Abstract
The current model of auxin-inducible transcription describes numerous regulatory interactions between AUXIN RESPONSE FACTORs (ARFs) and Aux/IAAs. However, specific relationships between individual members of these families in planta remain largely uncharacterized. Using a systems biology approach, the entire suite of Aux/IAA genes directly regulated by the developmentally pivotal ARF MONOPTEROS (MP) was recently determined for multiple Arabidopsis tissue types. This study showed that MP directly targets distinct subclades of Aux/IAAs, revealing potential regulatory modules of redundantly acting Aux/IAAs involved in MP-dependent processes. Further, functional analyses indicated that the protein products of these targeted Aux/IAAs negatively feedback on MP. Thus, comprehensive identification of Aux/IAAs targeted by individual ARFs will generate biologically meaningful networks of ARF-Aux/IAA regulatory modules controlling distinct plant pathways.
Keywords: Arabidopsis development, Aux/IAA genes, AUXIN RESPONSE FACTOR (ARF), functional genomics, transcriptional regulatory network
Abbreviations
- ARF
Auxin Response Factor
- GR
glucocorticoid receptor
- MP
MONOPTEROS
For over a decade, a model detailing the interactions between 2 families of transcriptional regulators has provided an important framework explaining how the phytohormone auxin modulates gene expression in plant growth and development.1 Members of the first family, the AUXIN RESPONSE FACTORS (ARFs), contain a conserved B3-type DNA binding domain that facilitates protein dimerization and mediates binding to Auxin Response Elements of target genes. While ARFs are functionally characterized as either activators or repressors (depending on the nature of their weakly conserved middle domain), the influence of auxin on gene expression is best understood for activator ARFs. This influence stems from physical interactions between ARFs and a second family of transcriptional regulators, the Aux/IAAs. Aux/IAAs directly bind ARFs through shared dimerization domains III and IV and confer transcriptional repression through their domain I. Auxin destabilizes these repressors by promoting interaction between Aux/IAA domain II and members of the TIR family of F-box proteins.2 In this way, auxin frees activator ARFs from the effects of Aux/IAAs, allowing rapid upregulation of auxin-inducible transcripts. Here, it is predicted that the upregulated genes include the Aux/IAAs themselves, the protein products of which then feedback upon ARF activity (Fig. 1A). This ensures that Aux/IAA-conferred repression of ARF activity is promptly re-established once auxin levels subside. Substantial biochemical evidence has led to the establishment of this molecular model, which effectively shows how an auxin input can trigger a transcriptional response, often detectable within only a few minutes.1 However, the model is usually presented in a general form lacking details regarding which individual ARFs and Aux/IAAs are participating in these regulatory relationships and in which tissue types. Without such detail, an overwhelming array of combinatorial interactions appears possible within the context of the model, given the numerous members of each protein family (23 ARFs and 29 Aux/IAAs in Arabidopsis). This regulatory potential has been suggested to contribute to the versatility of auxin, whose activity modulates essentially every facet of plant growth and development.2 Even so, it would be unrealistic to assume that all ARFs interact with all Aux/IAAs in planta, especially since gene expression analyses show that these factors vary in their spatial distribution.3,4 Therefore, to fully understand how auxin exerts its widespread influence on plant growth responses, relationships between specific, individual ARFs and Aux/IAAs must be established. Only then can we start to replace popular depictions of generalized ARF-Aux/IAA models, which imply seemingly unlimited combinations of ARF and Aux/IAA interactions, with more focused, biologically meaningful schemes.
Figure 1.
