Abstract
Thanks to their distinctive mode of action in a coordinated switch-like way, their multi-tiered signaling cascades and their involvement in cell responses to multiple internal and external stimuli, MAP kinases offer a remarkable possibility to be assembled into what we can call “MAPK transgenic circuits” to improve cell functions. Such circuit could be used to enhance cell signaling efficiency and boost cell functions for several purposes in plant biotechnology, medicine, and pharmaceutical industry.
Keywords: MAP kinase, MAPK transgenic circuit, mitogen activated protein kinase
Abbreviations
- MAP
Mitogen activated protein
- MAPK
MAP kinase
- MAPKK
MAPK kinase
- MAPKKK
MAPKK kinase
MAP Kinases and Environmental Stresses
Mitogen-Activated-Protein Kinases (MAPKs) are ubiquitous components of signaling pathways that convey biological information from receptors to target molecules in response to multiple intra- and extracellular signals. Accumulated knowledge confirms important roles of MAPK signaling pathways in most cell activities from cell nascence to cell death, including embryogenesis, cell division, hormone signaling, and adaptation to diverse environmental stresses. Plant adaptation is an important qualitative and quantitative trait controlled by multiple genes and complex signaling network. Biotechnology programs should move from the single gene manipulation to multiple genes approach. Particularly, it would be crucial to identify and quantify interacting molecules that act synergistically to increase plant adaptation to severe and multiple environmental stresses.
Scientific literature abounds with studies reporting positive effects of plant transformation with single genes on stress tolerance under controlled conditions (i.e. in vitro). Recently, several genes of the MAPKKK gene family have been identified in canola (Brassica napus).1 Some of them (BnaMAPKKK18 and BnaMAPKKK19 for instance) induce hypersensitivity response (HR)-like cell death and protect tobacco cells against biotic stress damages. Other genes of the same family are regulated by both biotic and abiotic stress treatments. Similarly, Wang et al. (2014) reported that the ectopic expression of the maize MAP kinase ZmSIMK1 (a gene of the group B MAPKs) results in an improved tolerance to both biotic (e.g. Pseudomonas syringae) and abiotic stresses (salt and drought).2 Moreover, transgenic tobacco expressing ZmSIMK1 exhibits an increased rate of seed germination and high levels of antioxidant enzymes with reduced accumulation of reactive oxygen species (ROS). The ZmSIMK1 also triggers a systemic acquired resistance (SAR) by inducing the expression of pathogen-related (PR) genes and hypersensitive response (HR) to protect plant cells from biotic stress injuries.2 A novel MAP kinase gene, called ZmMKK1 of the MAPKK family, group A, has also been recently identified in maize, conferring tolerance to chilling and defense responses to pathogens by increasing antioxidant enzyme activities and enhancing the accumulation of osmolytes.3 The overexpression of another maize MAPK gene, ZmMPK5, induces the defense responses in tobacco and confers tolerance to salt stress with healthier phenotype compared to wild type.4,5 In Arabidopsis, the interchangeable MAPK genes, MPK3/MPK6 seem to regulate the activity of the heat shock factor HSFA4A, conferring tolerance to salt and oxidative stresses.6
These findings and many others, recently reviewed,7,8 confirm the role of MAP kinases in environmental stress responses and highlight a great potential of MAP kinases in improving plant-tolerance through biotechnology programs. However, despite these positive outputs, results under field conditions are still below expectations, suggesting that single genes approach is not so effective to produce inherently stress-adapted plants, due to the differences between one simple variable lab condition and more complex multivariable field conditions. The reason behind this discrepancy would be that genetic transformation with a single gene may result in an overproduction of the corresponding transgene product compared to unchanged concentration of its target, yielding in disproportional concentrations between the interacting partners. Although a signal amplification may exist, the signal amplification may do not reach the threshold required to trigger effective cellular responses. This assumption was confirmed recently by a report showing that augmenting the concentrations of sequentially interacting MAP kinases increases the sensitivity of a signaling module and reduces the activation threshold.9
Subsequently, to insure a functional equilibrium and obtain a powerful genetic circuit system, simultaneous genetic transformation with a set of functionally-related genes would produce ‘calibrated’ concentrations of the interacting molecules and guarantee an efficient interaction between transgenes and their targets involved in the same signaling pathway. MAP kinases are good candidates for such an approach for 3 main reasons. First, MAP kinases are ubiquitously involved in developmental and stress signaling pathways. Second, MAP kinases, with phosphatases, operate as On/Off cell-signaling switchers with a distinctive mode of action in a coordinated switch-like way.10 Third, MAP kinases are remarkably “wired” to each other in a multi-tiered cascade, where each kinase activates another in a sequential way: MAPKKK→MAPKK→MAPK, where MAPKKK, MAPKK and MAPK are genes belonging to different MAP kinase gene families. Moreover, an efficient signaling system should be composed of elements optimized to function in their natural context, not in the context of the synthetic circuit,11 which is the case of MAP kinases. Three MAP kinases, plus a receptor-encoding gene, if available or predictable, and a target gene can thus be rewired into one signaling module to form a “MAPK transgenic circuit” (Fig. 1) to generate new genotypes with new characteristics to enhance cell functions and signaling efficacy for example to improve plant adaptation to its environment, or other biotechnology applications.
