosteric regulation of enzyme activities. Circadian rhythms are maintained by internal clocks and mediate physiology and metabolism in plants. Accumulating experimental evidence supports a role for the circadian clock in leaves and petals, stomatal opening, diurnal changes in photosynthetic activities and also photoperiodicity-dependent control of flowering time.21 There is not experimental evidence to support that circadian clocks have impacts on pollen tube growth. Recently, Danny et al. give the evidence which supports a role for the parent-of-origin effects on CCA1 expression during early stages of embryo development. Their results support that the circadian clock may function with embryo growth rates during embryo and seed development. However, for deeper understanding of the role of the circadian clock in the coordination of metabolism with other physiological processes for facilitating pollen tube growth, further evidence for the correlation between transcript and metabolite levels involved in the fast-growing pollen tube should be provided using reverse genetic approaches, such as enhancing or inhibiting the transcript levels (by T-DNA insertion and/or RNAi) of the genes involved in regulating the circadian clock to elucidate their biological functions. And, the clock-regulated components are modulated by not only transcriptional but also post-transcriptional, translational, and/or post-translational processes. It is also necessary to analyze its effects on the regulation of protein levels, protein modifications and metabolite amounts.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by the National Major Program of Transgenic Research in China (Grant No. 2013CB945100), the National Natural Science Foundation, China (Grant Nos. 31171475 and 31170293), and the State Key Laboratory of Crop Science, China (Grant No. 2013KF15). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nuwan U, Sella Kapu, Daniel J. Cosgrove changes in growth and cell wall extensibility of maize silks following pollination. J Exp Bot 2010; 14:4097-107; PMID:20656797; http://dx.doi.org/ 10.1093/jxb/erq225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hiscock SJ, Allen AM. Diverse cell signaling pathways regulate pollen-stigma interactions: the search for consensus. New Phytol 2008; 179:286-317; PMID:19086285; http://dx.doi.org/ 10.1111/j.1469-8137.2008.02457.x [DOI] [PubMed] [Google Scholar]
- 3. Obermeyer G, Fragner L, Lang V, Weckwerth W. Dynamic adaption of metabolic pathways during germination and growth of lily pollen tubes after inhibition of the electron transport chain. Plant Physiol 2013; 162:1822-33; PMID:23660836; http://dx.doi.org/ 10.1104/pp.113.219857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, Vasic B, Johnson MA, Palanivelu R. Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet 2009; 5(8):e1000621; PMID:19714218; http://dx.doi.org/ 10.1371/journal.pgen.1000621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin SY, Chen PW, Chuang MH, Juntawong P, Bailey-Serres J, Jauh GY. Profiling of translatomes of in vivo grown pollen tubes reveals genes with roles in micropylar guidance during pollination in Arabidopsis. Plant Cell 2014; 26:602-18; PMID:24532595; http://dx.doi.org/ 10.1105/tpc.113.121335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yue X, Gao X-Q, Wang F, Dong Y, Li X, Zhang XS. Transcriptional evidence for inferred pattern of pollen tube-stigma metabolic coupling during pollination. PLoS One 2014; 9:e107046; PMID:25215523; http://dx.doi.org/ 10.1371/journal.pone.0107046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chow BY, Kay SA. Global approaches for telling time: omics and the Arabidopsis circadian clock. Semin Cell Dev Biol 2013; 24:383-92; PMID:23435351; http://dx.doi.org/ 10.1016/j.semcdb.