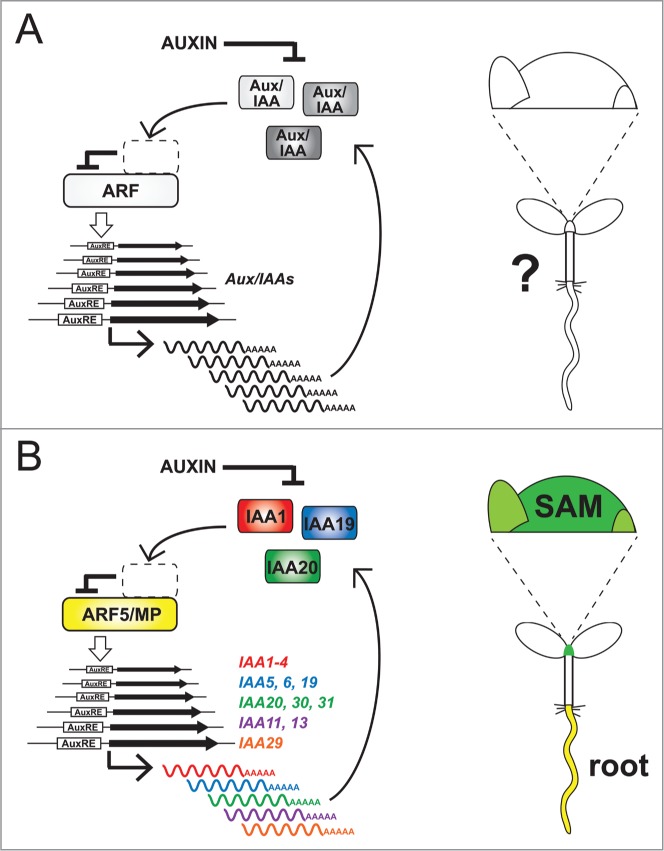
Models of auxin-mediated transcription. (A) Left: General model of ARF-Aux/IAA regulatory interactions and their influence on gene expression. In the absence of auxin, Aux/IAA repressors bind and repress activator ARFs, preventing upregulation of target genes. Auxin promotes Aux/IAA protein destabilization, allowing ARF-mediated upregulation of auxin-responsive transcripts, including the Aux/IAAs themselves. Right: Tissue-specific activities of ARF-Aux/IAA regulatory modules are not well established. (B) Left: ARF5/MP-specific model of auxin-inducible transcription. ARF5/MP directly activates numerous Aux/IAA genes, most of which can be organized into phylogenetically related groups. Where tested, protein products of these target genes (including IAA1, 19 and 20) negatively feedback onto MP function. Right: ARF5/MP directly regulates the same suite of Aux/IAA genes in both shoot apical meristem (SAM) and root tissue.
Given the large number of potential factors involved, systems biology approaches appear best suited to elucidate the authentic network of ARF-Aux/IAA relationships. As a first step, we have recently applied such an approach to interrogate the regulatory interactions associated with the developmentally important ARF ARF5/MONOPTEROS (MP),5 which mediates auxin signal transduction and organ formation in Arabidopsis.6 We sought to elucidate the entire suite of Aux/IAA genes directly regulated by MP, and whether the protein products of these targets feedback upon MP activity.
To this end, we established a steroid-inducible form of MP by fusing it to the hormone binding domain of the mammalian glucocorticoid receptor (GR)7 to generate MP::MP-GR. We assessed the transcriptional effects of MP-GR activation and established that MP directly regulates nearly half of all Arabidopsis Aux/IAA genes, implicating this ARF as a central player in auxin-mediated transcription.5 Importantly, the phylogenetic relationships between the regulated genes were not random. Rather, previously established clades of related Aux/IAAs8-10 were coordinately activated (including IAA1-4; IAA5, 6 and 19; IAA20, 30 and 31). This observation is noteworthy because it hints at presumptive clades of structurally and functionally related Aux/IAAs that modulate MP-dependent pathways. While many Aux/IAAs have been genetically defined by gain-of-function mutations in domain II that stabilize protein abundance, widespread functional redundancy is predicted to have masked the isolation of loss-of-function mutations.11 Thus, targeted mutation of small groups of related genes coordinately regulated by an ARF offers a promising strategy to genetically identify authentic Aux/IAA functions. Finally, these Aux/IAA target genes displayed expression patterns that were auxin-inducible and overlapped with and depended upon MP expression whenever tested (Fig 2 and 3).5
Figure 2.