Figure 1.
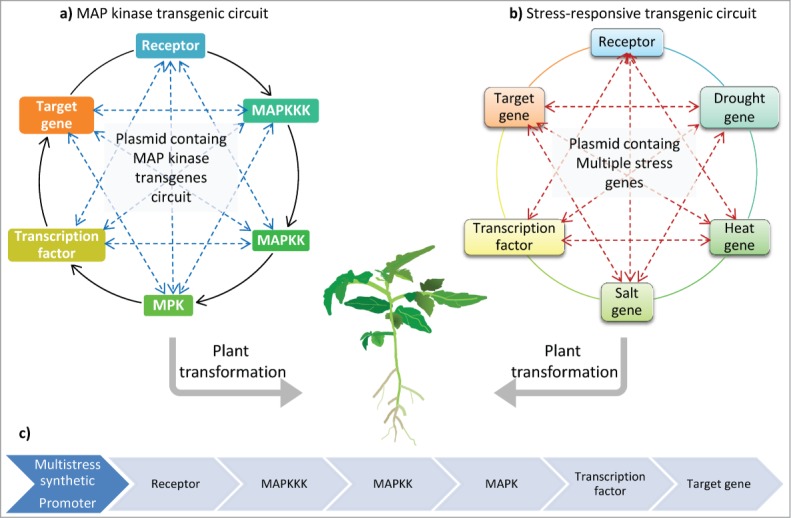
Schema of conceptual ‘transgenic circuits’ to enhance plant adaptation to environmental stresses. Plants are simultaniously exposed to multiple environmental constraints. Transformation with multiple stress-functionaly-related genes such as MAP kinases may provide an efficient solution to develop sustainablly stress tolerant spieces. (A) MAP kinase trangenic circuit composed of receptor, functionally related MAP kinases (MAPKKK ◊ MAPKK ◊ MAPK genes), transcription factor and a target gene. (B) Multi-stress transgenes circuit composed of receptor, drought responsive gene, salt responsive gene, heat (or cold) responsive gene, transcription factor and target gene. Dotted arrows indicate potential crosstalk between different-stress genes. (C) The transgenes can be assembled under the control of constitutive or inducible promoter on one or multiple vectors to be targeted to chloroplasts or mitochondria.
In abiotic stress context, a short part of MAPK stress cascade composed of 3 MAPK kinases (MEKK1→MKK2→MPK4/MPK6) was identified.12 Although this is an incomplete MAP cascade, it would be an important hunt for transgenic circuit approach to assess plant tolerance to abiotic stresses using this kinase core. The potential of using such a short MAPK core is supported by a recent study demonstrating that a fine-tunable signal processing can be inherited to a minimal MAPK module composed of only 3 tiered MAPKs.9 The aforementioned short MAPK cascade (MEKK1→MKK2→MPK4/6) can thus be engineered into a transgenic circuit for abiotic stress assessment, despite lacking receptor and transcription factor. Fewer components limited to only a MAP kinase rewired to a receptor have also been reported to enhance potato tolerance to blight disease by insuring positive signaling feedback.13 To assess plant tolerance to biotic stresses (for example resistance to bacteria and fungi) a more tiered MAPK cascade starting with flagellin receptor (FLS2) and ending by WRKY transcription factor, with MAPK core composed of (MEKK1, MKK4/MKK5, MPK3/MPK6)14 represents a worthy choice for MAPK transgenic circuit approach for the assessment of sustainable pathogen resistance.