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Endo M, Shimizu H, Nohales MA, Araki T, Kay SA. Tissue-specific clocks in Arabidopsis show asymmetric coupling. Nature 2014; 515:419-22; PMID:25363766; http://dx.doi.org/ 10.1038/nature13919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cui X, Lu F, Li Y, Xue Y, Kang Y, Zhang S, Qiu Q, Cui X, Zheng S, Liu B, et al. Ubiquitin-specific proteases UBP12 and UBP13 act in circadian clock and photoperiodic flowering regulation in Arabidopsis. Plant Physiol 2013; 162:897-906.PMID:23645632; http://dx.doi.org/ 10.1104/pp.112.213009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farré EM, Weise SE. The interactions between the circadian clock and primary metabolism. Curr Opin Plant Biol 2012; 15:293-300; PMID:22305520; http://dx.doi.org/ 10.1016/j.pbi.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 11. Hwang H, Cho MH, Hahn BS, Lim H, Kwon YK, Hahn TR, Bhoo SH. Proteomic identification of rhythmic proteins in rice seedlings. Biochim Biophys Acta 2011; 1814:470-9; PMID:21300183; http://dx.doi.org/ 10.1016/j.bbapap.2011.01.010 [DOI] [PubMed] [Google Scholar]
- 12. Gass N, Glagotskaia T, Mellema S, Stuurman J, Barone M, Mandel T, Roessner-Tunali U, Kuhlemeier C. Pyruvate decarboxylase provides growing pollen tubes with a competitive advantage in petunia. Plant Cell 2005; 17:2355-68; PMID:15994907; http://dx.doi.org/ 10.1105/tpc.105.033290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braun P, Carvunis AR, Charloteaux B, Dreze M, Ecker JR, Hill DE, Roth FP, Vidal M, Galli M, Balumuri P, et al. Evidence for network evolution in an Arabidopsis interactome map. Science 2011; 333:601-7; PMID:21798944; http://dx.doi.org/ 10.1126/science.1203877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xue HW, Chen X , Mei Y. Function and regulation of phospholipid signalling in plants. Biochem 2009; 421:145-56; PMID:19552624; http://dx.doi.org/ 10.1042/BJ20090300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Helling D, Possart A, Cottier S, Klahre U, Kost B. Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant Cell 2006; 18:3519-34; PMID:17172355; http://dx.doi.org/ 10.1105/tpc.106.047373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ischebeck T, Stenzel I, Heilmann I. Type B phosphatidylinositol-4-phosphate 5-kinases mediate Arabidopsis and Nicotiana tabacum pollen tube growth by regulating apical pectin secretion. Plant Cell 2008; 20:3312-30; PMID:19060112; http://dx.doi.org/ 10.1105/tpc.108.059568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sousa E, Kost B, Malho R. Arabidopsis phosphatidylinositol-4-monophosphate 5-kinase 4 regulates pollen tube growth and polarity by modulating membrane recycling. Plant Cell 2008; 20:3050-64; PMID:19033528; http://dx.doi.org/ 10.1105/tpc.108.058826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Monteiro D, Liu Q, Lisboa S, Scherer GE, Quader H, Malhó R. Phosphoinositides and phosphatidic acid regulate pollen tube growth and reorientation through modulation of [Ca2+]c and membrane secretion. J Exp Bot 2005; 56:1665-74; PMID:15837704; http://dx.doi.org/ 10.1093/jxb/eri163 [DOI] [PubMed] [Google Scholar]
- 19. Xu N, Gao XQ, Zhao XY, Zhu DZ, Zhou LZ, Zhang XS. Arabidopsis AtVPS15 is essential for pollen development and germination through modulating phosphatidylinositol 3-phosphate formation. Plant Mol Biol 2011; 77:251-60; PMID:21833541; http://dx.doi.org/ 10.1007/s11103-011-9806-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gutierrez RA, Stokes TL, Thum K, Xu X, Obertello M, Katari MS, Tanurdzic M, Dean A, Nero DC, McClung CR, et al. Systems approach identifies an organic nitrogen-responsive gene network that is regulated by the master clock control gene CCA1. Proc Natl Acad Sci USA 2008; 25; 105:4939-44; PMID:18344319; http://dx.doi.org/ 10.1073/pnas.0800211105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu SX, Webb CJ, Knowles SM, Kim SH, Wang Z, Tobin EM.CCA1 and ELF3 interact in the control of hypocotyl length and flowering time in Arabidopsis. Plant Physiol 2012; 158:1079-1088; PMID:22190341; http://dx.doi.org/ 10.1104/pp.111.189670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng DW, Miller M, Yu HH, Huang TY, Kim ED, Lu J, Xie Q, McClung CR, Chen ZJ. A role for CHH methylation in the parent-of-origin effect on altered circadian rhythms and biomass heterosis in Arabidopsis intraspecific hybrids. Plant Cell 2014; 26:2430-40; PMID:24894042; http://dx.doi.org/ 10.1105/tpc.113.115980 [DOI] [PMC free article] [PubMed] [Google Scholar]