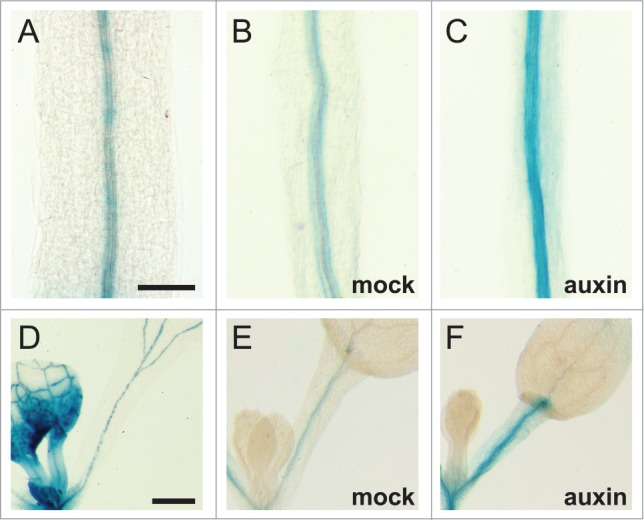
Expression patterns and auxin responsiveness of MP target genes IAA19 and IAA30. (A-C) 5 d after germination (DAG) hypocotyls expressing (A) MP::MP-GUS or (B, C) IAA19::GUS. (D-F) 5–6 DAG cotyledon petioles expressing (D) MP::MP-GUS or (E and F) IAA30::GUS. Tissues depicted in (C and F) were treated for 4h with auxin (10 μM indole-3-acetic acid) prior to GUS staining. Note that the expression patterns of IAA19 and IAA30 overlap with that of MP (A, B, D, and E) and are auxin inducible (compare C to B and F to E). 1701 basepairs of upstream non-coding sequence was used to generate the IAA30::GUS reporter. The IAA19::GUS reporter and GUS-staining protocol has been previously described.5 Bars: (A-C) 0.2 mm; (D-F) 0.5 mm.
Figure 3.
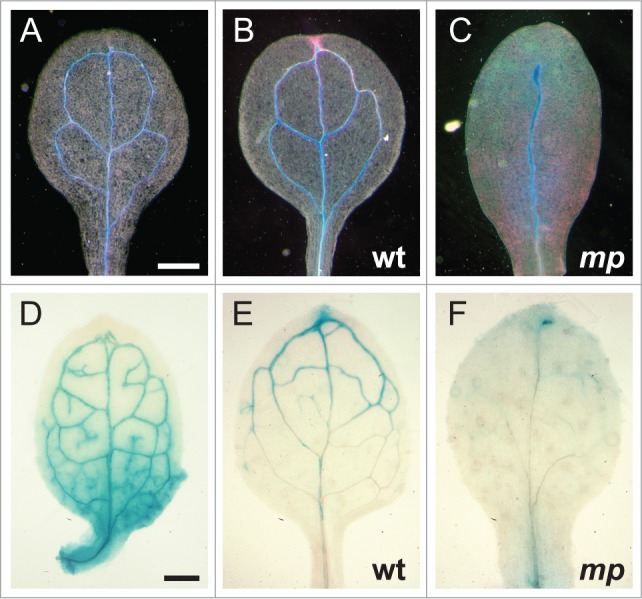
Expression of IAA1 and IAA20 overlaps with and depends upon MP. (A-C) Dark-field images of 6 d after germination (DAG) cotyledons expressing (A) MP::MP-GUS or (B and C) IAA1::GUS. Strong GUS expression appears blue, while weaker expression appears pink. Vascular strand xylem is clearly visible under dark-field conditions. (D-F) Bright-field images of 6 DAG first leaves expressing (D) MP::MP-GUS or (E, F) IAA20::GUS. Note that the expression patterns of IAA1 and IAA20 overlap with MP in lateral organ vasculature (A, B, D and E) and are reduced, along with the overall extent of vascularization, in an mp mutant (compare C to B and F to E). GUS reporter constructs and GUS-staining protocol has been previously described.5 Bars: (A-C) 0.5 mm; (D-F) 0.2 mm.
It is of note that the exact same suite of Aux/IAA genes was upregulated by MP in both shoot and root tissue.5 Since data of this type is available only for MP and not for other ARFs, it is difficult to make generalizations from these observations. Nonetheless, our results suggest that the complexity of the full ARF-Aux/IAA network may not be exacerbated by tissue-specific differences in the transcriptional activity of any given ARF.