Rewiring Multiple MAP Kinases to Construct “MAPK Transgenic Circuit”
To construct MAPK transgenic circuits, there are several methods. In the few last years, an important variety of transformation approaches and plasmid vectors have been engineered and commercialized to facilitate cloning, transfer, and expression of multiple genes. The candidate genes for transgenic circuits can be selected based on their involvements in plant stress-tolerance, or any other biological question of interest, and then stacked directly on one single vector or on different vectors, or they can be assembled indirectly by iterative rounds of genetic crossing between different lines carrying the desired single genes.15 However, the one single vector approach has the advantage that only one DNA molecule needs to be transferred and all the transgenes would be inherited together. Using bioactive-mediated transformation method, for example, the introduction of large DNA fragments into plant cells has been reported.16 More recently, 7 genes were assembled by an improved version of multiround Gateway technology.17 Another progress for multigenes transformation came from the use of site specific recombination with homing endonucleases to stack different genes into artificial chromosome vector18 or minichromosome.19
Which Promoters to Use?
Based on the strategy being used, the transgenic circuit can be driven by specific promoters and expressed as a constitutive, inducible or tissue-specific expression.20 Due to the segmental nature of gene promoter, cis-regulatory elements of different stress-responsive genes can be tethered by subunits into one block to construct synthetic promoters21 to lead the expression of the multigenes circuit. Fundamental research, however, will be required to determine the right inputs and outputs, the specific interacting MAPKs and their targets to define where and when these components may provide the best results. Developing transgenic circuit approach would give the possibility to switch the transgenic circuit on and off at the right moment by an exogenous application of appropriate activators/repressors, as it was demonstrated by O’Shaughnessy et al. through the application of the estradiol to assess the steady state-activation of MAPK signaling module.9
Where to Target the Expression of Transgenic Circuits?
Once engineered in appropriate vectors, transgenes circuits can be delivered into plant cells either to plastidal, mitochondrial or nuclear genomes. The nuclear genome, however, involves several challenges, which include (i) eukaryote nuclear genome does not process polycistronic genes, (ii) risk of gene silencing due to RNAi mechanisms, and (iii) random integration of the transgenes into the nuclear DNA, which may cause side position-effects on the development of the transgenic line (e.g., dwarfism or sterility). Different selection markers may also be required at each step of the obtention of multigenes lines, resulting in an extended selection process. Alternatively, and owing to the prokaryotic nature of the chloroplasts and mitochondria, multiples genes can be advantageously introduced as a single polycistronic operon into chloroplast or mitochonria.
An important question may rise about potential crosstalks of the transgenic circuit with the native pathways, which might represent a challenge for the new rewired modules. However, in bacteria (E. coli), the rewiring of promoters with different transcription or sigma factor genes in hundreds of combinations were tolerated by the bacteria genome with only slight effect on the growth.22 In plant, Navqi et al (2009) have created multiplex transgenic maize expressing at least 5 genes involved in vitamin metabolic pathways with no undesirable effects observed.23 In Arabidopsis, Kristensen et al (2005) also engineered an entire metabolic pathways without negative major effects on the metabolome and transcriptome.24
Regardless of these findings, one of the possible strategies to circumvent any potentially undesirable crosstalk is to design transgenic circuit as an orthogonal or heteroulogous module, which does not crosstalk with native pathways.
Finally, by constructing MAPK transgenic circuit and evaluating cell responses, we boost our comprehension of how MAPK modules work and how to exploit them in important applications in biopharmaceutical industry, medicine, and plant biotechnology. The good comprehension of the mechanisms of reception and signal transduction pathways involved in activating cell adaptive responses contribute considerably to the aim of developing an effective and maintainable plant stress tolerance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Sun Y, Wang C, Yang B, Wu F, Hao X, Liang W, Niu F, Yan J, Zhang H, Wang B, et al. Identification and functional analysis of mitogen-activated protein kinase kinase kinase (MAPKKK) genes in canola (Brassica napus L.). J Exp Bot 2014; 65:2171-88; PMID:24604738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang L, Liu Y, Cai G, Jiang S, Pan J, Li D. Ectopic expression of ZmSIMK1 leads to improved drought tolerance and activation of systematic acquired resistance in transgenic tobacco. J Biotechnol 2014; 172:18-29; PMID:24291188; http://dx.doi.org/ 10.1016/j.jbiotec.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 3. Cai G, Wang G, Wang L, Pan J, Liu Y, Li D. ZmMKK1, a novel group A mitogen-activated protein kinase kinase gene in maize, conferred chilling stress tolerance and was involved in pathogen defense in transgenic tobacco. Plant Sci 2014; 214:57-73; PMID:24268164; http://dx.doi.org/ 10.1016/j.plantsci.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 4. Zhang D, Jiang S, Pan J, Kong X, Zhou Y, Liu Y, Liu Y, Li D. The overexpression of a maize mitogen-activated protein kinase gene (ZmMPK5) confers salt stress tolerance and induces defence responses in tobacco. Plant Biol 2014; 16:558-70; PMID:23952812; http://dx.doi.org/ 10.1111/plb.12084 [DOI] [PubMed] [Google Scholar]
- 5. Zhang D, Jiang S, Pan J, Kong X, Zhou Y, Liu Y, Liu Y, Li D. The overexpression of a maize mitogen-activated protein kinase gene (ZmMPK5) confers salt stress tolerance and induces defence responses in tobacco. Plant Biol 2013; 16:558-70; PMID:23952812 [DOI] [PubMed] [Google Scholar]
- 6. Pérez-Salamó I, Papdi C, Gábor R, Zsigmond L, Vilela B, Lumbreras V, Nagy I, Horváth B, Domoki M, Darula Z, et al. The Heat Shock Factor HSFA4A confers salt tolerance and is regulated by oxidative stress and the MAP kinases, MPK3 and MPK6. Plant Physiol 2014; 165:319-34; PMID:24676858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moustafa K, AbuQamar S, Jarrar M, Al-Rajab AJ, Tremouillaux-Guiller J. MAPK cascades and major abiotic stresses. Plant Cell Rep 2014; 33:1217-25; PMID:24832772; http://dx.doi.org/ 10.1007/s00299-014-1629-0 [DOI] [PubMed] [Google Scholar]
- 8. Moustafa K. Improving plant stress-tolerance: potential applications of engineered MAPK cascades. Trends Biotechnol 2014; 32:389-90, PMID:24986255; http://dx.doi.org/ 10.1016/j.tibtech.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 9. O’Shaughnessy EC, Palani S, Collins JJ, Sarkar CA. Tunable signal processing in synthetic MAP kinase cascades. Cell 2011; 144:119-31; PMID:21215374; http://dx.doi.org/ 10.1016/j.cell.2010.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrell JE, Jr. Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends Biochem Sci 1996; 21:460-6; PMID:9009826; http://dx.doi.org/ 10.1016/S0968-0004(96)20026-X [DOI] [PubMed] [Google Scholar]
- 11. Haseltine EL, Arnold FH. Synthetic gene circuits: design with directed evolution. Ann Rev Biophys Biomol Struct 2007; 36:1-19; PMID:17243895; http://dx.doi.org/ 10.1146/annurev.biophys.36.040306.132600 [DOI] [PubMed] [Google Scholar]