Finally, we performed genetic analyses to assess how frequently the protein products of Aux/IAAs negatively feedback onto the activator ARF responsible for their expression. Because mutants are not available for the majority of the Aux/IAA genes targeted by MP, we chose one representative gene from each Aux/IAA clade mentioned above (namely, IAA1, 19 and 20) to perform these assays. In each case, antagonism between the target Aux/IAA and MP protein function was apparent.5 This suggests that coordinately regulated Aux/IAA gene clades operate redundantly by collectively modulating MP-dependent processes, including organ growth and vascular patterning.
Investigating the regulatory relationships between a single ARF (MP) and the Aux/IAA family has allowed the insertion of specific players into the generalized model for auxin-responsive gene expression (Fig. 1B). Moreover, these relationships have been established for multiple tissue types, including both shoot and root (Fig. 1B). Phylogenetic evidence and expression patterns indicate that clades of functionally related Aux/IAAs form regulatory modules with an individual ARF. This will facilitate targeted genetic analyses to establish further regulatory relationships controlling auxin-mediated transcription. Two other obvious directions follow from these results. First, similar studies on other ARFs may establish novel functional modules associated with the regulation of particular traits. As a result, each protein family member will no longer be characterized by its regulatory potential, but rather by its actual biological role in the organism. Second, applying similar strategies in other plants will establish how conserved ARF-Aux/IAA functional modules are, which will provide tremendous opportunities to convert these insights into practical advantages in the optimization of crop plants.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Guilfoyle TJ, Hagen G. Getting a grasp on domain III/IV responsible for Auxin Response Factor-IAA protein interactions. Plant Sci 2012; 190:82-8; PMID:22608522; http://dx.doi.org/ 10.1016/j.plantsci.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 2. Chapman EJ, Estelle M. Mechanism of auxin-regulated gene expression in plants. Annu Rev Genet 2009; 43:265-285; PMID:19686081; http://dx.doi.org/ 10.1146/annurev-genet-102108-134148 [DOI] [PubMed] [Google Scholar]
- 3. Rademacher EH, Moller B, Lokerse AS, Llavata-Peris CI, van den Berg W, Weijers D. A cellular expression map of the Arabidopsis AUXIN RESPONSE FACTOR gene family. Plant J 2011; 68:597-606; PMID:21831209; http://dx.doi.org/ 10.1111/j.1365-313X.2011.04710.x [DOI] [PubMed] [Google Scholar]
- 4. Vernoux T, Brunoud G, Farcot E, Morin V, Van den Daele H, Legrand J, Oliva M, Das P, Larrieu A, Wells D, et al. The auxin signalling network translates dynamic input into robust patterning at the shoot apex. Mol Syst Biol 2011; 7:508; PMID:21734647; http://dx.doi.org/ 10.1038/msb.2011.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krogan NT, Yin X, Ckurshumova W, Berleth T. Distinct subclades of Aux/IAA genes are direct targets of ARF5/MP transcriptional regulation. New Phytol 2014; 204:474-83; PMID:25145395; http://dx.doi.org/ 10.1111/nph.12994 [DOI] [PubMed] [Google Scholar]
- 6. Hardtke CS, Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J 1998; 17:1405-11; PMID:9482737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang R, Zhou X, Wang X. Chemically regulated expression systems and their applications in transgenic plants. Transgenic Res 2003; 12:529-40; PMID:14601652; http://dx.doi.org/ 10.1023/A:1025852307127 [DOI] [PubMed] [Google Scholar]
- 8. Liscum E, Reed JW. Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol Biol 2002; 49:387-400; PMID:12036262; http://dx.doi.org/ 10.1023/A:1015255030047 [DOI] [PubMed] [Google Scholar]
- 9. Remington DL, Vision TJ, Guilfoyle TJ, Reed JW. Contrasting modes of diversification in the Aux/IAA and ARF gene families. Plant Phys 2004; 135:1738-52; PMID:15247399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Overvoorde PJ, Okushima Y, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Liu A, Onodera C, Quach H, et al. Functional genomic analysis of the AUXIN/INDOLE-3-ACETIC ACID gene family members in Arabidopsis thaliana. Plant Cell 2005; 17:3282-300; PMID:16284307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Woodward AW, Bartel B. Auxin: regulation, action, and interaction. Ann Bot 2005; 95:707-35; PMID:15749753; http://dx.doi.org/ 10.1093/aob/mci083 [DOI] [PMC free article] [PubMed] [Google Scholar]