- 12. Teige M, Scheikl E, Eulgem T, Doczi R, Ichimura K, Shinozaki K, Shinozaki K, Dangl JL, Hirt H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell 2004; 15:141-52; PMID:15225555; http://dx.doi.org/ 10.1016/j.molcel.2004.06.023 [DOI] [PubMed] [Google Scholar]
- 13. Yamamizo C, Kuchimura K, Kobayashi A, Katou S, Kawakita K, Jones JD, Doke N, Yoshioka H. Rewiring mitogen-activated protein kinase cascade by positive feedback confers potato blight resistance. Plant Physiol 2006; 140:681-92; PMID:16407438; http://dx.doi.org/ 10.1104/pp.105.074906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002; 415:977-83; PMID:11875555; http://dx.doi.org/ 10.1038/415977a [DOI] [PubMed] [Google Scholar]
- 15. Ma JK, Hiatt A, Hein M, Vine ND, Wang F, Stabila P, van Dolleweerd C, Mostov K, Lehner T. Generation and assembly of secretory antibodies in plants. Science (New York, NY) 1995; 268:716-9; PMID:7732380; http://dx.doi.org/ 10.1126/science.7732380 [DOI] [PubMed] [Google Scholar]
- 16. Wada N, Kajiyama S, Akiyama Y, Kawakami S, No D, Uchiyama S, Otani M, Shimada T, Nose N, Suzuki G, et al. Bioactive beads-mediated transformation of rice with large DNA fragments containing Aegilops tauschii genes. Plant Cell Rep 2009; 28:759-68; PMID:19214515; http://dx.doi.org/ 10.1007/s00299-009-0678-2 [DOI] [PubMed] [Google Scholar]
- 17. Buntru M, Gartner S, Staib L, Kreuzaler F, Schlaich N. Delivery of multiple transgenes to plant cells by an improved version of MultiRound Gateway technology. Transgenic Res 2013; 22:153-67; PMID:22972476; http://dx.doi.org/ 10.1007/s11248-012-9640-0 [DOI] [PubMed] [Google Scholar]
- 18. Lin L, Liu YG, Xu X, Li B. Efficient linking and transfer of multiple genes by a multigene assembly and transformation vector system. Proc Natl Acad Sci U S A 2003; 100:5962-7; PMID:12719540; http://dx.doi.org/ 10.1073/pnas.0931425100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Houben A, Mette MF, Teo CH, Lermontova I, Schubert I. Engineered plant minichromosomes. Int J Dev Biol 2013; 57:651-7; PMID:24166447; http://dx.doi.org/ 10.1387/ijdb.130144ah [DOI] [PubMed] [Google Scholar]
- 20. Gurr SJ, Rushton PJ. Engineering plants with increased disease resistance: how are we going to express it? Trends Biotechnol 2005; 23:283-90; PMID:15922080; http://dx.doi.org/ 10.1016/j.tibtech.2005.04.009 [DOI] [PubMed] [Google Scholar]
- 21. Venter M. Synthetic promoters: genetic control through cis engineering. Trends Plant Sci 2007; 12:118-24; PMID:17292658; http://dx.doi.org/ 10.1016/j.tplants.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 22. Isalan M, Lemerle C, Michalodimitrakis K, Horn C, Beltrao P, Raineri E, Garriga-Canut M, Serrano L. Evolvability and hierarchy in rewired bacterial gene networks. Nature 2008; 452:840-5; PMID:18421347; http://dx.doi.org/ 10.1038/nature06847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Naqvi S, Zhu C, Farre G, Ramessar K, Bassie L, Breitenbach J, Perez Conesa D, Ros G, Sandmann G, Capell T, et al. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc Natl Acad Sci U S A 2009; 106:7762-7; PMID:19416835; http://dx.doi.org/ 10.1073/pnas.0901412106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kristensen C, Morant M, Olsen CE, Ekstrom CT, Galbraith DW, Moller BL, Bak S. Metabolic engineering of dhurrin in transgenic Arabidopsis plants with marginal inadvertent effects on the metabolome and transcriptome. Proc Natl Acad U S A 2005; 102:1779-84; PMID:15665094; http://dx.doi.org/ 10.1073/pnas.0409233102 [DOI] [PMC free article] [PubMed] [Google Scholar]


